An Algorithmic Approach Is Superior to the 99th Percentile Upper Reference Limits of High Sensitivity Troponin as a Threshold for Safe Discharge from the Emergency Department
Abstract
:1. Introduction
2. Materials and Methods
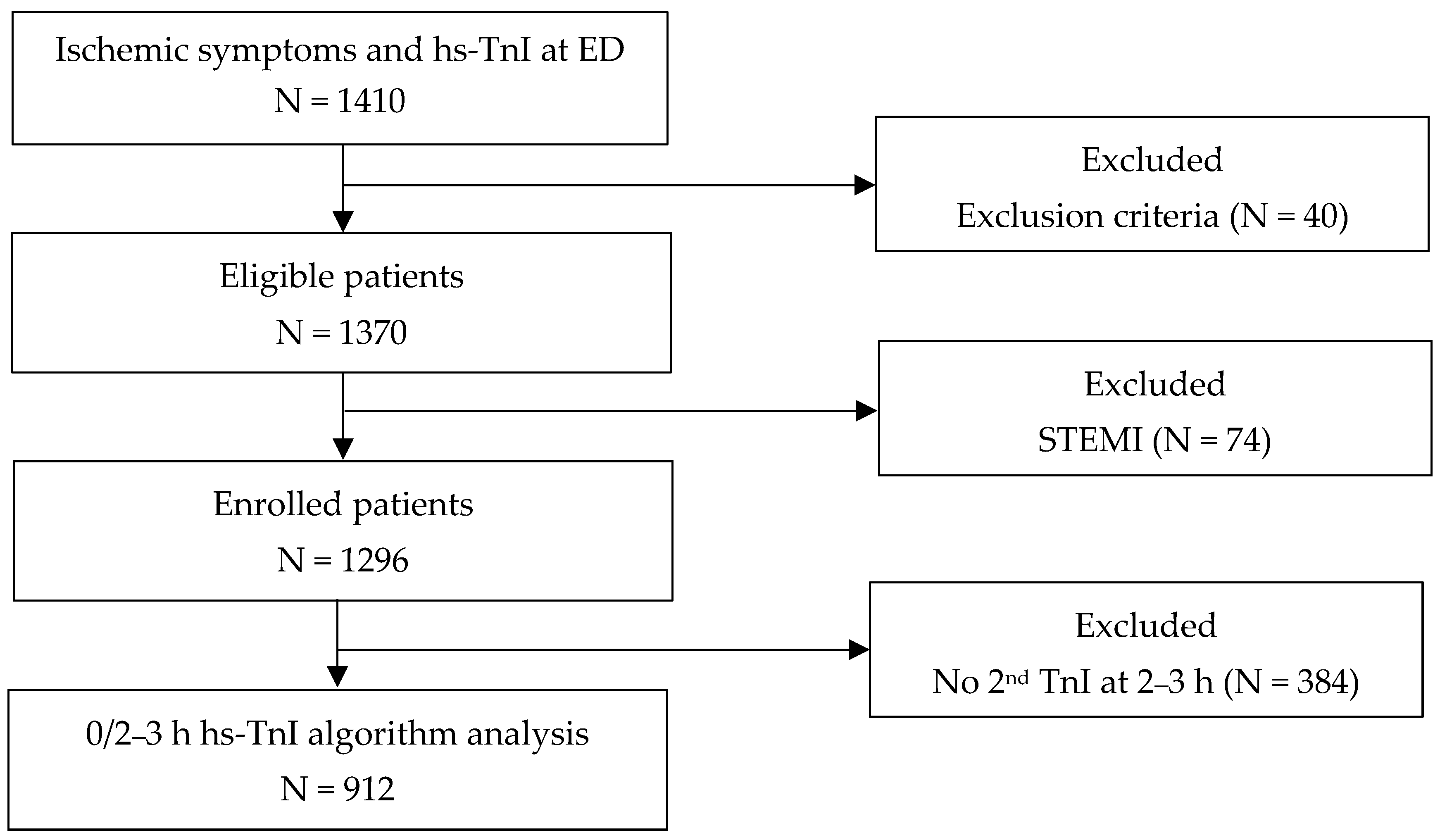
2.1. Study Design and Population
2.2. Data Collection and Clinical Assessment
2.3. Clinical Performance Evaluations of Access hs-TnI
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Final Adjudicated Diagnosis
3.3. Concentrations of hs-TnI According to Final Diagnosis
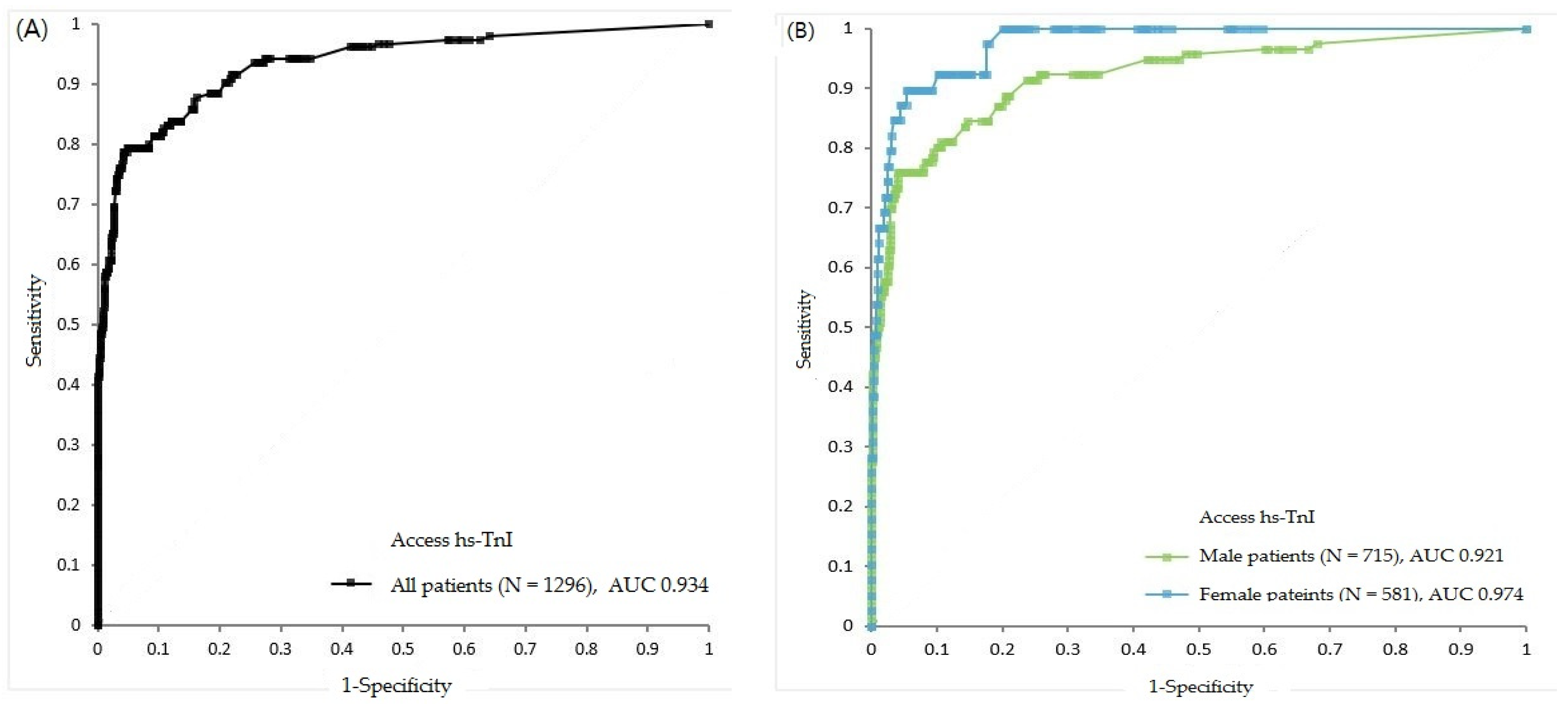
3.4. Clinical Performance of Baseline Access hs-TnI Using Different Cut-Offs
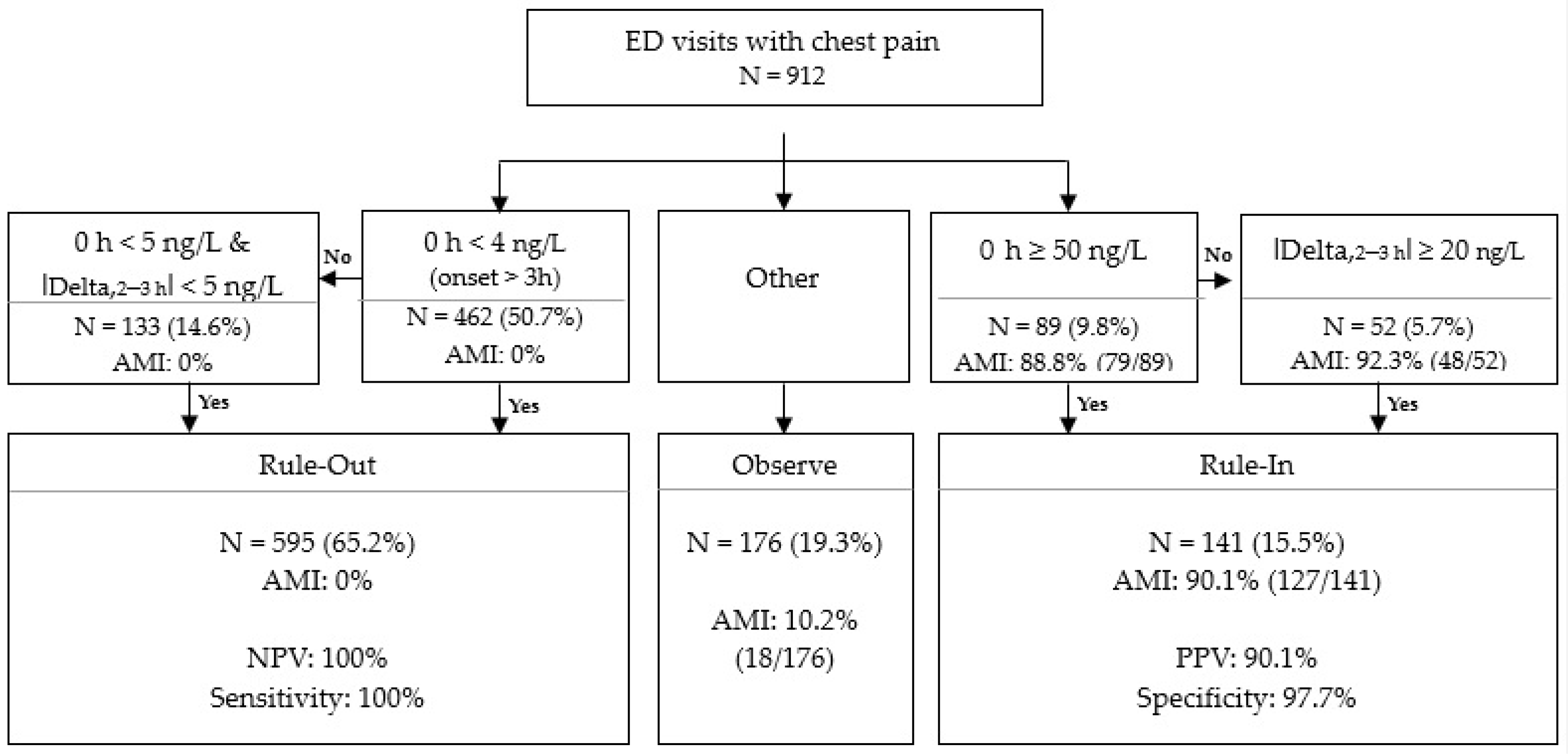
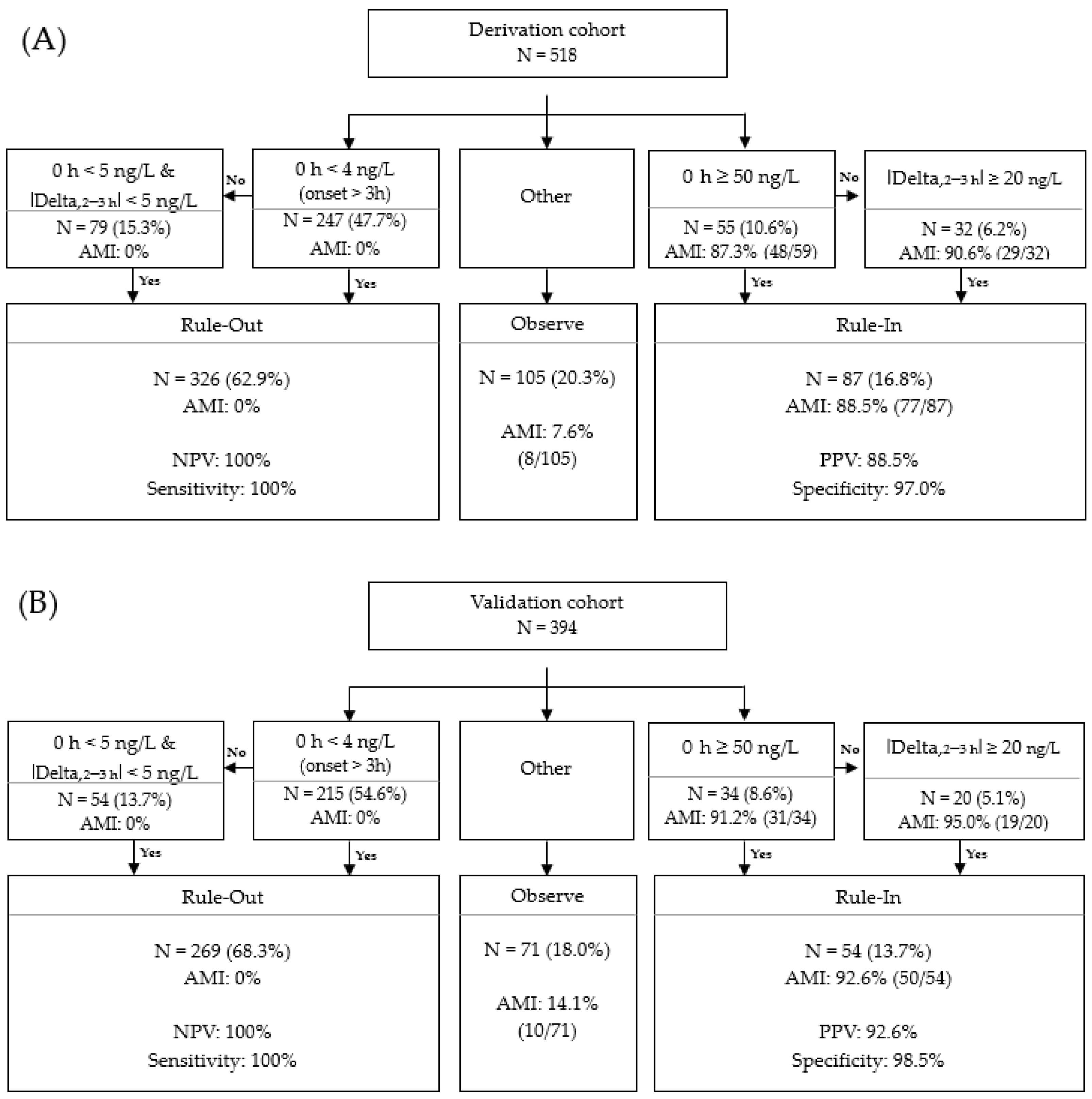
3.5. Validation of Access hs-TnI Algorithmic Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | emergency department |
| cTn | cardiac troponin |
| hs-cTn | high-sensitivity cardiac troponin |
| hs-TnI | high-sensitivity troponin I |
| STEMI | ST-elevation myocardial infarction |
| NSTEMI | non-ST segment elevation myocardial infarction |
| MI | myocardial infarction |
| AMI | acute myocardial infarction |
| URL | upper reference limit |
| IQR | interquartile range |
| ROC | receiver operating characteristic |
| AUC | area under curve |
| NPV | negative predictive value |
| PPV | positive predictive value |
| ECG | electrocardiogram |
| CT | computed tomography |
| eGFR | estimated glomerular filtration rate |
References
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.; Brotons, C.; Chew, D.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2015, 37, 267–315. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Giannitsis, E.; Mueller, C.; Lindahl, B.; Blankenberg, S.; Huber, K.; Plebani, M.; Biasucci, L.M.; Tubaro, M.; et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur. Heart J. 2012, 33, 2252–2257. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.S.; Anderson, J.L.; Adams, C.D.; Bridges, C.R.; Casey, D.; Ettinger, S.M.; Fesmire, F.M.; Ganiats, T.G.; Jneid, H.; Lincoff, A.M.; et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non–ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 57, e215–e367. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Newby, L.K. High-sensitivity troponin assays: Evidence, indications, and reasonable use. J. Am. Heart Assoc. 2014, 3, e000403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apple, F.S.; Collinson, P.O.; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin. Chem. 2012, 58, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Yoo, S.J.; Kim, J. Evaluation of the new Beckman Coulter Access hsTnI: 99th percentile upper reference limits according to age and sex in the Korean population. Clin. Biochem. 2020, 79, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van De Werf, F.; et al. Fourth universal definition of myocardial infarction. Eur. Heart J. 2018, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, Y.; Smith, S.W.; Sexter, A.; Gunsolus, I.L.; Schulz, K.; Apple, F.S. Clinical features and outcomes of emergency department patients with high-sensitivity cardiac troponin I concentrations within sex-specific reference intervals. Circulation 2019, 139, 1753–1755. [Google Scholar] [CrossRef]
- Pickering, J.W.; Young, J.M.; George, P.; Aldous, S.; Cullen, L.; Greenslade, J.H.; Richards, A.M.; Troughton, R.; Ardagh, M.; Frampton, C.M.; et al. The utility of presentation and 4-hour high sensitivity troponin I to rule-out acute myocardial infarction in the emergency department. Clin. Biochem. 2015, 48, 1219–1224. [Google Scholar] [CrossRef]
- Boeddinghaus, J.; Nestelberger, T.; Koechlin, L.; Wussler, D.; Lopez-Ayala, P.; Walter, J.E.; Troester, V.; Ratmann, P.D.; Seidel, F.; Zimmermann, T.; et al. Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J. Am. Coll. Cardiol. 2020, 75, 1111–1124. [Google Scholar] [CrossRef]
- Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Koechlin, L.; Meier, M.; Troester, V.; Wussler, D.; Badertscher, P.; Wildi, K.; Puelacher, C.; et al. High-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin. Chem. 2019, 65, 893–904. [Google Scholar] [CrossRef]
- Mueller, C.; Giannitsis, E.; Christ, M.; Ordóñez-Llanos, J.; Defilippi, C.; McCord, J.; Body, R.; Panteghini, M.; Jernberg, T.; Plebani, M.; et al. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann. Emerg. Med. 2016, 68, 76–87.e4. [Google Scholar] [CrossRef] [Green Version]
- Boeddinghaus, J.; Twerenbold, R.; Nestelberger, T.; Badertscher, P.; Wildi, K.; Puelacher, C.; Lavallaz, J.D.F.D.; Keser, E.; Giménez, M.R.; Wussler, D.N.; et al. Clinical validation of a novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin. Chem. 2018, 64, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestelberger, T.; Boeddinghaus, J.; Greenslade, J.; Parsonage, W.A.; Than, M.; Wussler, D.; Lopez-Ayala, P.; Zimmermann, T.; Meier, M.; Troester, V.; et al. Two-hour algorithm for rapid triage of suspected acute myocardial infarction using a high-sensitivity cardiac troponin I assay. Clin. Chem. 2019, 65, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.M.; Christenson, R.H.; Jacobsen, G.; McCord, J.; Apple, F.S.; Singer, A.J.; Limkakeng, A.; Peacock, W.F.; Defilippi, C.R. Performance of novel high-sensitivity cardiac troponin I assays for 0/1-hour and 0/2- to 3-hour evaluations for acute myocardial infarction: Results from the HIGH-US Study. Ann. Emerg. Med. 2020, 76, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, H.; Yun, Y.-M.; Lee, K.R.; Kim, A.H.J. Absolute change in high-sensitivity cardiac troponin I at three hours after presentation is useful for diagnosing acute myocardial infarction in the emergency department. Ann. Lab. Med. 2020, 40, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J. Cardiac troponin assays: Guide to understanding analytical characteristics and their impact on clinical care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef]
- Wu, A.H.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2018, 64, 645–655. [Google Scholar] [CrossRef]
- Hickman, P.E.; Abhayaratna, W.P.; Potter, J.M.; Koerbin, G. Age-related differences in hs-cTnI concentration in healthy adults. Clin. Biochem. 2019, 69, 26–29. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Anand, A.; Sandoval, Y.; Lee, K.K.; Smith, S.W.; Adamson, P.D.; Chapman, A.; Langdon, T.; Sandeman, D.; Vaswani, A.; et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study. Lancet 2015, 386, 2481–2488. [Google Scholar] [CrossRef] [Green Version]

| All Patients | AMI | Non-AMI | p-Values | |
|---|---|---|---|---|
| Number of patients–N (%) | 1296 | 155 (12.0%) | 1141 (88.0%) | |
| Age, years–median (IQR) | 59.0 (25–69) | 62.0 (52–70) | 58.0 (46–69) | 0.0008 |
| Female gender–N (%) | 581 (44.8%) | 39 (25.2%) | 542 (47.5%) | <0.0001 |
| Early presenters (<3 h from onset)–N (%) | 510 (39.4%) | 82 (52.9%) | 428 (37.5%) | 0.0008 |
| History and risk factors–N (%) | ||||
| Coronary artery disease | 385 (29.7%) | 58 (37.4%) | 327 (28.7%) | 0.0485 |
| Diabetes | 228 (17.6%) | 37 (23.9%) | 191 (16.7%) | 0.0540 |
| Hypertension | 508 (39.2%) | 71 (45.8%) | 437 (38.3%) | 0.1123 |
| Heart failure | 43 (3.3%) | 9 (5.8%) | 34 (3.0%) | 0.1133 |
| Chronic kidney disease | 36 (2.8%) | 5 (3.2%) | 31 (2.7%) | 0.9549 |
| eGFR < 60 mL/min/1.73 m2–N (%) | 143 (11.0%) | 28 (18.1%) | 115 (10.1%) | 0.0045 |
| Baseline hs-TnI, ng/L–median (IQR) | 3.5 (2.3–8.0) | 50.8 (21.2–421.9) | 3.0 (2.3–6.0) | <0.0001 |
| Group | Cut–Offs | Sensitivity | NPV | Specificity | PPV |
|---|---|---|---|---|---|
| Overall | 99th URL by manufacturer, 17.5 ng/L | 79.4 (72.1–85.4) | 97.1 (96.1–97.9) | 95.0 (93.6–96.2) | 68.3 (62.4–73.9) |
| 99th URL in our population, 9.5 ng/L | 83.9 (77.1–89.3) | 97.5 (96.5–98.3) | 86.8 (84.7–88.7) | 46.3 (42.3–50.5) | |
| Cut-off from ROC in this study *, 18.4 ng/L | 78.7 (71.4–84.9) | 97.1 (96.1–97.8) | 95.7 (94.4–96.8) | 71.3 (95.3–97.9) | |
| Men | 99th URL by manufacturer, 19.8 ng/L | 73.3 (64.3–81.1) | 94.9 (93.2–96.2) | 96.0 (94.1–97.4) | 78.0 (70.2–84.2) |
| 99th URL in our population, 11.3 ng/L | 78.5 (69.8–85.5) | 95.6 (93.9–96.9) | 90.5 (87.9–92.7) | 61.5 (55.0–67.5) | |
| Cut-off from ROC in this study, 18.8 ng/L | 75.9 (67.0–83.8) | 95.4 (93.7–96.6) | 96.0 (94.1–97.4) | 78.7 (70.9–84.6) | |
| Women | 99th URL by manufacturer, 11.6 ng/L | 92.3 (79.1–89.4) | 99.4 (98.2–99.8) | 88.6 (85.6–91.1) | 36.7 (31.1–42.7) |
| 99th URL in our population, 7.8 ng/L | 100 (91.0–100) | 100 | 79.5 (75.9–82.8) | 26.0 (22.9–29.3) | |
| Cut-off from ROC in this study, 17.5 ng/L | 89.7 (75.8–97.1) | 99.2 (98.1–99.7) | 94.6 (92.4–96.4) | 54.7 (45.4–65.6) | |
| ≥50 years | 99th URL, overall, by manufacturer, 17.5 ng/L | 80.0 (71.9–86.6) | 96.7 (95.4–97.7) | 93.3 (91.4–95.0) | 65.4 (58.9–71.3) |
| 99th URL in our population, 12.0 ng/L | 81.6 (73.7–88.0) | 96.7 (95.4–97.7) | 86.3 (83.7–88.6) | 48.3 (43.5–53.1) | |
| Cut-off from ROC in this study, 16.8 ng/L | 80.0 (71.9–86.6) | 96.7 (95.4–97.7) | 92.6 (90.5–94.3) | 62.9 (56.6–68.7) | |
| <50 years | 99th URL, overall, by manufacturer, 17.5 ng/L | 76.7 (57.7–90.0) | 98.0 (94.3–99.0) | 98.9 (97.1–99.7) | 85.2 (67.9–93.9) |
| 99th URL in our population, 8.5 ng/L | 83.3 (65.3–94.4) | 98.5 (96.8–99.3) | 95.4 (92.6–97.4) | 61.0 (48.4–72.0) | |
| Cut-off from ROC in this study, 6.9 ng/L | 86.7 (69.3–96.2) | 98.8 (97.0–99.5) | 82.8 (89.9–95.4) | 51.0 (41.6–61.4) |
| Study | Boeddinghaus et al. [12] | Nestelberger et al. [15] | Boeddinghous et al. [11] | Nowak et al. [16] | This Study | |
|---|---|---|---|---|---|---|
| High-sensitivity troponin I assays | Beckman, Access hs-TnI | Beckman, Access hs-TnI | Quidel, TrageTrue hs-TnI | Siemens, Atellica hs-cTn | Beckman, Access hs-TnI | |
| Study population | APACE study * | APACE study * | APACE study * | 29 US medical centers | One Korean center | |
| No. of participants | 680 | 1280 | 545 | 2113 | 1916 | 912 |
| AMI cases, % | 15.4 | 6.9 | 14.0 | 11.8 | 11.8 | 12.0 |
| Algorithm | 0/1 h | 0/2 h | 0/1 h | 0/1 h | 0/2–3 h | 0/2-3 h |
| Rule-out cut-off | ||||||
| 0 h hs-TnI for direct rule out, ng/L | 4 | 4 | 4 | 3 | 3 | 4 |
| 0 h hs-TnI for algorithmic rule out, ng/L | 5 | 5 | 5 | 6 | 7 | 5 |
| Delta hs-TnI | 4 | 5 | 3 | 3 | 7 | 5 |
| Rule-in cut-off | ||||||
| Baseline hs-TnI, ng/L | 50 | 50 | 60 | 120 | 120 | 50 |
| Delta hs-TnI, ng/L | 15 | 20 | 8 | 12 | 20 | 20 |
| Rule-out, % | 60.0 | 77.9 | 55.0 | 50.4 | 55.6 | 66.1 |
| Rule-in, % | 14.0 | 5.8 | 18.0 | 12.6 | 13.3 | 15.5 |
| Sensitivity, % | 98.9 | 97.7 | 100 | 98.7 | 99.1 | 100 |
| NPV, % | 99.8 | 99.8 | 100 | 99.7 | 99.9 | 100 |
| Specificity, % | 95.9 | 98.6 | 95 | 95.7 | 95.5 | 97.7 |
| PPV, % | 73.9 | 77.0 | 76.8 | 69.4 | 69.7 | 90.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.; Kim, G.S.; Byun, Y.S.; Kim, J.; Kim, S.; Chang, J.; Yoo, S.J. An Algorithmic Approach Is Superior to the 99th Percentile Upper Reference Limits of High Sensitivity Troponin as a Threshold for Safe Discharge from the Emergency Department. Medicina 2021, 57, 1083. https://doi.org/10.3390/medicina57101083
Kang T, Kim GS, Byun YS, Kim J, Kim S, Chang J, Yoo SJ. An Algorithmic Approach Is Superior to the 99th Percentile Upper Reference Limits of High Sensitivity Troponin as a Threshold for Safe Discharge from the Emergency Department. Medicina. 2021; 57(10):1083. https://doi.org/10.3390/medicina57101083
Chicago/Turabian StyleKang, Taekyung, Gwang Sil Kim, Young Sup Byun, Jongwoo Kim, Sollip Kim, Jeonghyun Chang, and Soo Jin Yoo. 2021. "An Algorithmic Approach Is Superior to the 99th Percentile Upper Reference Limits of High Sensitivity Troponin as a Threshold for Safe Discharge from the Emergency Department" Medicina 57, no. 10: 1083. https://doi.org/10.3390/medicina57101083
APA StyleKang, T., Kim, G. S., Byun, Y. S., Kim, J., Kim, S., Chang, J., & Yoo, S. J. (2021). An Algorithmic Approach Is Superior to the 99th Percentile Upper Reference Limits of High Sensitivity Troponin as a Threshold for Safe Discharge from the Emergency Department. Medicina, 57(10), 1083. https://doi.org/10.3390/medicina57101083






