Muscle Regeneration and Function in Sports: A Focus on Vitamin D
Abstract
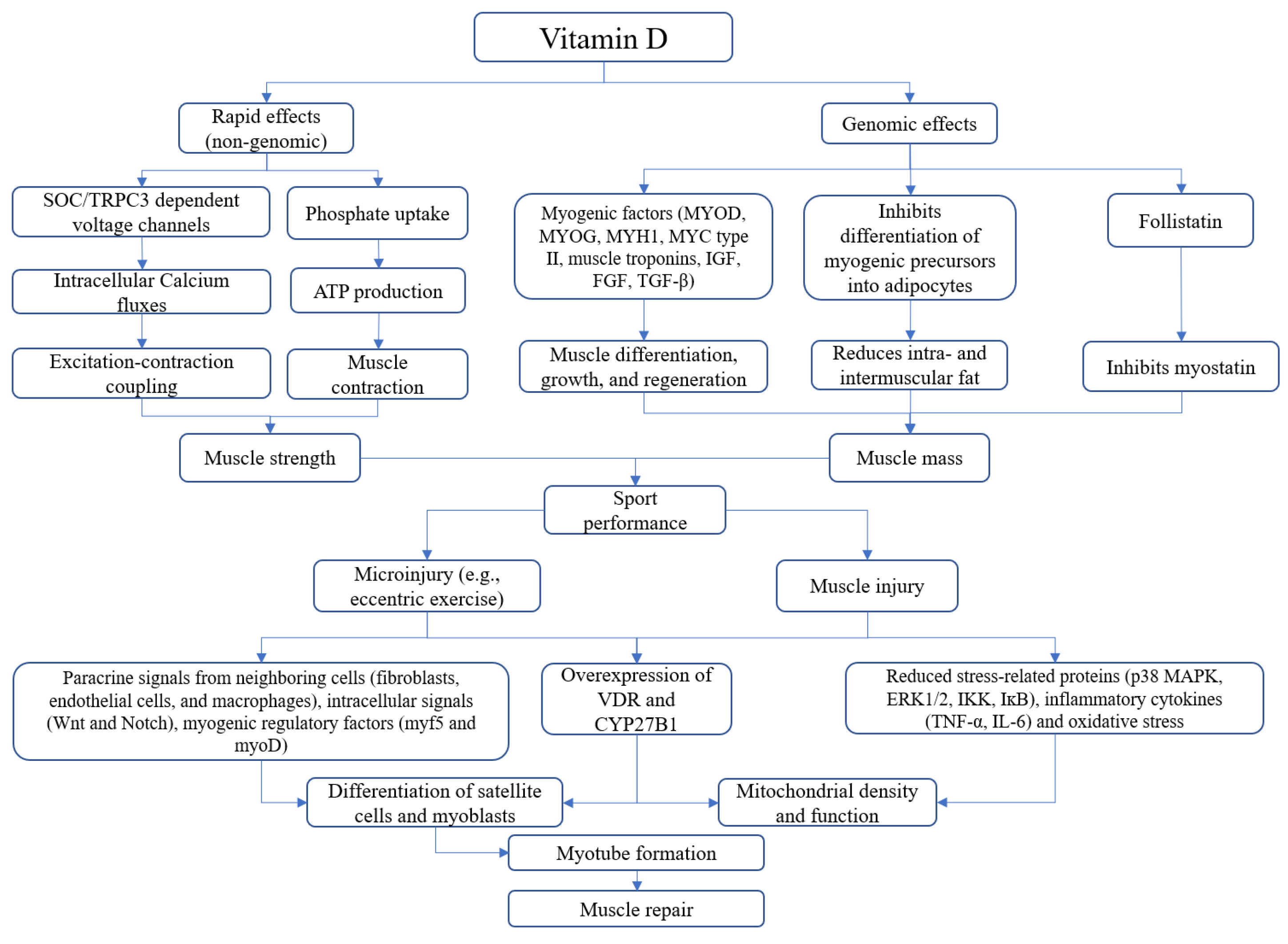
:1. Introduction
2. Vitamin D and Skeletal Muscle: Basic Concepts
3. Vitamin D and Sport-Related Muscle Injury: From Biology to Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dawson-Hughes, B. Vitamin D and muscle function. J. Steroid Biochem. Mol. Biol. 2017, 173, 313–316. [Google Scholar] [CrossRef]
- Valerio, M.S.; Janakiram, N.B.; Goldman, S.M.; Dearth, C.L. Pleiotropic actions of Vitamin D in composite musculoskeletal trauma. INJ 2020, 51, 2099–2109. [Google Scholar] [CrossRef]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Narayanan, R.; Sepulveda, V.A.; Falzon, M.; Weigel, N.L. The functional consequences of cross-talk between the vitamin D re-ceptor and ERK signaling pathways are cell-specific. J. Biol. Chem. 2004, 279, 47298–47310. [Google Scholar] [CrossRef] [Green Version]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport 2015, 18, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Mauro, G.L.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; De Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicentre retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gimigliano, F.; Moretti, A.; De Sire, A.; Calafiore, D.; Iolascon, G. The combination of vitamin D deficiency and overweight affects muscle mass and function in older post-menopausal women. Aging Clin. Exp. Res. 2018, 30, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; De Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, R.; Gimigliano, F. Hypovitaminosis D is associated with a reduction in upper and lower limb muscle strength and physical performance in post-menopausal women: A retrospective study. Aging Clin. Exp. Res. 2015, 27, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Moretti, A.; De Sire, A.; Calafiore, D.; Gimigliano, F. Effectiveness of Calcifediol in Improving Muscle Function in Post-Menopausal Women: A Prospective Cohort Study. Adv. Ther. 2017, 34, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Vitamin D and Sport Performance. Nutrients 2020, 12, 841. [Google Scholar] [CrossRef] [Green Version]
- von Hurst, P.; Conlon, C.; Foskett, A. Vitamin D status predicts hand-grip strength in young adult women living in Auckland, New Zealand. J. Steroid Biochem. Mol. Biol. 2013, 136, 330–332. [Google Scholar] [CrossRef]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A Review on Its Effects on Muscle Strength, the Risk of Fall, and Frailty. BioMed Res. Int. 2015, 2015, 953241. [Google Scholar] [CrossRef] [Green Version]
- Yagüe, M.D.L.P.; Yurrita, L.C.; Cabañas, M.C.; Cenzual, M.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [Green Version]
- Książek, A.; Dziubek, W.; Pietraszewska, J.; Słowińska-Lisowska, M. Relationship between 25(OH)D levels and athletic performance in elite Polish judoists. Biol. Sport 2018, 35, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes—A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports Health Benefits of Vitamin D. Sports Health Multidiscip. Approach 2012, 4, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, B. Vitamin D and Athletic Performance: The Potential Role of Muscle. Asian J. Sports Med. 2011, 2, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozłowska, M.; Żurek, P.; Rodziewicz, E.; Góral, K.; Żmijewski, P.; Lipińska, P.; Laskowski, R.; Walentukiewicz, A.K.; Antosiewicz, J.; Ziemann, E. Immunological Response and Match Performance of Professional Tennis Players of Different Age Groups During a Competitive Season. J. Strength Cond. Res. 2021, 35, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.A.R.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef] [Green Version]
- Mackey, A.L.; Kjaer, M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet. Muscle 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Smerdu, V.; Mizrachi, I.K.; Campione, M.; Leinwand, L.; Schiaffino, S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am. J. Physiol. Physiol. 1994, 267, C1723–C1728. [Google Scholar] [CrossRef]
- Scott, W.; Stevens, J.; Binder–Macleod, S.A. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle WIREs. Syst. Biol. Med. 2012, 4, 457–473. [Google Scholar]
- Silverthorn, D.U. Human Physiology; Jones & Bartlett Publishers: Burlington, MA, USA, 2015. [Google Scholar]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Mahmassani, Z.S.; Reidy, P.T.; McKenzie, A.I.; Stubben, C.; Howard, M.T.; Drummond, M.J. Disuse-induced insulin resistance susceptibility coincides with a dysregulated skeletal muscle metabolic transcriptome. J. Appl. Physiol. 2019, 126, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.I.; Priego, T.; López-Calderón, A. Hormones and Muscle Atrophy. Viral Entry Host Cells 2018, 1088, 207–233. [Google Scholar] [CrossRef]
- Fappi, A.; Neves, J.D.C.; Sanches, L.N.; E Silva, P.V.M.; Sikusawa, G.Y.; Brandão, T.P.C.; Chadi, G.; Zanoteli, E. Skeletal Muscle Response to Deflazacort, Dexamethasone and Methylprednisolone. Cells 2019, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Ucci, S.; Renzini, A.; Russi, V.; Mangialardo, C.; Cammarata, I.; Cavioli, G.; Santaguida, M.G.; Virili, C.; Centanni, M.; Adamo, S.; et al. Thyroid Hormone Protects from Fasting-Induced Skeletal Muscle Atrophy by Promoting Metabolic Adaptation. Int. J. Mol. Sci. 2019, 20, 5754. [Google Scholar] [CrossRef] [Green Version]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Girgis, C.M. Vitamin D and Skeletal Muscle: Emerging Roles in Development, Anabolism and Repair. Calcif. Tissue Int. 2019, 106, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr. Connect. 2017, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- de Boland, A.R.; Gallego, S.; Boland, R. Effects of vitamin D-3 on phosphate and calcium transport across and composition of skeletal muscle plasma cell membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1983, 733, 264–273. [Google Scholar] [CrossRef]
- Ryan, K.J.P.; Daniel, Z.C.T.R.; Craggs, L.; Parr, T.; Brameld, J.M. Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J. Endocrinol. 2013, 217, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Cha, K.M.; Houweling, P.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Hirunsai, M.; Charoenphandhu, N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D Signaling Regulates Proliferation, Differentiation, and Myotube Size in C2C12 Skeletal Muscle Cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.-K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef] [Green Version]
- Downes, M.; Mynett-Johnson, L.; E Muscat, G. The retinoic acid and retinoid X receptors are differentially expressed during myoblast differentiation. Endocrinology 1994, 134, 2658–2661. [Google Scholar] [CrossRef]
- Makanae, Y.; Ogasawara, R.; Sato, K.; Takamura, Y.; Matsutani, K.; Kido, K.; Shiozawa, N.; Nakazato, K.; Fujita, S. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp. Physiol. 2015, 100, 1168–1176. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Le Grand, F.; Jones, A.E.; Seale, V.; Scimè, A.; Rudnicki, M.A. Wnt7a Activates the Planar Cell Polarity Pathway to Drive the Symmetric Expansion of Satellite Stem Cells. Cell Stem Cell 2009, 4, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Renault, V.M.; Rando, T.A. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 2005, 16, 612–622. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Kim, J.; Kostin, S.; Lepper, C.; Fan, C.-M.; Braun, T. Myf5-Positive Satellite Cells Contribute to Pax7-Dependent Long-Term Maintenance of Adult Muscle Stem Cells. Cell Stem Cell 2013, 13, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Hyldahl, R.D.; Olson, T.; Welling, T.; Groscost, L.; Parcell, A.C. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol. 2014, 5, 485. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 498. [Google Scholar] [CrossRef]
- Srikuea, R.; Hirunsai, M. Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J. Appl. Physiol. 2016, 120, 1381–1393. [Google Scholar] [CrossRef] [Green Version]
- Puangthong, C.; Sukhong, P.; Saengnual, P.; Srikuea, R.; Chanda, M. A single bout of high-intensity exercise modulates the expression of vitamin D receptor and vitamin D-metabolising enzymes in horse skeletal muscle. Equine Vet-J. 2021, 53, 796–805. [Google Scholar] [CrossRef]
- Montenegro, K.R.; Carlessi, R.M.; Cruzat, V.F.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Kowaltowski, A.J. Satellite cell self-renewal in endurance exercise is mediated by inhibition of mito-chondrial oxygen consumption. J. Cachexia. Sarcopenia Muscle 2020, 11, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Latil, M.; Rocheteau, P.; Châtre, L.; Sanulli, S.; Mémet, S.; Ricchetti, M.; Tajbakhsh, S.; Chrétien, F. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat. Commun. 2012, 3, 903. [Google Scholar] [CrossRef] [Green Version]
- Esteca, M.V.; Severino, M.B.; Silvestre, J.G.; Dos Santos, G.P.; Tamborlin, L.; Luchessi, A.D.; Moriscot, A.S.; Gustafsson, Å.B.; Baptista, I.L. Loss of Parkin Results in Altered Muscle Stem Cell Differentiation during Regeneration. Int. J. Mol. Sci. 2020, 21, 8007. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Boland, R.L. In vitro muscle phosphate uptake. Characteristics and action of vitamin D3 metabolites. In Vitamin D: A Chemical, Biochemical, and Clinical Update; Norman, A.W., Schaefer, H., Grigoleit, H.G., Eds.; Walter de Gruyter: Berlin, Germany, 1985; pp. 590–591. [Google Scholar]
- Birge, S.J.; Haddad, J.G. 25-hydroxycholecalciferol stimulation of muscle metabolism. J. Clin. Investig. 1975, 56, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; E Zerwekh, J.; Nicar, M.J.; McCoy, K.; Buja, L.M. Effect of chronic vitamin D deficiency on chick heart mitochondrial oxidative phosphorylation. J. Mol. Cell. Cardiol. 1981, 13, 171–183. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the Vitamin D Status of Vitamin D Deficient Adults Is Associated With Improved Mitochondrial Oxidative Function in Skeletal Muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.; Wang, X.; Lanza, I.; Schaible, N.S.; Salisbury, J.; Nair, K.S.; Terzic, A.; Sieck, G.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.; Park, H.; Cho, S.; Lee, M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine 2013, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Kita, B.; Piotrowska, A.; Tota, Ł.; Maciejczyk, M.; Czerwińska-Ledwig, O.; Krepa, E.S.; Kita, S.; Pałka, T. The effect of vitamin D supplementation on the muscle damage after eccentric exercise in young men: A randomized, control trial. J. Int. Soc. Sports Nutr. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Barker, T.; Schneider, E.D.; Dixon, B.M.; Henriksen, V.T.; Weaver, L.K. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr. Metab. 2013, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Gabr, S.; Al-Eisa, E.S.; Alghadir, A.H. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging 2016, 11, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.-K.; Mittlmeier, T.; Vollmar, B. Vitamin D Increases Cellular Turnover and Functionally Restores the Skeletal Muscle after Crush Injury in Rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebolledo, B.J.; Bernard, J.A.; Werner, B.; Finlay, A.K.; Nwachukwu, B.U.; Dare, D.M.; Warren, R.F.; Rodeo, S.A. The Association of Vitamin D Status in Lower Extremity Muscle Strains and Core Muscle Injuries at the National Football League Combine. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Redzic, M.; Thomas, D.T. The Effects of Season-Long Vitamin D Supplementation on Collegiate Swimmers and Divers. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Sato, Y.; Kobayashi, T.; Kaneko, Y.; Ito, E.; Soma, T.; Okada, H.; Miyamoto, K.; Oya, A.; Matsumoto, M.; et al. Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice. Sci. Rep. 2020, 10, 12242. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iolascon, G.; Moretti, A.; Paoletta, M.; Liguori, S.; Di Munno, O. Muscle Regeneration and Function in Sports: A Focus on Vitamin D. Medicina 2021, 57, 1015. https://doi.org/10.3390/medicina57101015
Iolascon G, Moretti A, Paoletta M, Liguori S, Di Munno O. Muscle Regeneration and Function in Sports: A Focus on Vitamin D. Medicina. 2021; 57(10):1015. https://doi.org/10.3390/medicina57101015
Chicago/Turabian StyleIolascon, Giovanni, Antimo Moretti, Marco Paoletta, Sara Liguori, and Ombretta Di Munno. 2021. "Muscle Regeneration and Function in Sports: A Focus on Vitamin D" Medicina 57, no. 10: 1015. https://doi.org/10.3390/medicina57101015
APA StyleIolascon, G., Moretti, A., Paoletta, M., Liguori, S., & Di Munno, O. (2021). Muscle Regeneration and Function in Sports: A Focus on Vitamin D. Medicina, 57(10), 1015. https://doi.org/10.3390/medicina57101015






