Prognostic Factors for Post-Recurrence Survival in Stage II and III Colorectal Carcinoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Treatment and Follow-Up
2.3. Salvage Therapies
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Post-Recurrence Survival
3.3. Salvage Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Batut, M.J. (Ed.) Maligni tumori u Republici Srbiji; Institut za javno zdravlje Srbije: Beograd, Serbia, 2018; ISBN 978-86-7358-111-8. (In Serbian) [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.H.; Henson, D.E.; Hutter, R.V.P.; Nagle, R.B.; et al. Prognostic Factors in Colorectal Cancer. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.K.; Berlin, J.; Branton, P.; Burgart, L.J.; Carter, D.K.; Fitzgibbons, P.L.; Halling, K.; Frankel, W.; Jessup, J.; Kakar, S.; et al. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. Arch. Pathol. Lab. Med. 2009, 133, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, L.L.; Jessup, J.M.; Sargent, D.J.; Greene, F.L.; Stewart, A.K. Revised TN Categorization for Colon Cancer Based on National Survival Outcomes Data. J. Clin. Oncol. 2010, 28, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuniga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmaña, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Mason, M.J.; Sinicrope, F.A.; Phipps, A.I.; Tejpar, S.; Nesbakken, A.; Danielsen, S.A.; Sveen, A.; Buchanan, D.D.; Clendenning, M.; et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: A retrospective, pooled biomarker study. Ann. Oncol. 2017, 28, 1023–1031. [Google Scholar] [CrossRef]
- Reinert, T.; Schøler, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.-S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2015, 65, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Dienstmann, R.; Villacampa, G.; Sveen, A.; Mason, M.J.; Niedzwiecki, D.; Nesbakken, A.; Moreno, V.; Warren, R.S.; Lothe, R.A.; Guinney, J. Relative contribution of clinicopathological variables, genomic markers, transcriptomic subtyping and microenvironment features for outcome prediction in stage II/III colorectal cancer. Ann. Oncol. 2019, 30, 1622–1629. [Google Scholar] [CrossRef] [Green Version]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; André, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. J. Clin. Oncol. 2009, 27, 872–877. [Google Scholar] [CrossRef]
- Jeffery, M.; Hickey, B.; Hider, P.N.; See, A.M. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst. Rev. 2016, 11, CD002200. [Google Scholar] [CrossRef]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef] [Green Version]
- Goey, K.K.; Sørbye, H.; Glimelius, B.; Adams, R.A.; André, T.; Arnold, D.; Berlin, J.D.; Bodoky, G.; de Gramont, A.; Díaz-Rubio, E.; et al. Consensus statement on essential patient characteristics in systemic treatment trials for metastatic colorectal cancer: Supported by the ARCAD Group. Eur. J. Cancer 2018, 100, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumors, 7th ed.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. Carcinoma of the colon and rectum. In WHO Classification of Tumours of the Digestive System, 4th ed.; IARC Press: Lyon, France, 2010; pp. 134–144. [Google Scholar]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi64–vi72. [Google Scholar] [CrossRef] [PubMed]
- The R Foundation for Statistical Computing; Platform: x86_64-w64-mingw32/x64 (64-bit); “Sincere Pumpkin Patch”; Copyright (C). 2016. Available online: https://www.r-project.org/ (accessed on 21 January 2017).
- Joachim, C.; Macni, J.; Drame, M.; Pomier, A.; Escarmant, P.; Veronique-Baudin, J.; Vinh-Hung, V. Overall survival of colorectal cancer by stage at diagnosis. Medicine 2019, 98, e16941. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.H.; Renfro, L.A.; De Gramont, A.; Meyers, J.P.; Maughan, T.S.; Seymour, M.T.; Saltz, L.; Goldberg, R.M.; Sargent, D.J.; Eckhardt, S.G.; et al. Association of Age With Survival in Patients With Metastatic Colorectal Cancer: Analysis From the ARCAD Clinical Trials Program. J. Clin. Oncol. 2014, 32, 2975–2982. [Google Scholar] [CrossRef]
- Doat, S.; Thiébaut, A.; Samson, S.; Ricordeau, P.; Guillemot, D.; Mitry, E. Elderly patients with colorectal cancer: Treatment modalities and survival in France. National data from the ThInDiT cohort study. Eur. J. Cancer 2014, 50, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Shack, L.; Shah, A.; Lambert, P.; Rachet, B. Cure by age and stage at diagnosis for colorectal cancer patients in North West England, 1997–2004: A population-based study. Cancer Epidemiol. 2012, 36, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.R.; Park, G.E.; Johnson, E.K.; Martin, M.J.; Stojadinovic, A.; Maykel, J.A.; Causey, M.W. The Impact of Age on Colorectal Cancer Incidence, Treatment, and Outcomes in an Equal-Access Health Care System. Dis. Colon Rectum 2014, 57, 303–310. [Google Scholar] [CrossRef]
- Berian, J.R.; Benson, A.B.; Nelson, H.; Iii, A.B.B. Young Age and Aggressive Treatment in Colon Cancer. JAMA 2015, 314, 613–614. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A System-atic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [Green Version]
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H.; The GEKID Cancer Survival Working Group. Sex Differences in Colorectal Cancer Survival: Population-Based Analysis of 164,996 Colorectal Cancer Patients in Germany. PLoS ONE 2013, 8, e68077. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, G.; He, J.; Ren, S.; Wu, F.; Zhang, J.; Wang, F. Gender differences in colorectal cancer survival: A meta-analysis. Int. J. Cancer 2017, 141, 1942–1949. [Google Scholar] [CrossRef]
- Samawi, H.H.; Yin, Y.; Speers, C.H.; Cheung, W.Y. Sex Disparities in Outcomes of Early Stage Colorectal Cancer: A Population-Based Study. Clin. Colorectal Cancer 2018, 17, e711–e717. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Dai, W.; Li, Q.; Cai, S.; Peng, J. Prognostic Effect of Tumor Sidedness in Colorectal Cancer: A SEER-Based Analysis. Clin. Colorectal Cancer 2019, 18, e104–e116. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Poli, D.; Zaniboni, A.; Lonardi, S.; Labianca, R.; Sobrero, A.; Rosati, G.; Di Bartolomeo, M.; Scartozzi, M.; Zagonel, V.; et al. The prognostic impact of primary tumour location in patients with stage II and stage III colon cancer receiving adjuvant therapy. A GISCAD analysis from three large randomised trials. Eur. J. Cancer 2019, 111, 1–7. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, F.; Peng, J.; Wang, F.; Lin, Y.; Jiang, W.; Yang, X.; Li, L.; Lu, Z.; Wan, D.; et al. High pretreatment serum CA19-9 level predicts a poor prognosis for patients with stage III colon cancer after curative resection and adjuvant chemotherapy. J. Cancer 2019, 10, 3810–3818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Kotake, K.; Sugihara, K.; Takahashi, H.; Maeda, K.; Uyama, I. Clinicopathological Factors Associated with Recurrence and Prognosis after R0 Resection for Stage IV Colorectal Cancer with Peritoneal Metastasis. Dig. Surg. 2016, 33, 382–391. [Google Scholar] [CrossRef]
- Kang, H.; O’Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-Year Outcomes Evaluation of Mucinous and Signet-Ring Cell Carcinoma of the Colon and Rectum. Dis. Colon Rectum 2005, 48, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Loree, J.; Advani, S.M.; Ning, J.; Li, W.; Pereira, A.A.; Lam, M.; Raghav, K.; Morris, V.K.; Broaddus, R.; et al. Prognostic Implications of Mucinous Differentiation in Metastatic Colorectal Carcinoma Can Be Explained by Distinct Molecular and Clinicopathologic Characteristics. Clin. Colorectal Cancer 2018, 17, e699–e709. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, M.J.; Campbell, M.E.; Goldberg, R.M.; Grothey, A.; Seitz, J.-F.; Benedetti, J.K.; André, T.; Haller, D.G.; Sargent, D. Survival Following Recurrence in Stage II and III Colon Cancer: Findings From the ACCENT Data Set. J. Clin. Oncol. 2008, 26, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-R.; Chang, T.-C.; Lee, Y.-C.; Lee, P.-H.; Chang, K.-J.; Liang, J.-T. Pulmonary Resection for Colorectal Cancer Metastases: Duration Between Cancer Onset and Lung Metastasis as an Important Prognostic Factor. Ann. Surg. Oncol. 2009, 16, 1026–1032. [Google Scholar] [CrossRef]
- Gonzalez, M.; Robert, J.H.; Halkic, N.; Mentha, G.; Roth, A.; Perneger, T.; Ris, H.B.; Gervaz, P. Survival after Lung Metastasectomy in Colorectal Cancer Patients with Previously Resected Liver Metastases. World J. Surg. 2011, 36, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryuk, J.P.; Choi, G.-S.; Park, J.S.; Kim, H.J.; Park, S.Y.; Yoon, G.S.; Jun, S.H.; Kwon, Y.C. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann. Surg. Treat. Res. 2014, 86, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Broadbridge, V.T.; Karapetis, C.; Beeke, C.; Woodman, R.; Padbury, R.; Maddern, G.; Kim, S.W.; Roder, D.; Hakendorf, P.; Price, T.J. Do metastatic colorectal cancer patients who present with late relapse after curative surgery have a better survival? Br. J. Cancer 2013, 109, 1338–1343. [Google Scholar] [CrossRef] [Green Version]
- Brotto, K.; Malisic, E.; Cavic, M.; Krivokuca, A.; Jankovic, R. The Usability of Allele-Specific PCR and Reverse-Hybridization Assays for KRAS Genotyping in Serbian Colorectal Cancer Patients. Dig. Dis. Sci. 2012, 58, 998–1003. [Google Scholar] [CrossRef]
- Jakovljevic, K.; Malisic, E.; Cavic, M.; Krivokuca, A.; Dobricic, J.; Jankovic, R. KRAS and BRAF mutations in Serbian patients with colorectal cancer. J. BUON 2012, 17, 575–580. [Google Scholar]
- Cavic, M.; Krivokuca, A.; Boljevic, I.; Brotto, K.; Jovanovic, K.; Tanic, M.; Filipovic, L.; Zec, M.; Malisic, E.; Jankovic, R.; et al. Pharmacogenetics in cancer therapy—8 years of experience at the Institute for Oncology and Radiology of Serbia. J. BUON 2016, 21, 1287–1295. [Google Scholar] [PubMed]
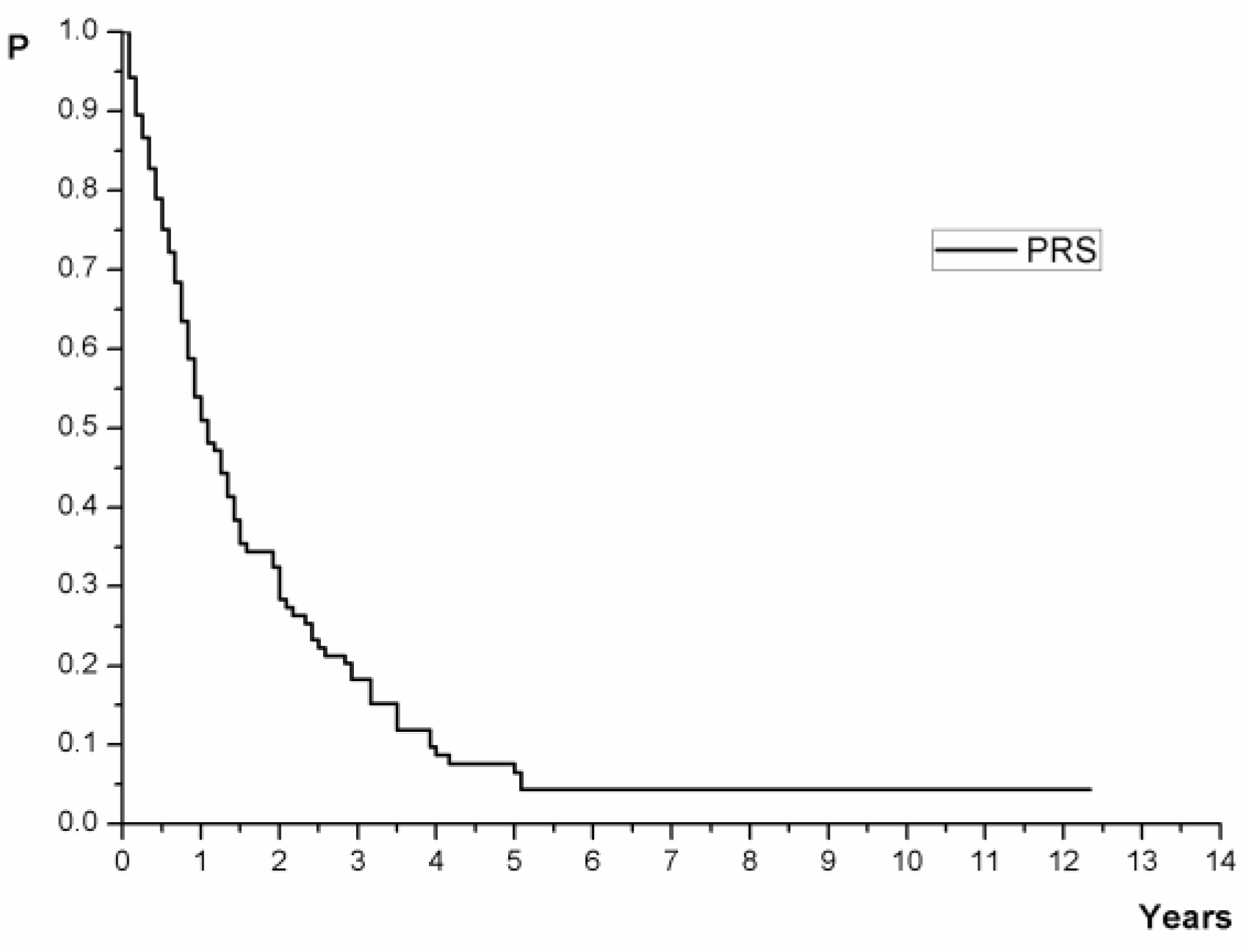
| Time | No in Risk | Cumulative Percentage with 95% CI |
|---|---|---|
| 1 year | 56 (53.33%) | 51.04 (42.28–61.60) |
| 2 year | 32 (30.48%) | 28.37 (20.85–38.60) |
| 3 year | 20 (19.05%) | 18.24 (12.06–27.60) |
| 4 year | 9 (8.57%) | 8.69 (4.54–16.60) |
| 5 years | 7 (6.67%) | 6.51 (3.04–13.90) |
| Characteristic | N (%) | Survival (Months) | Characteristic | N (%) | Survival (Months) | ||
|---|---|---|---|---|---|---|---|
| Median (95% CI) | Log-Rank Test | Median (95% CI) | Log-Rank Test | ||||
| Age (years) | Differentiation | ||||||
| Mean (SD) | 65.6 (10) | / | / | Low grade | 94 (89.5%) | 13 (11–17) | ns |
| Median (Range) | 67 (20–84) | High grade | 11 (10.48%) | 11 (≥6) | |||
| Age (categories) | Lymphatic invasion | ||||||
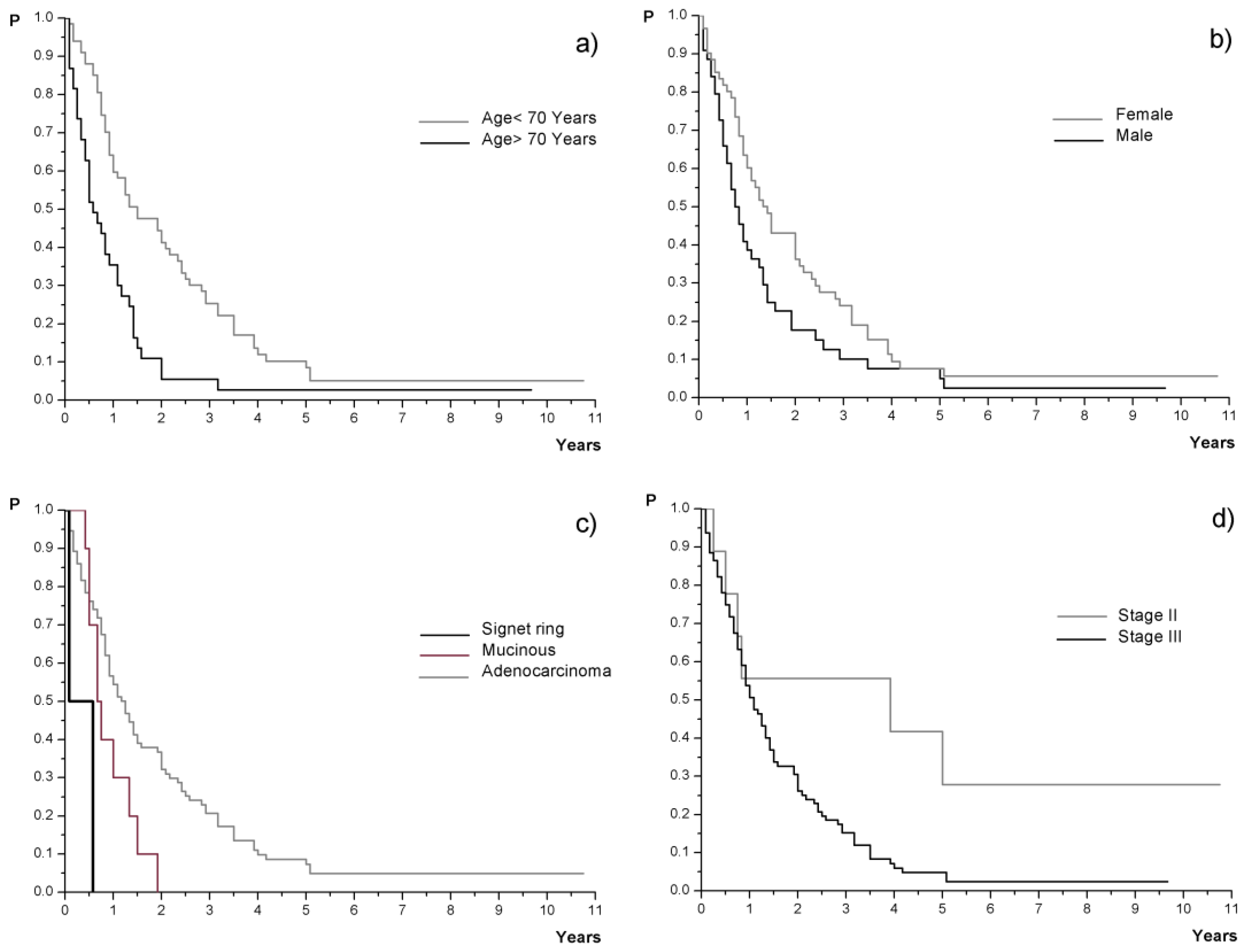
| <70 yrs | 67 (63.8%) | 18 (12–29) | <0.01 | Yes | 73 (69.5%) | 7.5 (≥5) | ns |
| ≥70 yrs | 38 (36.2%) | 7 (5–13) | No | 14 (13.4%) | 15 (11–18) | ||
| Gender | No data | 18 (17.1%) | / | ||||
| Male | 44 (41.9%) | 9.5 (7–16) | <0.05 | Vascular invasion | |||
| Female | 61 (58.1%) | 17 (12–25) | Yes | 44 (41.9%) | 11 (9–30) | ns | |
| Localization | No | 35 (33.3%) | 14 (9–18) | ||||
| Right side | 36 (34.3%) | 10 (8–14) | ns | No data | 26 (24.8%) | / | |
| Left side | 69 (65.7%) | 16 (12–24) | T stage | ||||
| Bowel obstruction | T2 | 4 (3.8%) | 13 (≥2) | ns | |||
| Yes | 33 (31.4%) | 13 (10–29) | ns | T3 | 84 (80%) | 13 (11–18) | |
| No | 72 (68.6%) | 13 (10–18) | T4a | 3 (2.9%) | 23 (≥5) | ||
| Bowel perforation | T4b | 14 (13.3%) | 9 (6–50) | ||||
| Yes | 12 (11.4%) | 13.5 (≥10) | ns | N stage | |||
| No | 93 (88.6%) | 13 (10–18) | N0 | 10 (9.5%) | 28.5 (≥6) | ns | |
| ECOG PS † | N1a | 12 (11.4%) | 20.5 (≥11) | ||||
| PS0 | 89 (84.8%) | 12 (10–17) | ns | N1b | 24 (22.9%) | 16 (11–34) | |
| PS1 | 16 (15.2%) | 16.5 (6–48) | N2a | 24 (22.9%) | 13 (10–24) | ||
| CEA | N2b | 33 (31.4%) | 9 (7–19) | ||||
| ≤5 mg/mL | 86 (81.9%) | 12 (10–18) | ns | No data | 2 (1.9%) | / | |
| >5 ng/mL | 16 (15.2%) | 14.5 (5–42) | Stage | ||||
| No data | 3 (2.9%) | / | II | 9 (8.6%) | 47 (≥9) | <0.05 | |
| Ca19-9 | III | 96 (91.4%) | 13 (10–17) | ||||
| ≤37 U/mL | 91 (86.7%) | 15 (11–18) | <0.01 | First recurrence | |||
| >37 U/mL | 9 (8.6%) | 8 (≥4) | Local | 18 (17.1%) | 20.5 (10–42) | ns | |
| No data | 5 (4.7%) | / | Systemic disease | 11 (10.5%) | 8 (≥5) | ||
| Adjuvant CHT | Combined | 76 (72.4%) | 12.5 (10–17) | ||||
| 5FU-LV | 37 (35.2%) | 23 (11–34) | ns | Early recurrence | |||
| Capecitabine | 68 (64.8%) | 11 (9–16) | <1 year | 39 (37.1%) | 11 (10–19) | ns | |
| Histology | ≥1 year | 66 (62.9%) | 13 (10–23) | ||||
| Adenocarcinoma | 93 (88.6%) | 15 (11–19) | <0.01 | Late recurrence | |||
| Mucinous | 10 (9.5%) | 8.5 (≥6) | ≥4 years | 13 (12.4%) | 10 (≥6) | ns | |
| Signet ring | 2 (1.9%) | 4 (≥1) | <4 years | 92 (87.6%) | 13.5 (12–18) | ||
| Parameters | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI) | Wald Test | HR (95% CI) | Likelihood Ratio Test | |
| Age ≥70 yrs vs. <70 yrs | 2.19 (1.42–3.38) | p < 0.01 | 2.43 (1.55–3.81) | p < 0.01 |
| Gender Male vs. Female | 1.45 (0.96–2.19) | ns | - | |
| Histology Mucinous vs. Adenocarcinoma Signet ring vs. Adenocarcinoma | 2.07 (1.02–4.20) 6.73 (1.58–28.58) | p < 0.05 p < 0.05 | 1.51 (0.73–3.10) 9.69 (2.23–42.0) | |
| Clinical stage Stage III vs. Stage II | 2.64 (1.13–6.17) | p < 0.05 | - | |
| Ca19–9 >37 U/ml vs. ≤37 U/mL | 2.71 (1.33–5.52) | p < 0.05 | 3.51 (1.68–7.37) | |
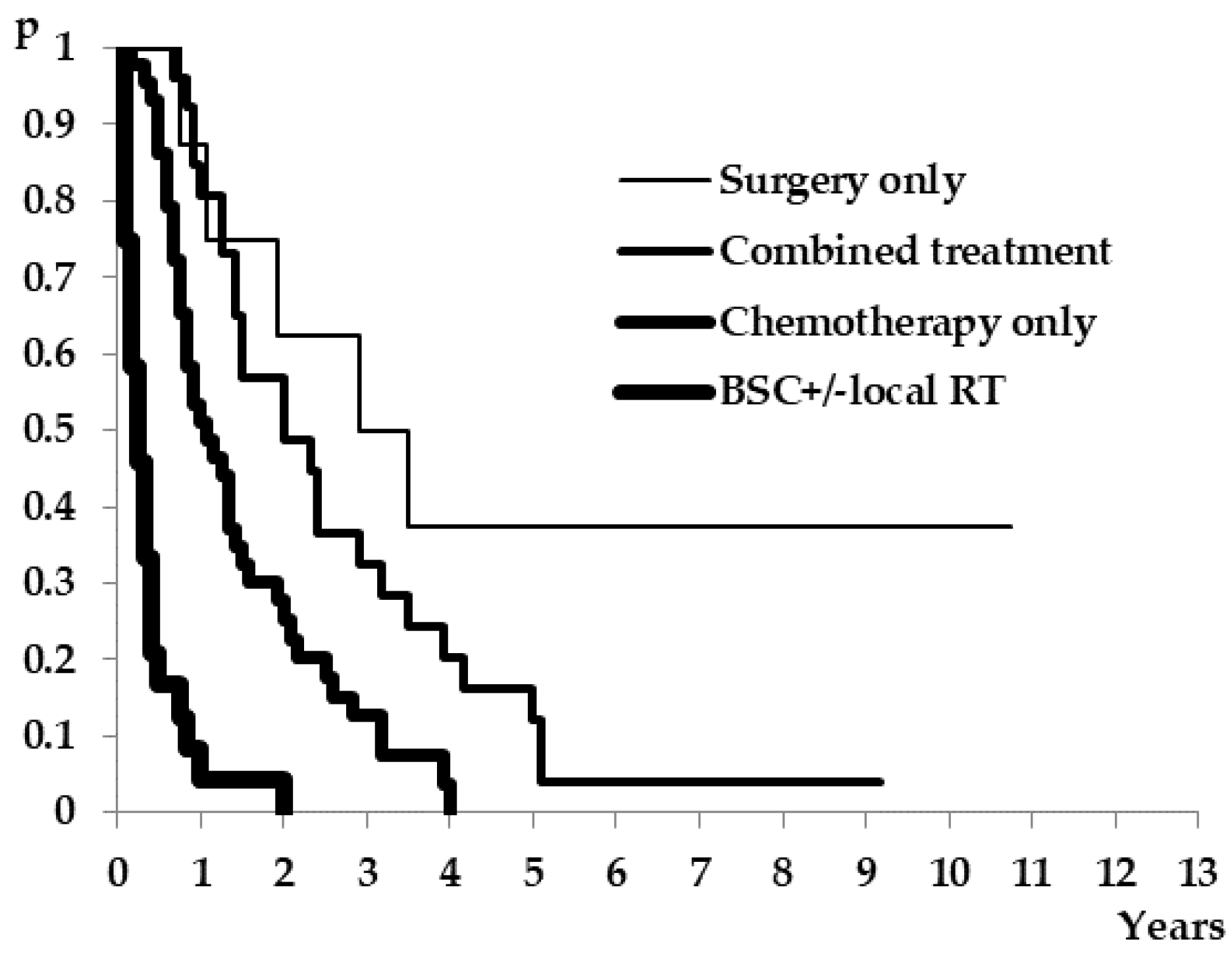
| Salvage Treatment | N (%) | Median Survival (Months) 95%CI | Log-Rank Test |
|---|---|---|---|
| BSC +/− local RT | 24 (22.8%) | 3 (2–5) | <0.01 |
| Surgery only | 8 (7.6%) | 38.5 (23-NR) | |
| Chemotherapy only | 41 (39%) | 13 (10–19) | |
| Combined treatment | 24 (22.9%) | 24 (17–42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, N.; Radosavljevic, D.; Gavrilovic, D.; Nikolic, V.; Stanic, N.; Spasic, J.; Cacev, T.; Castellvi-Bel, S.; Cavic, M.; Jankovic, G. Prognostic Factors for Post-Recurrence Survival in Stage II and III Colorectal Carcinoma Patients. Medicina 2021, 57, 1108. https://doi.org/10.3390/medicina57101108
Nikolic N, Radosavljevic D, Gavrilovic D, Nikolic V, Stanic N, Spasic J, Cacev T, Castellvi-Bel S, Cavic M, Jankovic G. Prognostic Factors for Post-Recurrence Survival in Stage II and III Colorectal Carcinoma Patients. Medicina. 2021; 57(10):1108. https://doi.org/10.3390/medicina57101108
Chicago/Turabian StyleNikolic, Neda, Davorin Radosavljevic, Dusica Gavrilovic, Vladimir Nikolic, Nemanja Stanic, Jelena Spasic, Tamara Cacev, Sergi Castellvi-Bel, Milena Cavic, and Goran Jankovic. 2021. "Prognostic Factors for Post-Recurrence Survival in Stage II and III Colorectal Carcinoma Patients" Medicina 57, no. 10: 1108. https://doi.org/10.3390/medicina57101108
APA StyleNikolic, N., Radosavljevic, D., Gavrilovic, D., Nikolic, V., Stanic, N., Spasic, J., Cacev, T., Castellvi-Bel, S., Cavic, M., & Jankovic, G. (2021). Prognostic Factors for Post-Recurrence Survival in Stage II and III Colorectal Carcinoma Patients. Medicina, 57(10), 1108. https://doi.org/10.3390/medicina57101108








