Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study
Abstract
:1. Introduction
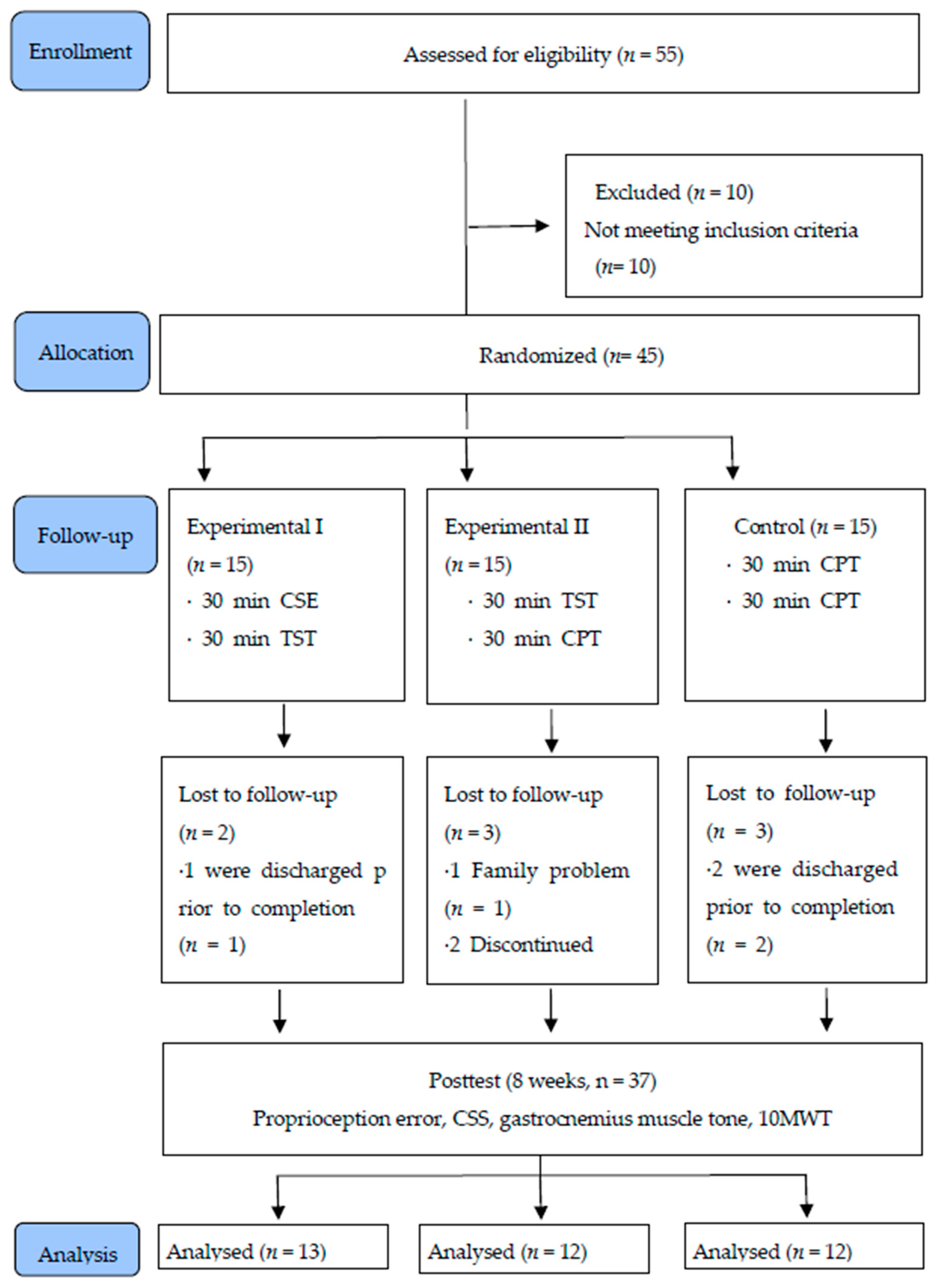
2. Materials and Methods
2.1. Participants
2.2. Sample Size Calculation
2.3. Test Design
2.4. Intervention
2.4.1. Experimental I Group
2.4.2. Experimental II Group
2.4.3. Control Group
2.5. Evaluation
2.5.1. Proprioception
2.5.2. Spasticity
2.5.3. MyotonPRO
2.5.4. 10 m Walk Test
2.6. Data Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Comparison of Proprioception Error between the Three Groups
3.3. Comparison of CSS and GMT among the Three Groups
3.4. Comparison of 10MWT among the Three Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sommerfeld, D.K.; Eek, E.U.B.; Svensson, A.K.; Holmqvist, L.W.; Von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohannon, R.W.; Andrews, A.W.; Smith, M.B. Rehabilitation goals of patients with hemiplegia. Int. J. Rehabil. Res. 1988, 11, 181–184. [Google Scholar] [CrossRef]
- Brincks, J.; Nielsen, J.F. Increased power generation in impaired lower extremities correlated with changes in walking speeds in sub-acute stroke patients. Clin. Biomech. 2012, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Rensink, M.; Schuurmans, M.; Lindeman, E.; Hafsteinsdottir, T. Task-oriented training in rehabilitation after stroke: Systematic review. J. Adv. Nurs. 2009, 65, 737–754. [Google Scholar] [CrossRef]
- Ganesh, G.S.; Kumari, R.; Pattnaik, M.; Mohanty, P.; Mishra, C.; Kaur, P.; Dakshinamoorthy, A. Effectiveness of Faradic and Russian currents on plantar flexor muscle spasticity, ankle motor recovery, and functional gait in stroke patients. Physiother. Res. Int. 2018, 23, e1705. [Google Scholar] [CrossRef]
- Jonsdottir, J.; Cattaneo, D.; Recalcati, M.; Regola, A.; Rabuffetti, M.; Ferrarin, M.; Casiraghi, A. Task-oriented biofeedback to improve gait in individuals with chronic stroke: Motor learning approach. Neurorehabilit. Neural Repair 2010, 24, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Outermans, J.C.; van Peppen, R.P.; Wittink, H.; Takken, T.; Kwakkel, G. Effects of a high-intensity task-oriented training on gait performance early after stroke: A pilot study. Clin. Rehabil. 2010, 24, 979–987. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, C.Y.; Kim, H.D. Short-term effects of whole-body vibration combined with task-related training on upper extremity function, spasticity, and grip strength in subjects with poststroke hemiplegia: A pilot randomized controlled trial. Am. J. Phys. Med. Rehabil. 2016, 95, 608–617. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. Transcutaneous electrical nerve stimulation combined with task-related training improves lower limb functions in subjects with chronic stroke. Stroke 2007, 38, 2953–2959. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.W.; Ng, G.Y.; Ng, S.S. Effectiveness of a combination of cognitive behavioral therapy and task-oriented balance training in reducing the fear of falling in patients with chronic stroke: Study protocol for a randomized controlled trial. Trials 2018, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Folkerts, M.A.; Hijmans, J.M.; Elsinghorst, A.L.; Mulderij, Y.; Murgia, A.; Dekker, R. Effectiveness and feasibility of eccentric and task-oriented strength training in individuals with stroke. NeuroRehabilitation 2017, 40, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Tseng, H.Y.; Liao, K.K.; Wang, C.J.; Lai, K.L.; Yang, Y.R. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: A randomized trial. Neurorehabilit. Neural Repair 2012, 26, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.W.; Wu, C.Y.; Wang, W.E.; Lin, K.C.; Chang, K.C.; Chen, C.C.; Liu, C.T. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: A pilot randomized controlled trial. Clin. Rehabil. 2017, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, C. Der Hemiplegische Patient Cognitiv-Therapeutische Uegungen, 1st ed.; Pflaum: Munich, Germany, 1997; pp. 71–106. [Google Scholar]
- Birbamer, G.; Beise, U. Das Gehirn denkt nicht in musklekontraktionen. Ars Med. 2001, 6, 282–285. [Google Scholar]
- Chanubol, R.; Wongphaet, P.; Chavanich, N.; Werner, C.; Hesse, S.; Bardeleben, A.; Merholz, J. A randomized controlled trial of Cognitive Sensory Motor Training Therapy on the recovery of arm function in acute stroke patients. Clin. Rehabil. 2012, 26, 1096–1104. [Google Scholar] [CrossRef]
- Haywood, K.; Getchell, N. Life Span Motor Development; Human Kinetics: Champaign, IL, USA, 2005. [Google Scholar]
- Wongphaet, P.; Sinlapawonsa, T.; Butrach, W.; Chira-Adisai, W.; Laothamatas, J.; Jitpraphai, C. Functional magnetic resonance imaging evidence of brain plasticity after cognitive sensory motor training in a stroke patient. Neurol. J. Thail. 2005, 5, 123–126. [Google Scholar]
- Díaz-Arribas, M.J.; Martín-Casas, P.; Cano-de-la-Cuerda, R.; Plaza-Manzano, G. Effectiveness of the Bobath concept in the treatment of stroke: A systematic review. Disabil. Rehabil. 2020, 42, 1636–1649. [Google Scholar] [CrossRef]
- Scrivener, K.; Dorsch, S.; McCluskey, A.; Schurr, K.; Graham, P.L.; Cao, Z.; Shepherd, R.; Tyson, S. Bobath therapy is inferior to task-specific training and not superior to other interventions in improving lower limb activities after stroke: A systematic review. J. Physiother. 2020, 66, 225–235. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Kesar, T.M.; Reisman, D.S.; Perumal, R.; Jancosko, A.M.; Higginson, J.S.; Rudolph, K.S.; Binder-Macleod, S.A. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture 2011, 33, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Cappellino, F.; Paolucci, T.; Zangrando, F.; Iosa, M.; Adriani, E.; Mancini, P.; Bellelli, A.; Saraceni, V. Neurocognitive rehabilitative approach effectiveness after anterior cruciate ligament reconstruction with patellar tendon. A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2012, 48, 17–30. [Google Scholar]
- Cheng, P.T.; Wu, S.H.; Liaw, M.Y.; Wong, A.M.; Tang, F.-T. Symmetrical body-weight distribution training in stroke patients and its effect on fall prevention. Arch. Phys. Med. Rehabil. 2001, 82, 1650–1654. [Google Scholar] [CrossRef]
- Dean, C.M.; Richards, C.L.; Malouin, F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: A randomized, controlled pilot trial. Arch. Phys. Med. Rehabil. 2000, 81, 409–417. [Google Scholar] [CrossRef]
- Nadeau, S.; Duclos, C.; Bouyer, L.; Richards, C.L. Guiding task-oriented gait training after stroke or spinal cord injury by means of a biomechanical gait analysis. Prog. Brain Res. 2011, 192, 161–180. [Google Scholar]
- Kwon, O.H.; Woo, Y.; Lee, J.S.; Kim, K.H. Effects of task-oriented treadmill-walking training on walking ability of stoke patients. Top. Stroke Rehabil. 2015, 22, 444–452. [Google Scholar] [CrossRef]
- Trans, T.; Aaboe, J.; Henriksen, M.; Christensen, R.; Bliddal, H.; Lund, H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee 2009, 16, 256–261. [Google Scholar] [CrossRef]
- Lim, C. Multi-Sensorimotor Training Improves Proprioception and Balance in Subacute Stroke Patients: A Randomized Controlled Pilot Trial. Front. Neurol. 2019, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Piriyaprasarth, P.; Morris, M.E.; Winter, A.; Bialocerkowski, A.E. The reliability of knee joint position testing using electrogoniometry. BMC Musculoskelet. Disord. 2008, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Meng, F.; Zhang, Y.; Xu, M.Y.; Yue, S.W. Full-movement neuromuscular electrical stimulation improves plantar flexor spasticity and ankle active dorsiflexion in stroke patients: A randomized controlled study. Clin. Rehabil. 2016, 30, 577–586. [Google Scholar] [CrossRef]
- Levin, M.F.; Hui-Chan, C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J. Neurol. 1993, 240, 63–71. [Google Scholar] [CrossRef]
- Poon, D.M.; Hui-Chan, C.W. Hyperactive stretch reflexes, co-contraction, and muscle weakness in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 128–135. [Google Scholar] [CrossRef]
- Bailey, L.; Samuel, D.; Warner, M.; Stokes, M. Parameters representing muscle tone, elasticity and stiffness of biceps brachii in healthy older males: Symmetry and within-session reliability using the MyotonPRO. J. Neurol. Disord. 2013, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Yu, Y.F.; Ding, W.L.; Yu, J.Y.; Song, L.; Feng, Y.N.; Zhang, Z.J. Quantification of the Masseter Muscle Hardness of Stroke Patients Using the MyotonPRO Apparatus: Intra-and Inter-Rater Reliability and Its Correlation with Masticatory Performance. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2021, 27, e928109. [Google Scholar]
- Green, J.; Forster, A.; Young, J. Reliability of gait speed measured by a timed walking test in patients one year after stroke. Clin. Rehabil. 2002, 16, 305–314. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Chung, Y.; Hwang, S. Weight-shift training improves trunk control, proprioception, and balance in patients with chronic hemiparetic stroke. Tohoku J. Exp. Med. 2014, 232, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Chiang, J.H. The effects of surface compliance on foot pressure in stance. Gait Posture 1996, 4, 122–129. [Google Scholar] [CrossRef]
- Wu, C.L.; Huang, M.H.; Lee, C.L.; Liu, C.W.; Lin, L.J.; Chen, C.H. Effect on spasticity after performance of dynamic-repeated-passive ankle joint motion exercise in chronic stroke patients. Kaohsiung J. Med. Sci. 2006, 22, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.J.; Kim, W.H.; Park, E.Y. Effect of task-oriented training for people with stroke: A meta-analysis focused on repetitive or circuit training. Top. Stroke Rehabil. 2015, 22, 34–43. [Google Scholar] [CrossRef]
- Lin, P.Y.; Yang, Y.R.; Cheng, S.J.; Wang, R.Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 562–568. [Google Scholar] [CrossRef]
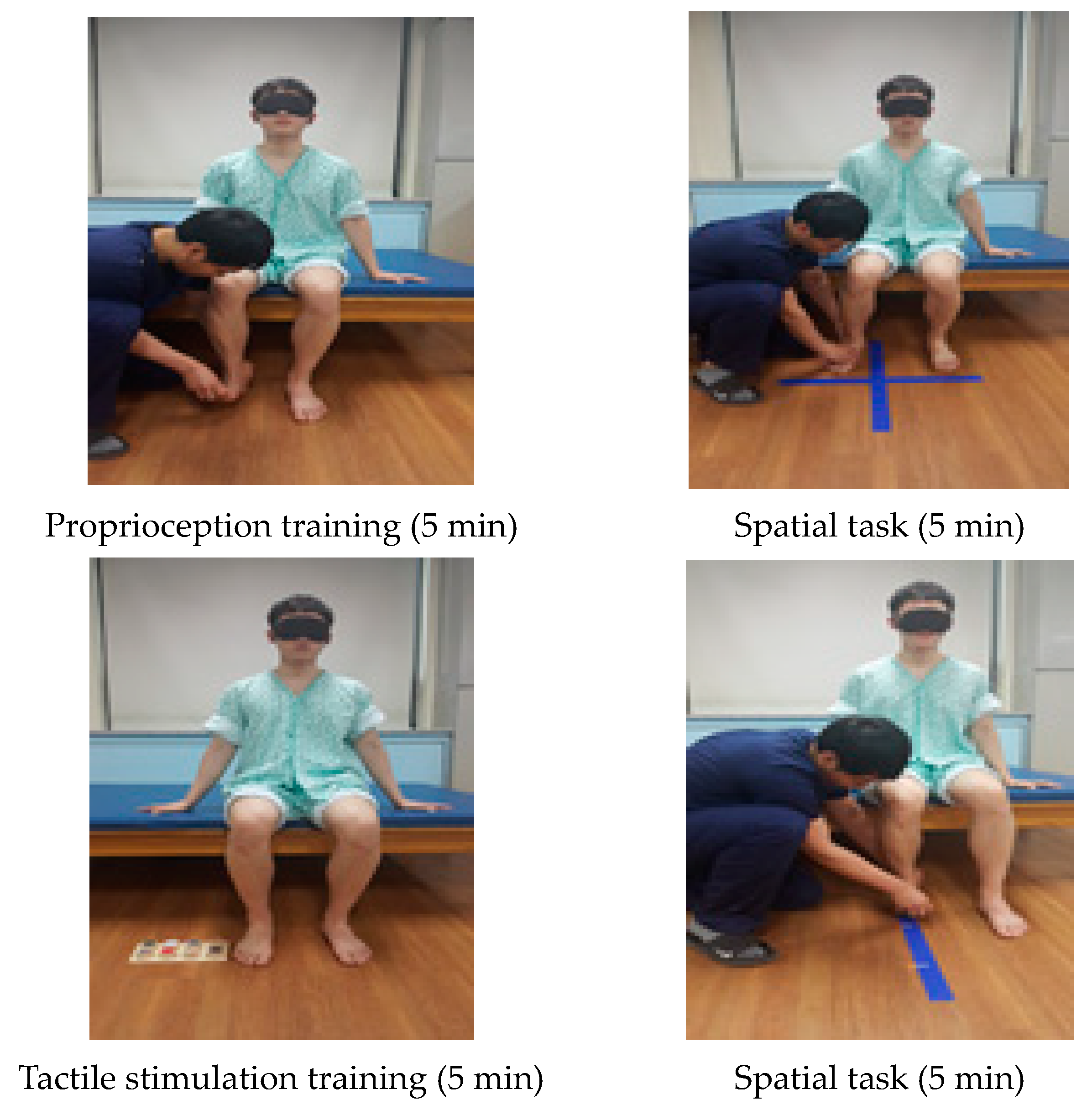
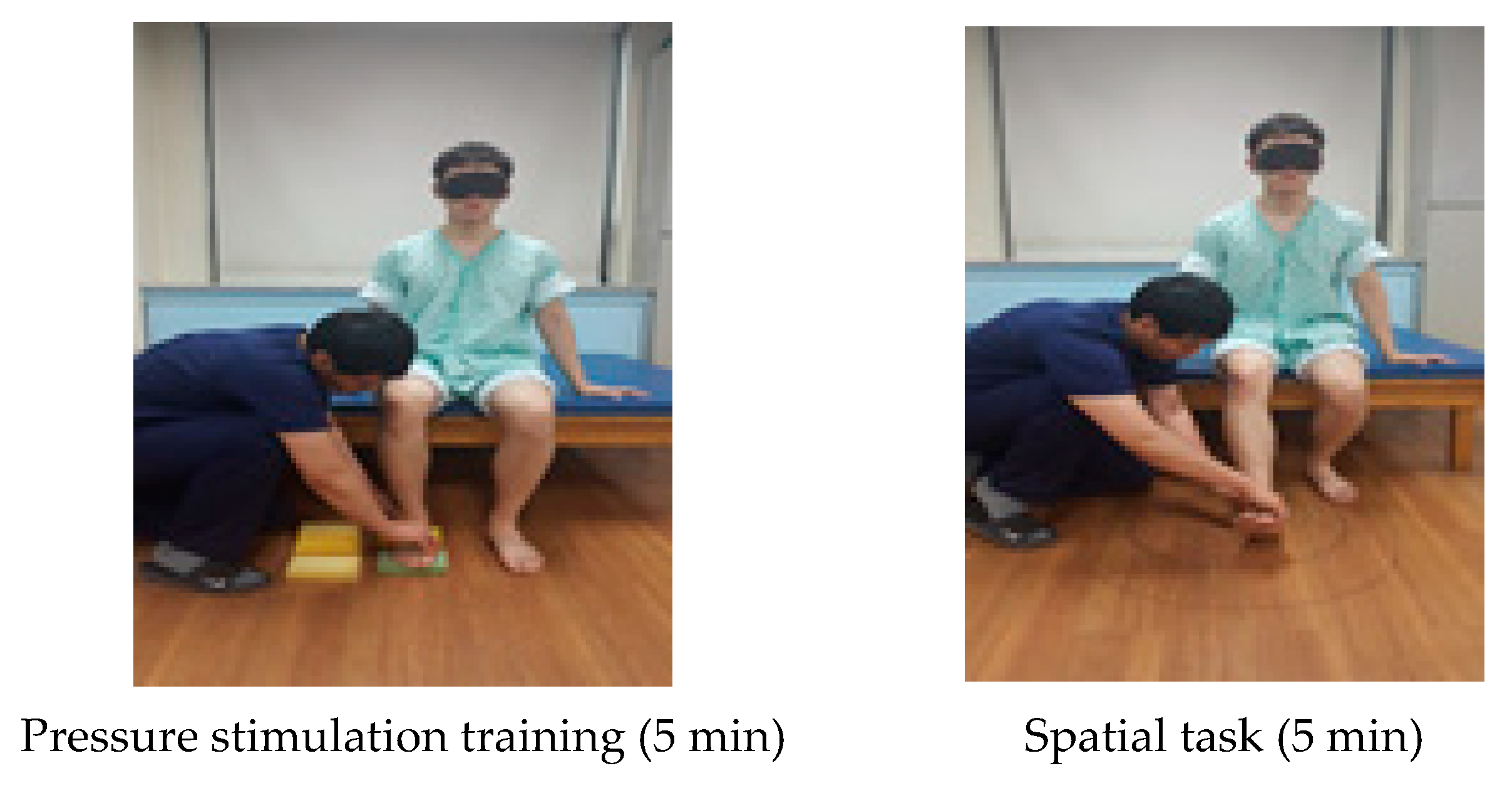
| Cognitive Sensorimotor Exercise Program | |
|---|---|
| Proprioception training (5 min) | The training was performed in the initial position and in the final position for proprioception after putting pressure on the ankle joint. The training was performed in the initial position and in the final position for position sense. |
| Tactile stimulation training (5 min) | CSE training using a tactile task was given to distinguish surface materials and friction sense. Subjects used visual and somatosensory techniques to differentiate each sense. |
| Pressure stimulation training (5 min) | Subjects presented a cognitive problem to distinguish the difference in the degree of the sponge pressure on the trunk in the sitting posture and asked the subjects to distinguish between the vision and somatosensory senses while conducting a task. |
| Spatial task (5 min) | A spatial task was given to distinguish where the patient’s foot was positioned in four areas. A line was drawn vertically on the floor straight from the patient’s affected knee and this line was horizontally divided in half. |
| Spatial task (5 min) | The patient’s heels were put together, and three lines were made depending on the knee angle. Training was performed to see if there were any changes in distance. If the patient was able to complete this task, a line was added. |
| Spatial task (5 min) | For the spatial cognition training, patients were instructed to trace an oval shape in front of them, and the position on the floor was expanded from a small one to bigger ones. |
| Experimental I (n = 13) | Experimental II (n = 12) | Control Group (n = 12) | p | |
|---|---|---|---|---|
| Height (cm) | 165.55 ± 7.51 | 164.93 ± 7.00 | 168.04 ± 4.70 | 0.474 a |
| Weight (kg) | 61.48 ± 9.17 | 65.83 ± 9.83 | 64.35 ± 9.55 | 0.512 a |
| Age (year) | 50.23 ± 14.89 | 52.75 ± 17.00 | 55.08 ± 10.55 | 0.704 a |
| MMSE-K (score) | 27.77 ± 1.64 | 27.17 ± 1.53 | 27.50 ± 1.09 | 0.586 a |
| K-NIHSS (score) | 9.61 ± 2.33 | 9.58 ± 2.43 | 9.75 ± 2.73 | 0.455 a |
| Onset (months) | 12.07 ± 3.57 | 13.17 ± 3.90 | 11.83 ± 3.71 | 0.649 a |
| Gender (male/female) | 7/6 | 7/5 | 8/4 | 0.805 b |
| Diagnosis (infarction/hemorrhage) | 6/7 | 6/6 | 8/4 | 0.556 b |
| Affected side (Left/Right) | 6/7 | 6/6 | 7/5 | 0.826 b |
| Brunnstrom recovery stage (3/4) | 8/5 | 8/4 | 6/6 | 0.695 b |
| Experimental I (n = 13, A) | Experimental II (n = 12, B) | Control Group (n = 12, C) | df | ES | F(p) | Post-Hoc | |
|---|---|---|---|---|---|---|---|
| Proprioception Error (Degree) | |||||||
| Pretest | 11.48 ± 1.58 | 11.84 ± 1.53 | 11.57 ± 1.57 | 2 | 0.0980 | 0.177 (0.839) | |
| Posttest | 8.38 ± 1.33 | 10.10 ± 1.38 | 10.99 ± 1.46 | 2 | 0.7870 | ||
| change | −3.10 ± 1.30 | −1.74 ± 1.06 | −0.58 ± 0.35 | 2 | 1.1525 | 20.054 (0.000) | A > B > C |
| t(p) | 8.606 (0.000) | 5.717 (0.000) | 5.619 (0.000) | ||||
| Experimental I (n = 13, A) | Experimental II (n = 12, B) | Control Group (n = 12, C) | df | ES | F(p) | Post-Hoc | |
|---|---|---|---|---|---|---|---|
| Composite Spasticity Score (Score) | |||||||
| Pretest | 11.23 ± 0.83 | 11.25 ± 0.87 | 11.17 ± 0.83 | 2 | 0.0400 | 0.032 (0.968) | |
| Posttest | 9.69 ± 1.03 | 10.25 ± 0.75 | 10.92 ± 0.79 | 2 | 0.5876 | ||
| change | −1.54 ± 0.78 | −1.00 ± 0.74 | −0.25 ± 0.45 | 2 | 0.8046 | 11.433 (0.000) | A, B > C |
| t(p) | 7.146 (0.000) | 4.690 (0.001) | 1.915 (0.082) | ||||
| Gastrocnemius Muscle Tone (Hz) | |||||||
| Pretest | 15.88 ± 1.96 | 15.18 ± 1.75 | 14.69 ± 1.50 | 2 | 0.2830 | 1.450 (0.249) | |
| Posttest | 15.10 ± 2.10 | 14.44 ± 1.74 | 14.42 ± 1.54 | 2 | 0.1787 | ||
| change | −0.77 ± 0.41 | −0.73 ± 0.38 | −0.28 ± 0.13 | 2 | 0.7129 | 8.397 (0.001) | A, B > C |
| t(p) | 6.774 (0.000) | 6.720 (0.000) | 7.545 (0.000) | ||||
| Experimental I (n = 13, A) | Experimental II (n = 12, B) | Control Group (n = 12, C) | df | ES | F(p) | Post-Hoc | |
|---|---|---|---|---|---|---|---|
| 10 m Walk Test (m/s) | |||||||
| Pretest | 0.72 ± 0.22 | 0.72 ± 0.22 | 0.71 ± 0.21 | 2 | 0.0213 | 0.007 (0.993) | |
| Posttest | 1.03 ± 0.23 | 0.97 ± 0.25 | 0.82 ± 0.26 | 2 | 0.3533 | ||
| change | 0.31 ± 0.08 | 0.25 ± 0.09 | 0.10 ± 0.06 | 2 | 1.1041 | 22.194 (0.000) | A, B > C |
| t(p) | 13.647 (0.000) | 9.537 (0.000) | 5.778 (0.000) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Jang, S.-H. Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study. Medicina 2021, 57, 1098. https://doi.org/10.3390/medicina57101098
Kim K-H, Jang S-H. Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study. Medicina. 2021; 57(10):1098. https://doi.org/10.3390/medicina57101098
Chicago/Turabian StyleKim, Kyung-Hun, and Sang-Hun Jang. 2021. "Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study" Medicina 57, no. 10: 1098. https://doi.org/10.3390/medicina57101098
APA StyleKim, K.-H., & Jang, S.-H. (2021). Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study. Medicina, 57(10), 1098. https://doi.org/10.3390/medicina57101098






