Effects of Colocasia antiquorum var. Esculenta Extract In Vitro and In Vivo against Periodontal Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Colocasia antiquorum var. Esculenta Extract (CA)
2.2. Antibacterial Activity of CA
2.3. Cell Cytotoxicity Assay
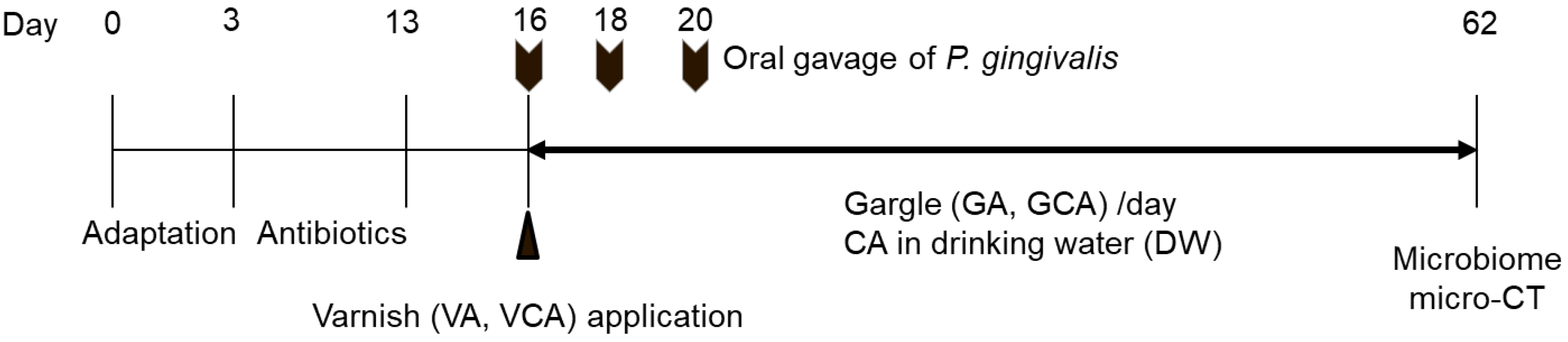
2.4. In Vivo Study Using a Mouse Model
2.5. Oral Microbiome
2.6. Quantification of Alveolar Bone Loss by Micro-Computed Tomography (CT)
2.7. Statistical Analysis
3. Results
3.1. Antibacterial Activity of CA
3.2. Cell Viability of CA
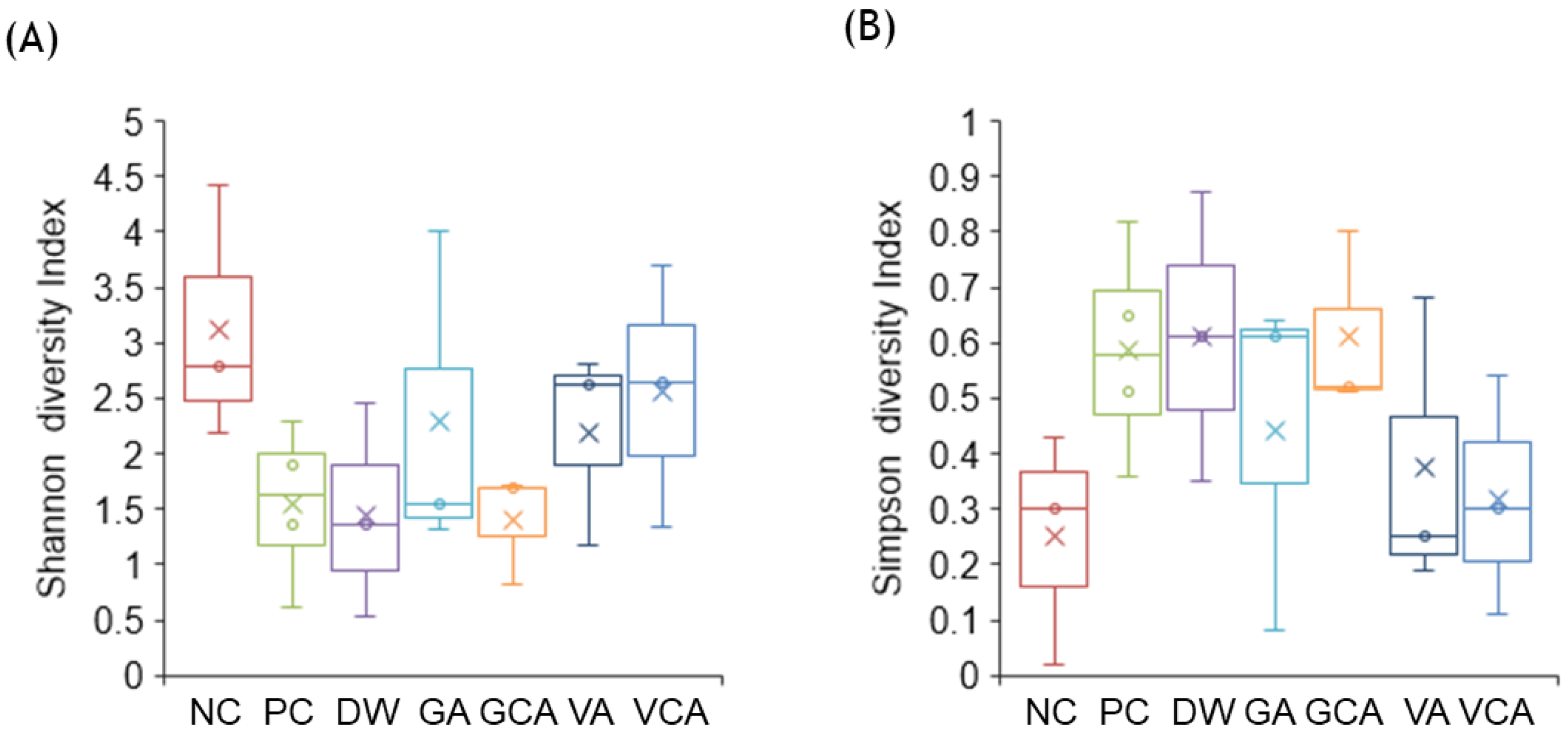
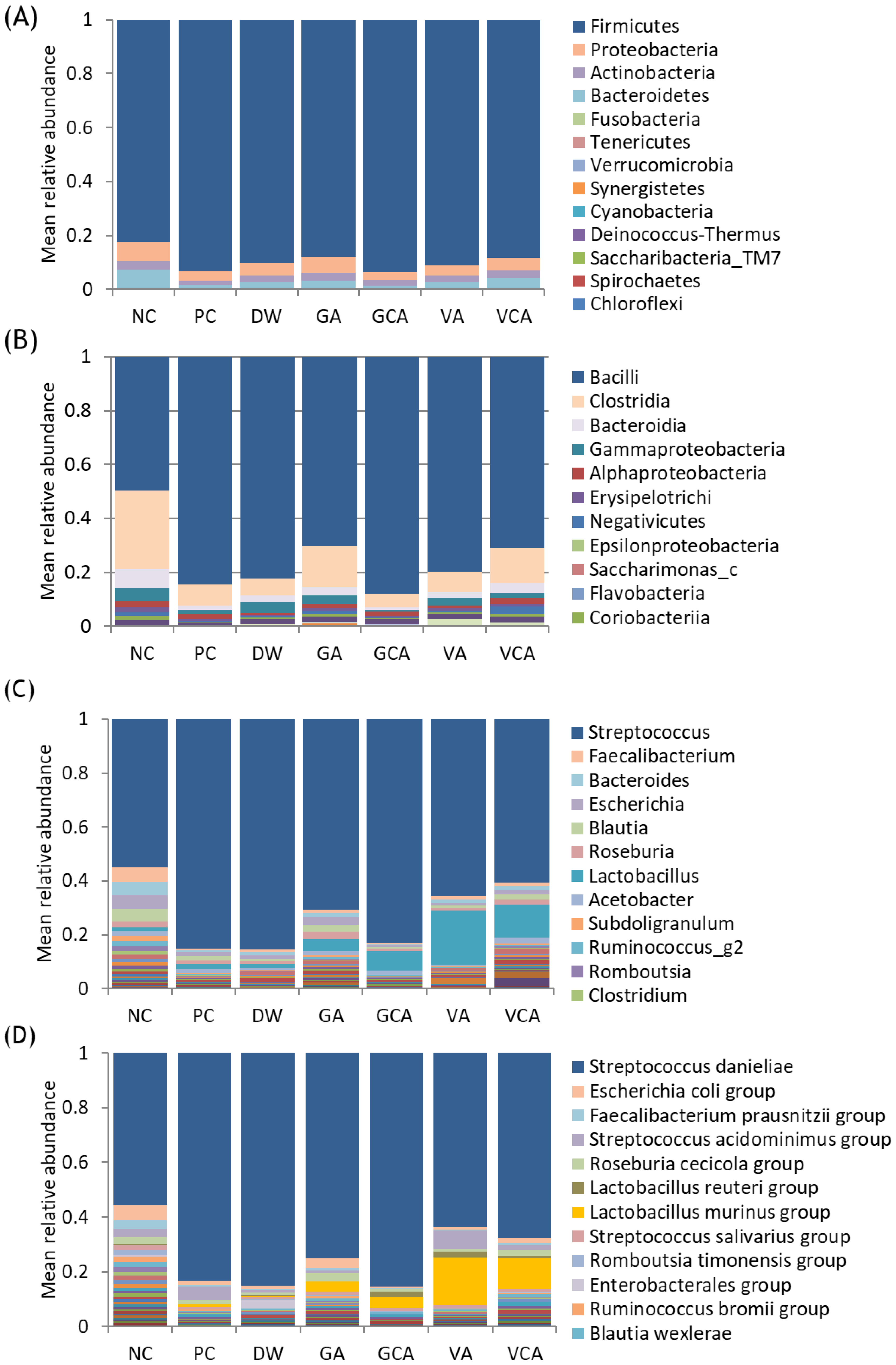
3.3. Microbiome Analysis
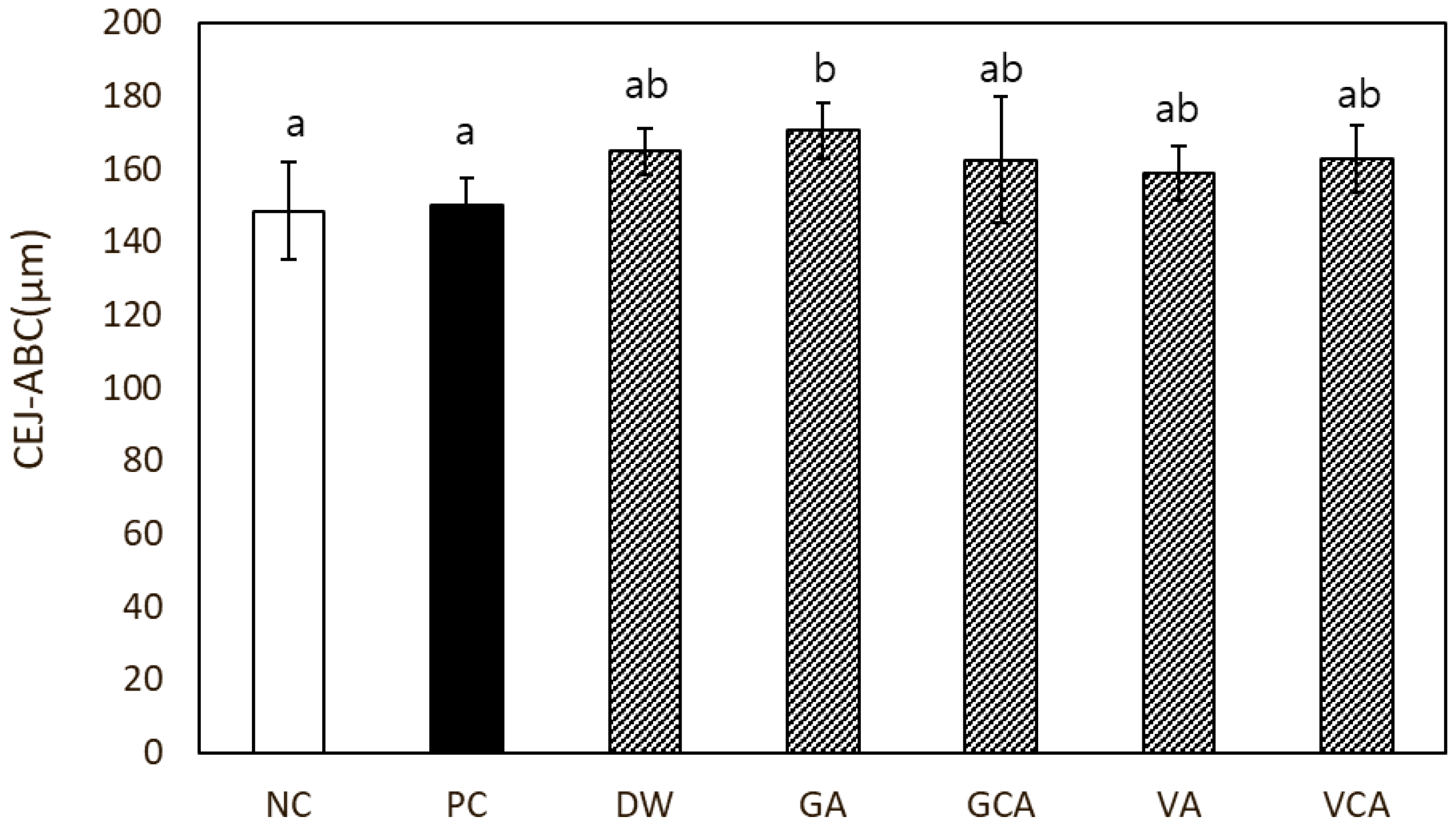
3.4. Alveolar Bone Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotstein, I.; Simon, J.H. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontology 2004, 34, 165–203. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Radnaabaatar, M.; Kim, Y.E.; Go, D.S.; Jung, Y.; Jung, J.; Yoon, S.J. Burden of dental caries and periodontal disease in South Korea: An analysis using the national health insurance claims database. Community Dent. Oral Epidemiol. 2019, 47, 513–519. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Della Vella, F.; Di Stasio, D.; Carinci, F.; Lucchese, A.; Petruzzi, M. Oral Health Status and Need for Oral Care in an Aging Population: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4558. [Google Scholar] [CrossRef] [Green Version]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Mustapha, I.Z.; Debrey, S.; Oladubu, M.; Ugarte, R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J. Periodontol. 2007, 78, 2289–2302. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.; Aduse-Opoku, J.; Hashim, A.; Hanski, E.; Streich, R.; Knowles, S.C.L.; Pedersen, A.B.; Wade, W.G.; Curtis, M.A. A 16S rRNA Gene and Draft Genome Database for the Murine Oral Bacterial Community. mSystems 2021, 6, e01222-20. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Abusleme, L.; Hong, B.-Y.; Hoare, A.; Konkel, J.E.; Diaz, P.I.; Moutsopoulos, N.M. Oral Microbiome Characterization in Murine Models. Bio. Protoc. 2017, 7, e2655. [Google Scholar] [CrossRef] [Green Version]
- Tangade, P.S.; Mathur, A.; Tirth, A.; Kabasi, S. Anti-gingivitis effects of Acacia arabica-containing toothpaste. Chin. J. Dent. Res. 2012, 15, 49–53. [Google Scholar]
- Dany, S.S.; Mohanty, P.; Tangade, P.; Rajput, P.; Batra, M. Efficacy of 0.25% Lemongrass Oil Mouthwash: A Three Arm Prospective Parallel Clinical Study. J. Clin. Diagn. Res. 2015, 9, ZC13–ZC17. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.K.; Ramberg, P. The effect of a dentifrice containing Magnolia extract on established plaque and gingivitis in man: A six-month clinical study. Int. J. Dent. Hyg. 2014, 12, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Ji, S.H.; Son, J.L.; Shin, S.J.; Oh, S.; Kim, S.H.; Bae, J.M. Antibacterial, anti-inflammatory, and anti-osteoclastogenic activities of Colocasia antiquorum var. esculenta: Potential applications in preventing and treating periodontal diseases. Dent. Mater. J. 2020, 39, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Boutaugh, N.R.; Tiffany, M.; Roopenian, D.C. B Cell IgD Deletion Prevents Alveolar Bone Loss Following Murine Oral Infection. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 864359. [Google Scholar] [CrossRef]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Dudek, A.; Pędziwiatr, M.; Major, P. Differences in Compositions of Oral and Fecal Microbiota between Patients with Obesity and Controls. Medicina 2021, 57, 678. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Gabet, Y.; Yumoto, H.; Houri-Haddad, Y.; Shapira, L. Three-dimensional quantification of alveolar bone loss in Porphyromonas gingivalis-infected mice using micro-computed tomography. J. Periodontol. 2005, 76, 1282–1286. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Wang, Y.; Wang, Z.; Ding, W.; Sun, X.; He, K.; Feng, Q.; Zhang, X. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging 2020, 12, 13090–13114. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Kim, H.D.; Moon, J.Y.; Zavras, A.I.; Zdanowicz, J.; Jang, S.J.; Jin, B.H.; Bae, K.H.; Paik, D.I.; Douglass, C.W. Periodontitis and the risk for non-fatal stroke in Korean adults. J. Periodontol. 2008, 79, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, R.H. Communities and Ecosystems; Macmillan Publishing Company, Inc.: New York, NY, USA, 1975. [Google Scholar]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The roles of osteocytes in alveolar bone destruction in periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef]
- Kumar, P.S. Microbial dysbiosis: The root cause of periodontal disease. J. Periodontol. 2021, 92, 1079–1087. [Google Scholar] [CrossRef]
- Mealey, B.L. Diabetes and periodontal disease: Two sides of a coin. Compend. Contin. Educ. Dent. 2000, 21, 943–946. [Google Scholar]
- Reddy, M.S. Oral osteoporosis: Is there an association between periodontitis and osteoporosis? Compend. Contin. Educ. Dent. 2002, 23, 21–28. [Google Scholar]
- Baker, P.J.; Dixon, M.; Evans, R.T.; Roopenian, D.C. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol. Immunol. 2000, 15, 27–32. [Google Scholar] [CrossRef]
- Clements, J.D.; Baker Pamela, J.; Dixon, M.; Roopenian Derry, C. Genetic Control of Susceptibility to Porphyromonas gingivalis-Induced Alveolar Bone Loss in Mice. Infect. Immun. 2000, 68, 5864–5868. [Google Scholar] [CrossRef] [Green Version]



| Group | Code | Treatment |
|---|---|---|
| Negative control | NC | Non-challenge with P. gingivalis |
| Positive control | PC | Oral challenge with P. gingivalis |
| CA in drinking water | DW | Oral challenge with P. gingivalis, 1 w/v% of CA in drinking water |
| Gargle | GA | Oral challenge with P. gingivalis, Garglin zero™ |
| Gargle with CA | GCA | Oral challenge with P. gingivalis, 5% CA in Garglin zero™ |
| Varnish | VA | Applied varnish, oral challenge with P. gingivalis |
| Varnish with CA | VCA | Applied varnish with 15% CA, oral challenge with P. gingivalis |
| NC | PC | DW | GA | GCA | VA | VCA | ||
|---|---|---|---|---|---|---|---|---|
| Class | Gammaproteobacteria | 5.112 (1.507) | 1.635 (1.009) | 4.171 (3.714) | 3.071 (2.711) | 0.7697 (0.298) | 2.875 (3.327) | 1.871 (1.126) |
| Genus | Lactobacillus | 1.027 (0.502) | 1.866 (1.483) | 1.623 (0.995) | 4.131 (2.115) | 7.021 (5.700) | 17.821 (16.630) | 11.131 (7.057) |
| Species | L. murinus | 0.026 (0.021) | 0.685 (0.883) | 0.307 (0.341) | 2.819 (2.965) | 3.667 (3.014) | 14.065 (19.074) | 8.790 (7.304) |
| S. danieliae | 42.150 (31.206) | 75.332 (13.578) | 76.763 (17.017) | 61.411 (30.131) | 77.308 (10.311) | 51.222 (29.464) | 52.389 (19.910) | |
| P. gingivalis | 0.001 (0.001) | 0.013 (0.020) | 0.000 (0.000) | 0.011 (0.002) | 0.005 (0.009) | 0.001 (0.002) | 0.006 (0.011) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-H.; Shin, S.-J.; Tae, H.-J.; Oh, S.-H.; Bae, J.-M. Effects of Colocasia antiquorum var. Esculenta Extract In Vitro and In Vivo against Periodontal Disease. Medicina 2021, 57, 1054. https://doi.org/10.3390/medicina57101054
Moon S-H, Shin S-J, Tae H-J, Oh S-H, Bae J-M. Effects of Colocasia antiquorum var. Esculenta Extract In Vitro and In Vivo against Periodontal Disease. Medicina. 2021; 57(10):1054. https://doi.org/10.3390/medicina57101054
Chicago/Turabian StyleMoon, Seong-Hee, Seong-Jin Shin, Hyun-Jin Tae, Seung-Han Oh, and Ji-Myung Bae. 2021. "Effects of Colocasia antiquorum var. Esculenta Extract In Vitro and In Vivo against Periodontal Disease" Medicina 57, no. 10: 1054. https://doi.org/10.3390/medicina57101054
APA StyleMoon, S.-H., Shin, S.-J., Tae, H.-J., Oh, S.-H., & Bae, J.-M. (2021). Effects of Colocasia antiquorum var. Esculenta Extract In Vitro and In Vivo against Periodontal Disease. Medicina, 57(10), 1054. https://doi.org/10.3390/medicina57101054







