Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy
Abstract
1. Introduction
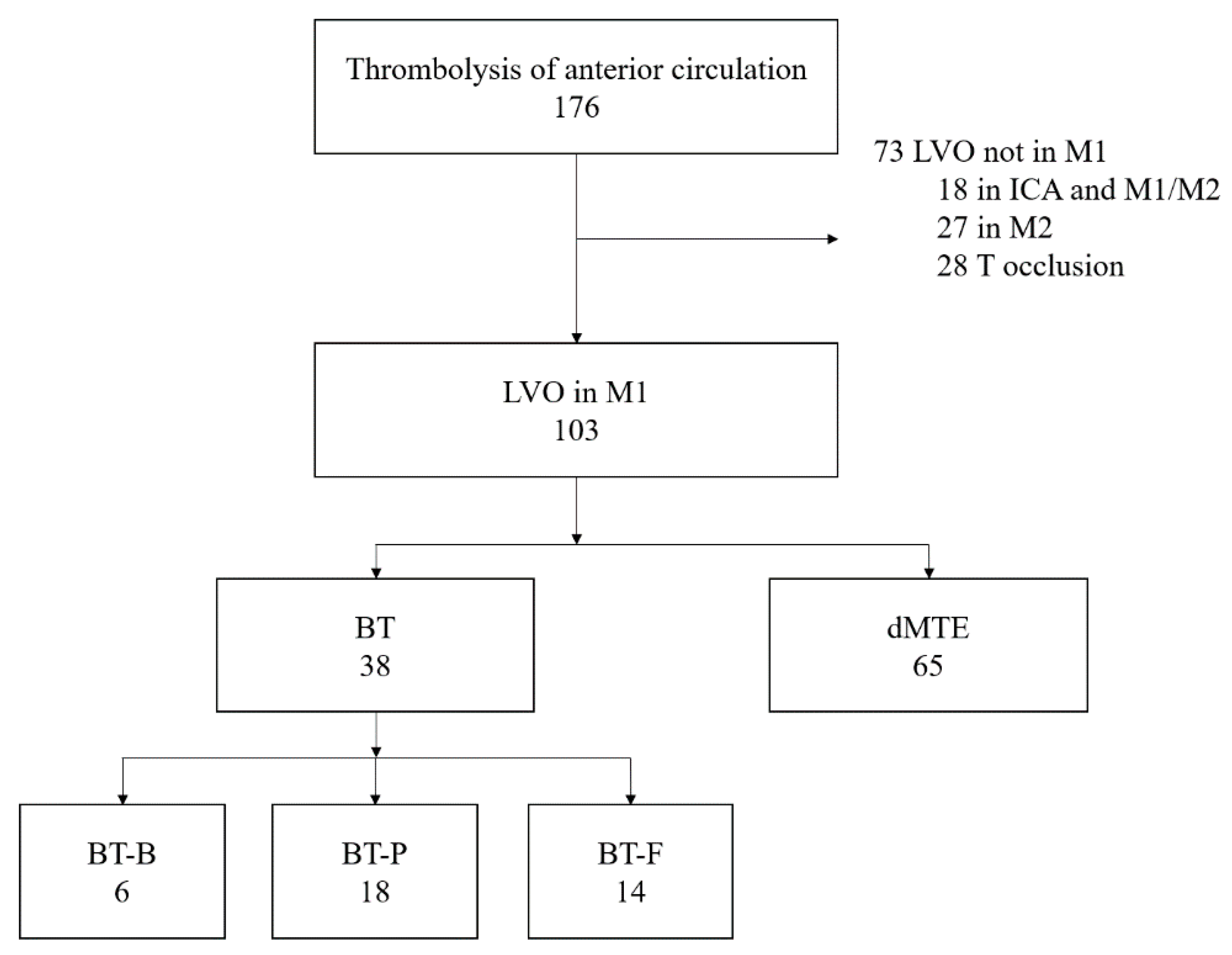
2. Materials and Methods
2.1. Outcome Measures
2.2. Statistical Analysis
3. Results
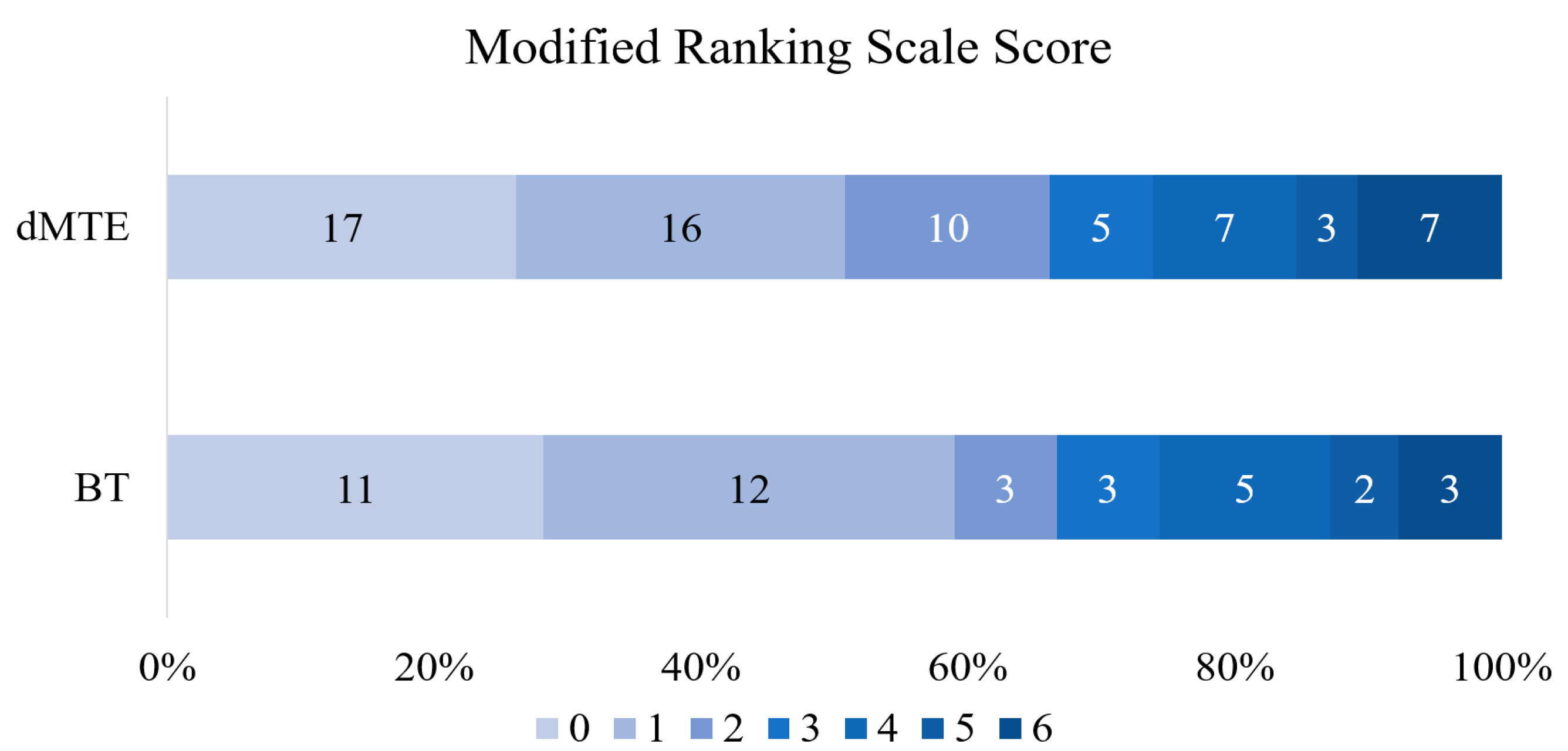
3.1. BT vs. dMTE
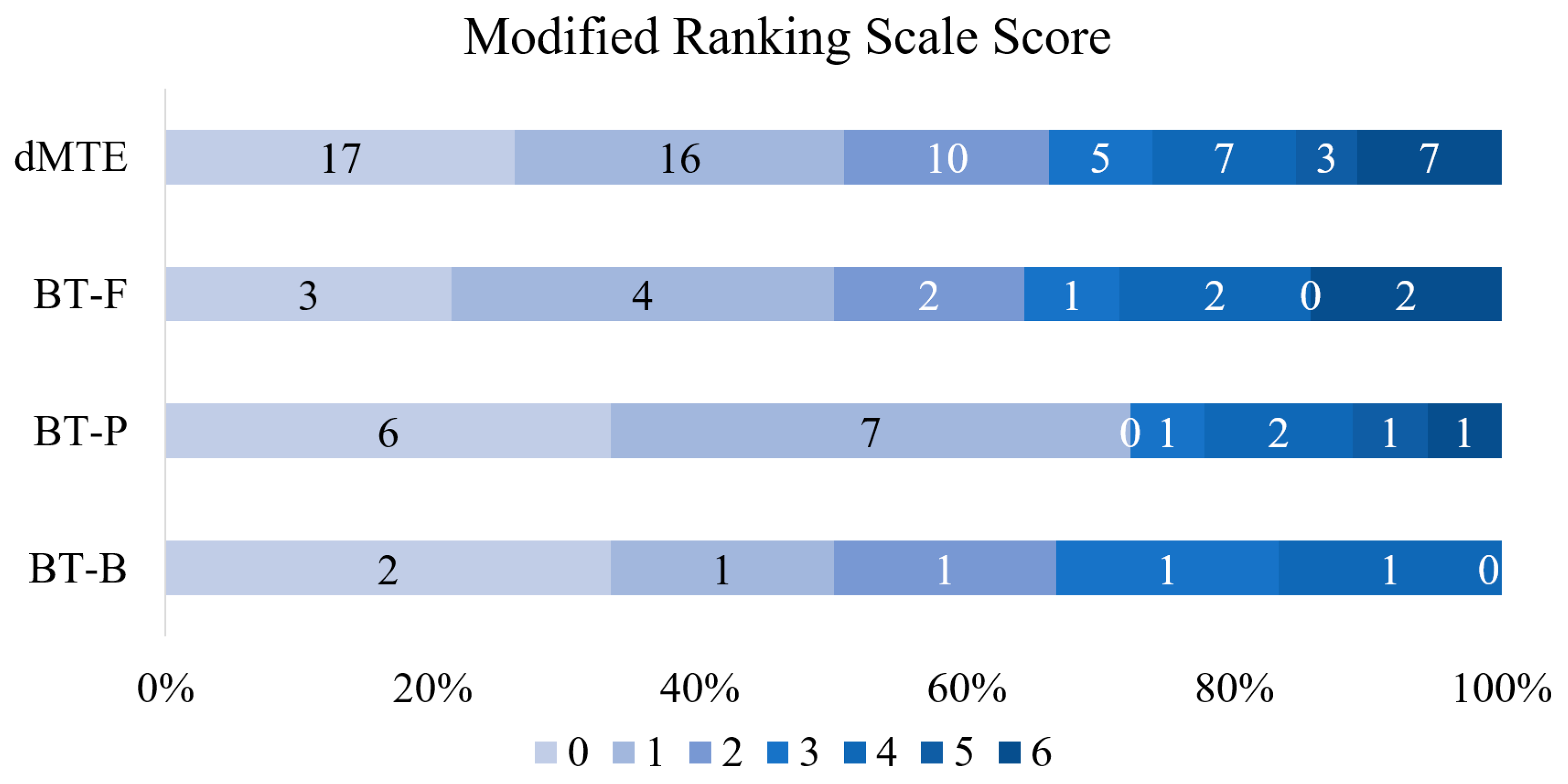
3.2. Various Dosages of r-tPA vs. dMTE
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Powers, W.J.; Derdeyn, C.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke 2015, 46, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- Bellwald, S.; Weber, R.; Dobrocky, T.; Nordmeyer, S.; Jung, S.; Hadisurya, J.; Mordasini, P.; Mono, M.-L.; Stracke, C.P.; Sarikaya, H.; et al. Direct Mechanical Intervention Versus Bridging Therapy in Stroke Patients Eligible for Intravenous Thrombolysis. Stroke 2017, 48, 3282–3288. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Kaesmacher, J.; Molina, C.A.; Selim, M.H.; Alexandrov, A.V.; Tsivgoulis, G. Primary Thrombectomy in tPA (Tissue-Type Plasminogen Activator) Eligible Stroke Patients with Proximal Intracranial Occlusions. Stroke 2017, 49, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.; Parsons, M.W.; Oxley, T.; et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; De Miquel, M.A.; Molina, C.A.; Rovira, À.; Román, L.S.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; Van Zwam, W.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.; Van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Y.; Zhang, L.; Zhang, Y.; Treurniet, K.M.; Chen, W.; Peng, Y.; Han, H.; Wang, J.; Wang, S.; et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N. Engl. J. Med. 2020, 382, 1981–1993. [Google Scholar] [CrossRef]
- Phan, K.; Dmytriw, A.A.; Maingard, J.; Asadi, H.; Griessenauer, C.J.; Ng, W.; Kewagamang, K.; Mobbs, R.J.; Moore, J.M.; Ogilvy, C.S.; et al. Endovascular Thrombectomy Alone versus Combined with Intravenous Thrombolysis. World Neurosurg. 2017, 108, 850–858.e2. [Google Scholar] [CrossRef]
- Mistry, E.A.; Mistry, A.M.; Nakawah, M.O.; Chitale, R.V.; James, R.F.; Volpi, J.J.; Fusco, M.R. Mechanical Thrombectomy Outcomes with and without Intravenous Thrombolysis in Stroke Patients. Stroke 2017, 48, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Damaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e138. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Higashida, R.; Wechsler, L.; Gent, M.; Rowley, H.; Kase, C.S.; Pessin, M.; Ahuja, A.; Callahan, F.; Clark, W.M.; et al. Intra-arterial Prourokinase for Acute Ischemic Stroke. JAMA 1999, 282, 2003–2011. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Mordasini, P.; Arnold, M.; López-Cancio, E.; Cerdà, N.; Boeckh-Behrens, T.; Kleine, J.F.; Goyal, M.; Hill, M.D.; Pereira, V.M.; et al. Direct mechanical thrombectomy in tPA-ineligible and -eligible patients versus the bridging approach: A meta-analysis. J. NeuroInterv. Surg. 2018, 11, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gariel, F.; Lapergue, B.; Bourcier, R.; Berge, J.; Barreau, X.; Mazighi, M.; Kyheng, M.; Labreuche, J.; Fahed, R.; Blanc, R.; et al. Mechanical Thrombectomy Outcomes with or without Intravenous Thrombolysis. Stroke 2018, 49, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zi, W.; Hao, Y.; Yang, D.; Shi, Z.; Lin, M.; Wang, S.; Liu, W.; Wang, Z.; Guo, F.; et al. Direct endovascular treatment: An alternative for bridging therapy in anterior circulation large-vessel occlusion stroke. Eur. J. Neurol. 2017, 24, 935–943. [Google Scholar] [CrossRef]
- Broeg-Morvay, A.; Mordasini, P.; Bernasconi, C.; Bühlmann, M.; Pult, F.; Arnold, M.; Schroth, G.; Jung, S.; Mattle, H.P.; Gralla, J.; et al. Direct Mechanical Intervention Versus Combined Intravenous and Mechanical Intervention in Large Artery Anterior Circulation Stroke. Stroke 2016, 47, 1037–1044. [Google Scholar] [CrossRef]
- Weber, R.; Nordmeyer, H.; Hadisurya, J.; Heddier, M.; Stauder, M.; Stracke, P.; Berger, K.; Chapot, R. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J. NeuroInterv. Surg. 2016, 9, 229–233. [Google Scholar] [CrossRef]
- Kass-Hout, T.; Kass-Hout, O.; Mokin, M.; Thesier, D.M.; Yashar, P.; Orion, D.; Jahshan, S.; Hopkins, L.N.; Siddiqui, A.H.; Snyder, K.V.; et al. Is Bridging with Intravenous Thrombolysis of Any Benefit in Endovascular Therapy for Acute Ischemic Stroke? World Neurosurg. 2014, 82, e453–e458. [Google Scholar] [CrossRef]
- Ferrigno, M.; Bricout, N.; Leys, D.; Estrade, L.; Cordonnier, C.; Personnic, T.; Kyheng, M.; Hénon, H. Intravenous Recombinant Tissue-Type Plasminogen Activator: Influence on Outcome in Anterior Circulation Ischemic Stroke Treated by Mechanical Thrombectomy. Stroke 2018, 49, 1377–1385. [Google Scholar] [CrossRef]
- Kellert, L.; Wollenweber, F.A.; Thomalla, G.; Nolte, C.H.; Fiehler, J.; Ringleb, P.A.; Dorn, F. Thrombolysis management in thrombectomy patients: Real-life data from German stroke centres. Eur. Stroke J. 2017, 2, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Abilleira, S.; Ribera, A.; Cardona, P.; Rubiera, M.; López-Cancio, E.; Amaro, S.; Rodríguez-Campello, A.; Camps-Renom, P.; Cánovas, D.; De Miquel, M.A.; et al. Outcomes After Direct Thrombectomy or Combined Intravenous and Endovascular Treatment Are Not Different. Stroke 2017, 48, 375–378. [Google Scholar] [CrossRef]
- Guedin, P.; Larcher, A.; Decroix, J.-P.; Labreuche, J.; Dreyfus, J.-F.; Evrard, S.; Wang, A.; Graveleau, P.; Tassan, P.; Picó, F.; et al. Prior IV Thrombolysis Facilitates Mechanical Thrombectomy in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Rha, J.-H.; Saver, J.L. The Impact of Recanalization on Ischemic Stroke Outcome. Stroke 2007, 38, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Behrens, L.; Möhlenbruch, M.; Stampfl, S.; Ringleb, P.A.; Hametner, C.; Kellert, L.; Pham, M.; Herweh, C.; Bendszus, M.; Rohde, S. Effect of thrombus size on recanalization by bridging intravenous thrombolysis. Eur. J. Neurol. 2014, 21, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, P.; Bücke, P.; Ganslandt, O.; Bäzner, H.; Henkes, H.; Pérez, M.A. Mechanical thrombectomy in patients with M1 occlusion and NIHSS score ≤5: A single-centre experience. Stroke Vasc. Neurol. 2016, 1, 165–171. [Google Scholar] [CrossRef] [PubMed]
| BT (n = 38) | dMTE (n = 65) | p Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Sex female, n (%) | 22 (57.9) | 39 (60) | 0.835 |
| Age, y mean (SD) | 67.1 (9.6) | 68.4 (11.8) | 0.347 |
| NIHSS on admission, median (range) | 14 (4–20) | 13 (2–24) | 0.635 |
| ASPECTS on admission CT, median (range) | 9 (6–10) | 9 (6–10) | 0.689 |
| Clot length mm, mean (SD) | 14.8 (8.2) | 15.7 (7.1) | 0.452 |
| Vascular and other risk factors, n (%) | |||
| Hypertension | 34 (89.5) | 53 (81.5) | 0.286 |
| Diabetes mellitus | 4 (10.5) | 14 (21.5) | 0.158 |
| Atrial fibrillation | 21 (58.3) | 33 (50.8) | 0.468 |
| Coronary heart disease | 17 (44.7) | 32 (49.2) | 0.661 |
| Heart failure | 15 (39.5) | 23 (35.4) | 0.680 |
| Use of anticoagulants, n (%) | 3 * (7.89) | 17 (28.33) | 0.022 |
| Vital signs | |||
| Systolic blood pressure, mean (SD) | 150.7 (25.4) | 153.9 (28.2) | 0.983 |
| Diastolic blood pressure, mean (SD) | 85.0 (12.3) | 85.7 (12.3) | 0.321 |
| Mean blood pressure, mean (SD) | 109.8 (14.4) | 108.5 (17.8) | 0.485 |
| Pulse, mean (SD) | 80.2 (14.9) | 82.3 (23.8) | 0.524 |
| Treatment | |||
| Median time from neurologist’s consultation to MTE, h (IQR) | 01:23:00 (00:30:45) | 01:12:00 (00:27:30) | 0.113 |
| Median time from image to MTE, h (IQR) | 00:46:30 (00:37:45) | 00:42:30 (00:26:15) | 0.625 |
| Median time from symptom onset to recanalization, h (IQR) | 04:00:00 (01:12:30) | 03:50:30 (02:07:30) | 0.865 |
| Median time from symptom onset to MTE, h (IQR) | 03:10:00 (01:14:00 | 03:00:00 (02:19:00) | 0.994 |
| Median duration of MTE, h (IQR) | 00:42:30 (00:30:00) | 00:30:00 (00:25:00) | 0.025 |
| Duration of hospitalization, days, mean (SD) | 24.5 (4.4) | 21.4 (2.6) | 0.036 |
| Outcome | |||
| Successful reperfusion (TICI 2b-3), n (%) | 33 (86.8) | 58 (89.2) | 0.717 |
| Complete reperfusion (TICI 3), n (%) | 22 9 (57.9) | 35 (53.9) | 0.691 |
| NIHSS change during first 24 h, median (range) | 5 (−5, 17) | 6 (−6, 19) | 0.665 |
| Complications, n (%) | |||
| sICH | 4 (10.5) | 1 (1.5) | 0.121 |
| aICH | 4 (10.5) | 7 (10.8) | 0.831 |
| Distal embolization | 5 (13.2) | 8 (12.3) | 0.787 |
| Clinical outcome after 3 months | |||
| Functional independence (mRS 0–2), n (%) | 26 (68.4) | 43 (66.2) | 0.814 |
| Excellent clinical outcome (mRS 0–1), n (%) | 23 (60.5) | 33 (50.8) | 0.340 |
| Mortality at 90 days, n (%) | 2 (5.3) | 5 (7.7) | 0.638 |
| BT-B (n = 6) | BT-P (n = 18) | BT-F (n = 14) | dMTE (n = 65) | p Value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Sex female, n (%) | 3 (50.0) | 12 (66.7) | 7 (50.0) | 39 (60.0) | 0.722 |
| Age, y mean (SD) | 65.3 (7.5) | 67.6 (10.3) | 67.4 (9.3) | 68.4 (11.8) | 0.649 |
| NIHSS on admission, median (range) | 9.5 (5–18) | 14 (4–20) | 13.5 (5–20) | 13 (2–24) | 0.581 |
| ASPECTS on admission CT, median (range) | 9 (7–10) | 9.5 (7–10) | 8.5 (6–10) | 9 (6–10) | 0.329 |
| Clot length mm, mean (SD) | 16.2 (8.3) | 13.6 (5) | 18.3 (8.4) | 14.8 (8.2) | 0.360 |
| Vascular and other risk factors, n (%) | |||||
| Hypertension | 5 (83.3) | 16 (88.9) | 13 (92.9) | 53 (81.5) | 0.697 |
| Diabetes mellitus | 1 (16.7) | 1 (5.6) | 2 (14.3) | 14 (21.5) | 0.459 |
| Atrial fibrillation | 2 (33.3) | 11 (61.1) | 8 (57.1) | 33 (50.8) | 0.729 |
| Coronary heart disease | 1 (16.7) | 9 (50.0) | 7 (50.0) | 32 (49.2 | 0.490 |
| Heart failure | 2 (33.3) | 7 (38.9) | 6 (42.9) | 23 (35.4) | 0.953 |
| Use of anticoagulants, n (%) | 0 (0.0) | 2 * (11.1) | 1 * (7.1) | 17 (28.3) | 0.134 |
| Vital signs | |||||
| Systolic blood pressure, mean (SD) | 146.0 (25.1) | 158.1 (28.7) | 153.7 (21.7) | 153.9 (28.2) | 0.711 |
| Diastolic blood pressure, mean (SD) | 82.6 (4.3) | 86.0 (13.4) | 90.6 (12.4) | 85.7 (12.3) | 0.449 |
| Mean blood pressure, mean (SD) | 103.7 (11.2) | 110.0 (16.3) | 111.6 (13.1) | 108.5 (17.8) | 0.608 |
| Pulse, mean (SD) | 71.6 (6.3) | 83.9 (19.0) | 78.3 (8.2) | 82.3 (23.8) | 0.402 |
| Treatment | |||||
| Median time from neurologist’s consultation to MTE, h (IQR) | 00:59:00 (00:31:00) | 01:21:00 (00:27:00) | 01:34:00 (00:45:00) | 01:12:00 (00:27:30) | 0.017 |
| Median time from image to MTE, h (IQR) | 00:28:00 (00:24:00) | 00:38:00 (00:20:00) | 01:13:00 (00:39:00) | 00:42:30 (00:26:15) | 0.005 |
| Median time from symptom onset to MTE, h (IQR) | 02:24:30 (01:50:00) | 03:05:00 (01:03:00) | 03:30:00 (01:17:00) | 03:00:00 (02:15:00) | 0.792 |
| Median time from symptom onset to r-tPA, h (IQR) | 01:57:00 (02:10:15) | 02:22:30 (01:26:15) | 01:49:00 (00:53:30) | 0.280 | |
| Median time from symptom onset to recanalization, h (IQR) | 03:22:00 (01:47:00) | 03:55:00 (01:04:00) | 04:12:00 (01:33:00) | 03:50:30 (02:07:30) | 0.531 |
| Median duration of MTE, h (IQR) | 00:50:00 (00:32:00) | 00:40:00 (00:35:00) | 00:45:00 (00:30:00) | 00:30:00 (00:25:00) | 0.125 |
| Duration of hospitalization (days), mean (SD) | 28.3 (14.6) | 21.3 (5.5) | 27.0 (7.9) | 21.4 (2.6) | 0.928 |
| Outcome | |||||
| Successful reperfusion (TICI 2b-3), n (%) | 5 (83.3) | 15 (83.3) | 13 (92.9) | 58 (89.2) | 0.825 |
| Complete reperfusion (TICI 3), n (%) | 3 (50.0) | 11 (61.1) | 8 (57.1) | 35 (53.8) | 0.943 |
| NIHSS change during first 24 h, median (range) | 6 (1–13) | 6.8 (−5, 17) | 6 (−5, 16) | 6 (−6, 19) | 0.990 |
| Complications, n (%) | |||||
| sICH | 0 (0) | 0 (0) | 4 (28.6) | 1 (1.5) | <0.001 |
| aICH | 0 (0) | 1 (5.6) | 3 (21.4) | 7 (10.8) | 0.410 |
| Distal embolization | 1 (16.7) | 3 (16.7) | 1 (7.1) | 8 (12.3) | 0.865 |
| Clinical outcome after 3 months | |||||
| Functional independence (mRS 0–2), n (%) | 4 (66.7) | 13 (72.2) | 9 (64.3) | 43 (66.2) | 0.427 |
| Excellent clinical outcome (mRS 0–1), n (%) | 3 (50) | 13 (72.2) | 7 (50) | 33 (50.8) | 0.926 |
| Mortality at 90 days, n (%) | 0 (0) | 1 (5.6) | 1 (7.1) | 5 (7.7) | 0.905 |
| Groups Pairs | dMTE and BT-B | dMTE and BT-P | dMTE and BT-F | BT-B and BT-P | BT-B and BT-F | BT-P and BT-F | |
|---|---|---|---|---|---|---|---|
| Dependent Variable | |||||||
| sICH | 1.000 | 1.000 | <0.001 | 1.000 | 0.040 | 0.001 | |
| Median time from neurologist’s consultation to MTE | 1.000 | 1.000 | 0.036 | 0.719 | 0.041 | 0.597 | |
| Median time from image to MTE | 0.932 | 1.000 | 0.029 | 1.000 | 0.019 | 0.015 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurminas, M.; Berūkštis, A.; Misonis, N.; Blank, K.; Tamošiūnas, A.E.; Jatužis, D. Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy. Medicina 2020, 56, 357. https://doi.org/10.3390/medicina56070357
Kurminas M, Berūkštis A, Misonis N, Blank K, Tamošiūnas AE, Jatužis D. Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy. Medicina. 2020; 56(7):357. https://doi.org/10.3390/medicina56070357
Chicago/Turabian StyleKurminas, Marius, Andrius Berūkštis, Nerijus Misonis, Karmela Blank, Algirdas Edvardas Tamošiūnas, and Dalius Jatužis. 2020. "Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy" Medicina 56, no. 7: 357. https://doi.org/10.3390/medicina56070357
APA StyleKurminas, M., Berūkštis, A., Misonis, N., Blank, K., Tamošiūnas, A. E., & Jatužis, D. (2020). Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy. Medicina, 56(7), 357. https://doi.org/10.3390/medicina56070357






