Cannabis Dopaminergic Effects Induce Hallucinations in a Patient with Parkinson’s Disease
Abstract
:1. Introduction
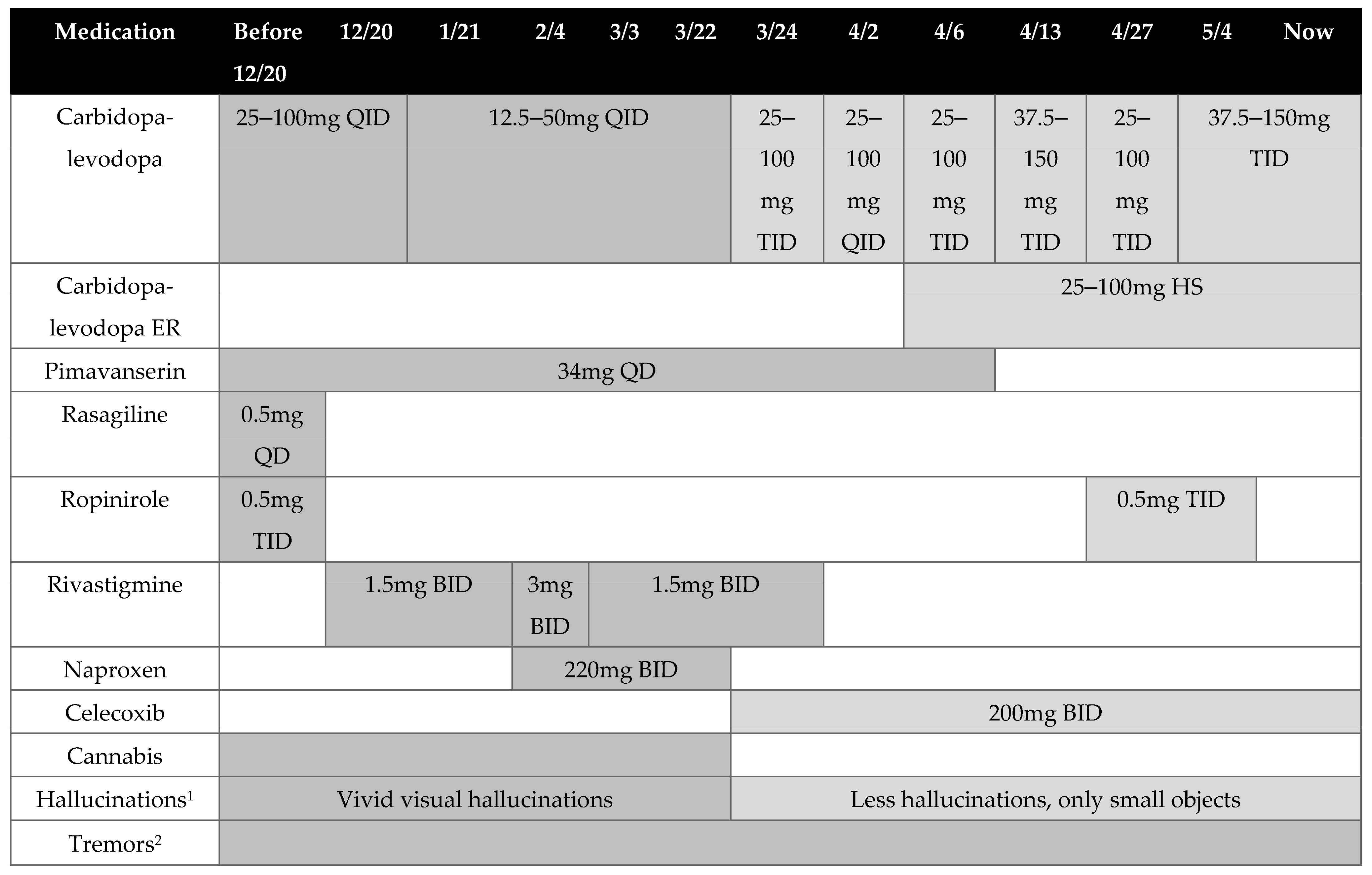
2. Case Presentation
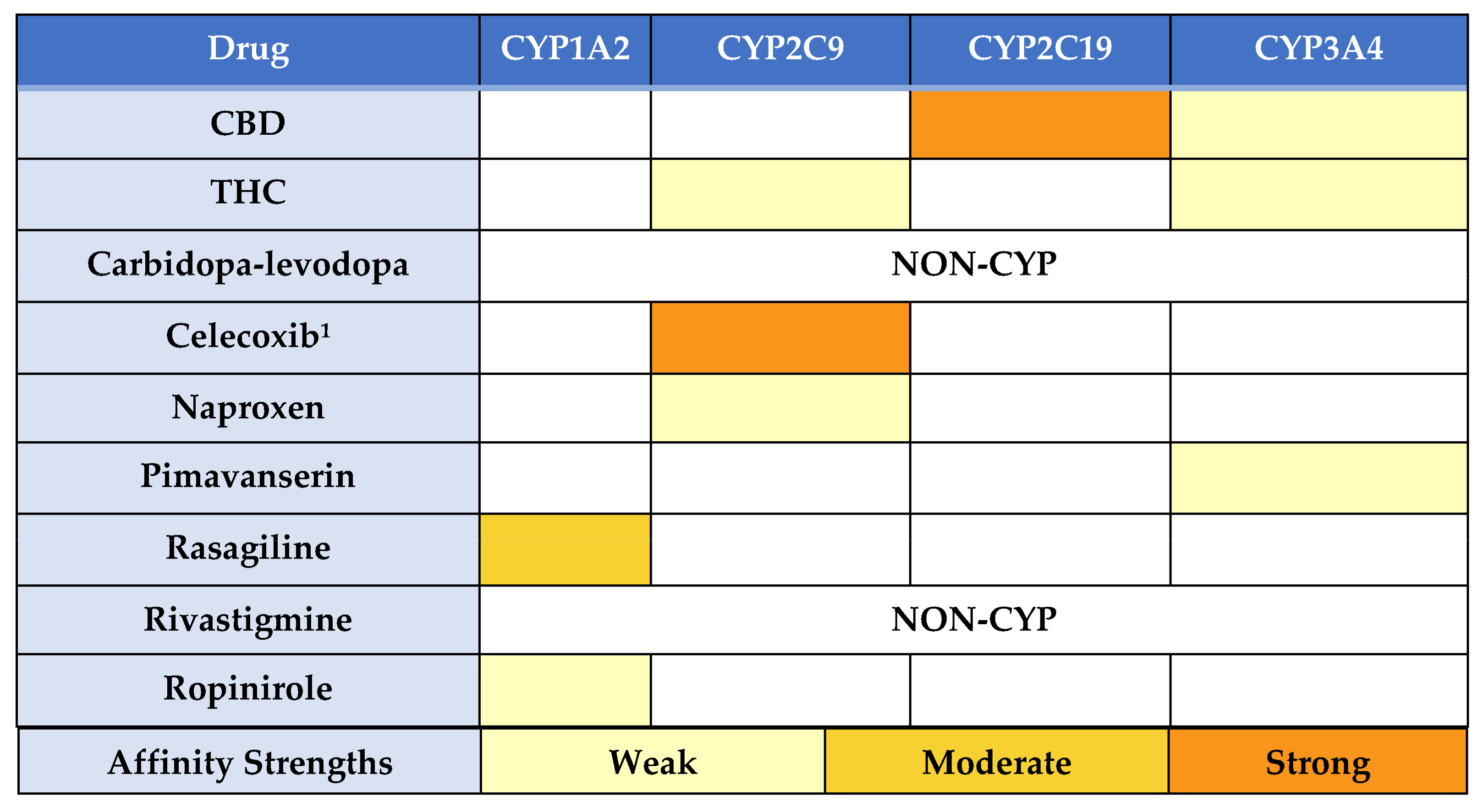
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; McCormack, S. Medical Cannabis for the Treatment of Chronic Pain: A Review of Clinical Effectiveness and Guidelines; Canada Agency Drugs Technology Health: Ottawa, ON, Canada, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546424/pdf/Bookshelf_NBK546424.pdf (accessed on 25 June 2021).
- Methaneethorn, J.; Poomsaidorn, C.; Naosang, K.; Kaewworasut, P.; Lohitnavy, M. A delta-9-tetrahydrocannabinol physiologically-based pharmacokinetic model development in humans. Eur. J. Drug Metab. Pharm. 2020, 45, 495–511. [Google Scholar] [CrossRef]
- Wang, G.S.; Bourne, D.W.A.; Klawitter, J.; Sempio, C.; Chapman, K.; Knupp, K.; Wempe, M.F.; Borgelt, L.; Christians, U.; Heard, K.; et al. Disposition of oral delta-9 tetrahydrocannabinol (THC) in children receiving cannabis extracts for epilepsy. Clin. Toxicol. 2019, 58, 124–128. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Thacker, D.L. A brief background on cannabis: From plant to medical indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Ashok, A.H.; Volkow, N.D. The effects of delta-9-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, S.C.; Sopory, P.; Reeta, K.H. Chronic neurological disorders: Genetic and epigenetic markers for monitoring of pharmacotherapy. Neuro India 2021, 69, 252–259. [Google Scholar] [CrossRef]
- Parkinson’s Foundation. Available online: https://www.parkinson.org/ (accessed on 21 June 2021).
- Koonrungsesomboon, N.; Khatsri, R.; Wongchompoo, P.; Teekachunhatean, S. The impact of genetic polymorphisms on CYP1A2 activity in humans: A systemic review and meta-analysis. Pharm. J. 2018, 18, 760–768. [Google Scholar] [CrossRef]
- Klein, K.; Zanger, U.M. Pharmacogenomics of cytochrome P450 3A4: Recent progress toward the “missing heritability” problem. Front Genet. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epidiolex [Package Insert]; Greenwich Biosciences: Carlsbad, CA, USA, 2020.
- Cravanas, B.; Frei, K. The effects of cannabis on hallucinations in Parkinson’s disease patients. J. Neurol. Sci. 2020, 419, 117206. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 32–360. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef] [PubMed]
- Bland, T.M.; Haining, R.L.; Tracy, T.S.; Callery, P.S. CYP2C-catalyzed delta9-tetrahydrocannabinol metabolism: Kinetics, pharmacogenetics and interaction with phenytoin. Biochem. Pharmacol. 2005, 70, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.; Arwood, M.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 inhibition: Understanding drug-drug interactions due to mechanism-based inhibition in clinical practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Oleszkiewicz, B.; Wiela-Hojeńska, A. CYP2C19 polymorphism in relation to the pharmacotherapy optimization of commonly used drugs. Pharmazie 2018, 73, 619–624. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Kraft, B.; Frickey, N.A.; Kaufmann, R.M.; Reif, M.; Frey, R.; Gustorff, B.; Kress, H.G. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008, 109, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.P. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef]
- Schmack, K.; Bosc, M.; Ott, T.; Sturgill, J.F.; Kepecs, A. Striatal dopamine mediates hallucination-like perception in mice. Science 2021, 372. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kim, J.S.; Lee, P.H. Effect of rivastigmine on behavioral and psychiatric symptoms of Parkinson’s disease dementia. J. Mov. Disord. 2015, 8, 98–102. [Google Scholar] [CrossRef]
- Ko, J.H.; Antonelli, F.; Monchi, O.; Ray, N.; Rusjan, P.; Houle, S.; Lang, A.; Christopher, L.; Strafella, A.P. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson’s disease. Hum. Brain Mapp. 2013, 34, 1591–1604. [Google Scholar] [CrossRef] [Green Version]
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Hindley, G.; Beck, K.; Borgan, F.; Ginestet, C.E.; McCutcheon, R.; Kleinloog, D.; Ganesh, S.; Radhakrishnan, R.; D’Souza, D.C.; Howes, O.D. Psychiatric symptoms caused by cannabis constituents: A systematic review and meta-analysis. Lancet Psychiatry 2020, 7, 344–353. [Google Scholar] [CrossRef]
- Henquet, C.; Rosa, A.; Delespaul, P.; Papiol, S.; Faňanás, L.; Van Os, J.; Myin-Germeys, I. COMT Val158Met moderation of cannabis-induced psychosis: A momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr. Scand. 2009, 119, 156–160. [Google Scholar] [CrossRef]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef] [Green Version]
- Akil, M.; Kolachana, B.S.; Rothmond, D.A.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J. Neurosci. 2003, 23, 2008–2013. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.S.; Rodnitzky, R.L.; Uc, E. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev. Neurosci. 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Schloss, P. The cannabinoid CB1 receptor is expressed on serotonergic and dopaminergic neurons. Eur. J. Pharmacol. 2008, 578, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Viñals, X.; Moreno, E.; Lanfumey, L.; Cordomí, A.; Pastor, A.; de La Torre, R. Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015, 13, e1002194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin. Pharm. Ther. 2020, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Lonsdorf, T.B.; Schalling, M.; Kosek, E.; Ingvar, M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT Val158Met polymorphism. PLoS ONE 2009, 4, e6016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Genotype | Phenotype |
|---|---|---|
| CYP1A21 | *1F|*1F | Possible Normal Metabolizer |
| CYP2B6 | *1|*1 | Normal Metabolizer |
| CYP2C9 | *1|*1 | Normal Metabolizer |
| CYP2C19 | *1|*1 | Normal Metabolizer |
| CYP2D6 | *1|*4 | Intermediate Metabolizer |
| CYP3A42 | *1|*1 | Undetermined |
| CYP3A5 | *3|*3 | Poor Expresser Metabolizer |
| COMT | rs4680 AA (Met/Met) | Low Activity |
| DRD2 | rs1799978 AA | Normal Receptor Expression |
| HTR2A | rs7997012 GG | Altered Receptor Function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzolato, K.; Thacker, D.; Toro-Pagán, N.D.; Hanna, A.; Turgeon, J.; Matos, A.; Amin, N.; Michaud, V. Cannabis Dopaminergic Effects Induce Hallucinations in a Patient with Parkinson’s Disease. Medicina 2021, 57, 1107. https://doi.org/10.3390/medicina57101107
Pizzolato K, Thacker D, Toro-Pagán ND, Hanna A, Turgeon J, Matos A, Amin N, Michaud V. Cannabis Dopaminergic Effects Induce Hallucinations in a Patient with Parkinson’s Disease. Medicina. 2021; 57(10):1107. https://doi.org/10.3390/medicina57101107
Chicago/Turabian StylePizzolato, Katie, David Thacker, Nicole Del Toro-Pagán, Abeer Hanna, Jacques Turgeon, Adriana Matos, Nishita Amin, and Veronique Michaud. 2021. "Cannabis Dopaminergic Effects Induce Hallucinations in a Patient with Parkinson’s Disease" Medicina 57, no. 10: 1107. https://doi.org/10.3390/medicina57101107
APA StylePizzolato, K., Thacker, D., Toro-Pagán, N. D., Hanna, A., Turgeon, J., Matos, A., Amin, N., & Michaud, V. (2021). Cannabis Dopaminergic Effects Induce Hallucinations in a Patient with Parkinson’s Disease. Medicina, 57(10), 1107. https://doi.org/10.3390/medicina57101107









