Factors Associated with Procedural Thromboembolisms after Mechanical Thrombectomy for Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
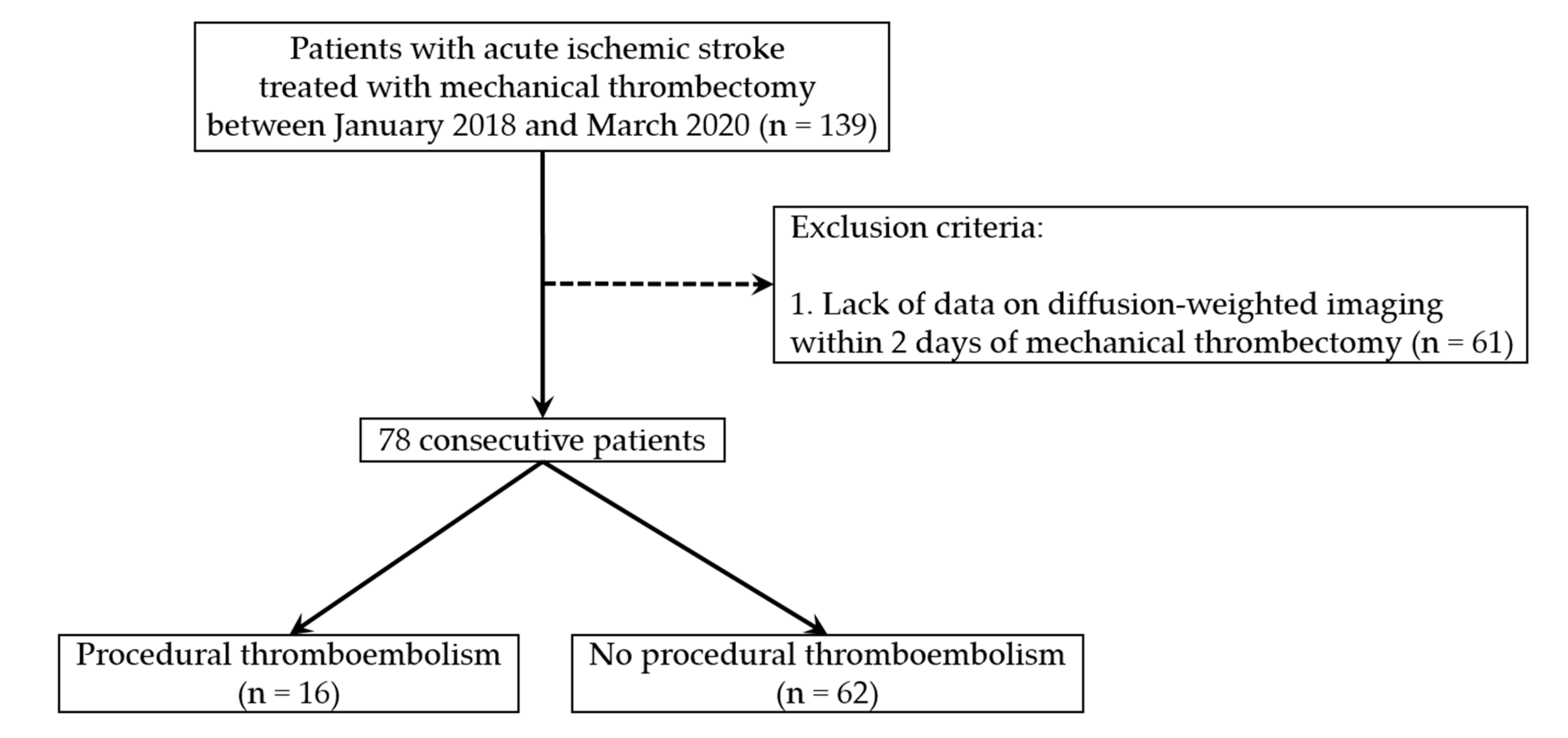
2.1. Patient Selection Criteria
2.2. Data Collection and Patient Characteristics
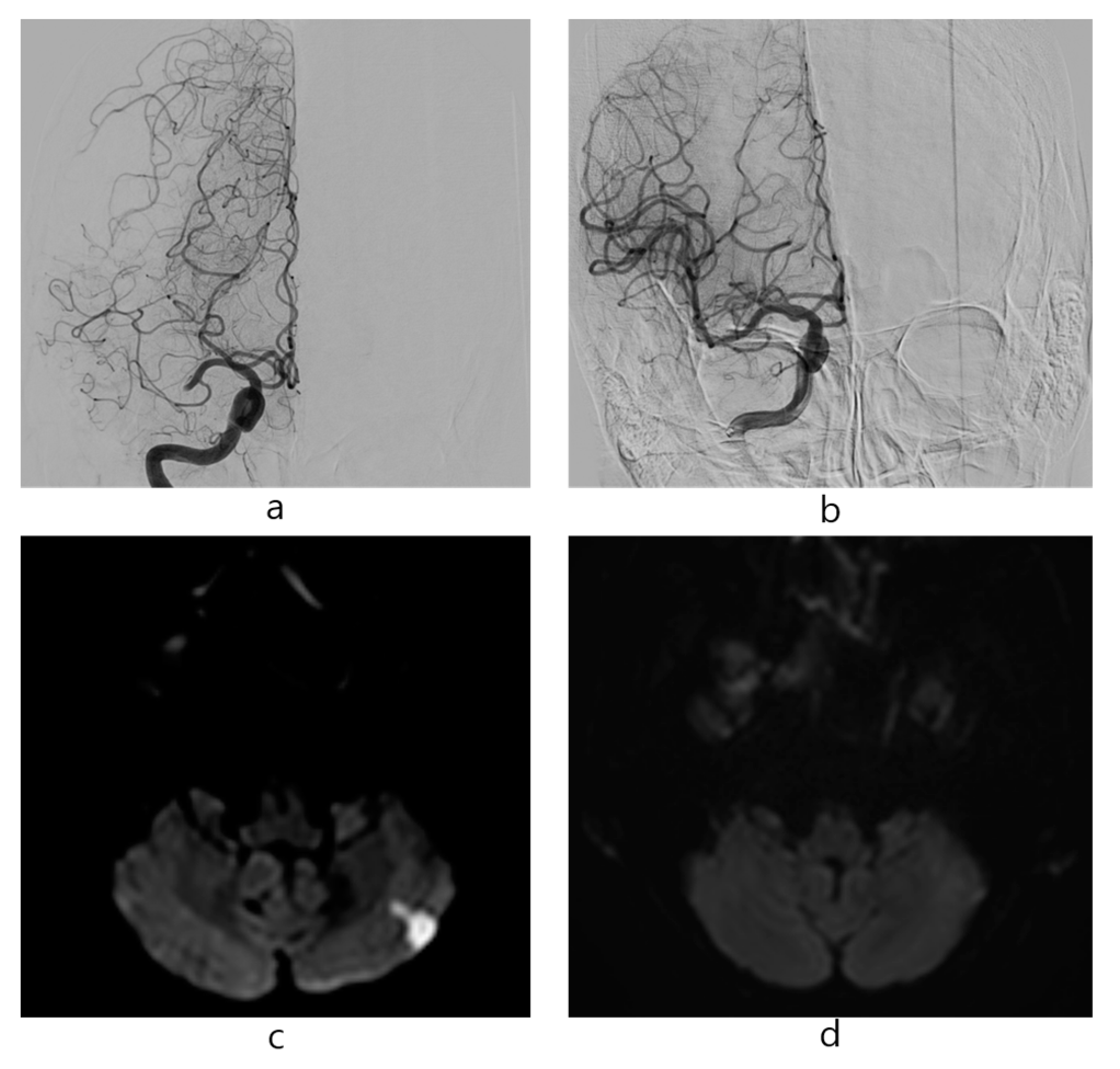
2.3. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-Retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; Román, L.S.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. New Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, B.M.; Kim, D.J.; Suh, S.H.; Kim, D.I.; Kim, Y.S.; Huh, S.K.; Park, J.; Lee, J.W.; Kim, Y.B. MR-DWI-Positive lesions and symptomatic ischemic complications after coiling of unruptured intracranial aneurysms. Stroke 2013, 44, 789–791. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, M.U.; Kang, J.; Kim, Y.J.; Kim, C.; Sohn, J.H.; Yang, J.; Jeon, J.P.; Cho, Y.; Choi, H.J. Impact of reducing the procedure time on thromboembolism after coil embolization of cerebral aneurysms. Front. Neurol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Song, J.; Shin, Y.S. Antiplatelet drug resistance did not increase the thromboembolic events after stent-assisted coiling of unruptured intracranial aneurysm: A single center experience of 99 cases. Neurol. Sci. 2017, 38, 879–885. [Google Scholar] [CrossRef]
- Xu, X.; Feng, Y.; Bai, X.; Ma, Y.; Wang, Y.; Chen, Y.; Yang, B.; Ling, F.; Zhang, X.; Jiao, L. Risk factors for silent new ischemic cerebral lesions following carotid artery stenting. Interv. Neuroradiol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Fieschi, C.; Toni, D.; Lesaffre, E.; von Kummer, R.; Boysen, G.; Bluhmki, E.; Höxter, G.; Mahagne, M.H.; et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995, 274, 1017–1025. [Google Scholar] [CrossRef]
- Kim, M.S.; Jo, K.I.; Yeon, J.Y.; Kim, J.S.; Kim, K.H.; Jeon, P.; Hong, S.C. Association between postprocedural infarction and antiplatelet drug resistance after coiling for unruptured intracranial aneurysms. AJNR Am. J. Neuroradiol. 2016, 37, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Ihn, Y.K.; Shin, S.H.; Baik, S.K.; Choi, I.S. Complications of endovascular treatment for intracranial aneurysms: Management and prevention. Interv. Neuroradiol. 2018, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Lee, D.H.; Kim, J.K.; Ahn, J.S.; Kwun, B.D.; Kim, D.Y.; Choi, C.G. Microembolism after endovascular coiling of unruptured cerebral aneurysms: Incidence and risk factors. J. Neurosurg. 2016, 124, 777–783. [Google Scholar] [CrossRef]
- Alawieh, A.; Vargas, J.; Fargen, K.M.; Langley, E.F.; Starke, R.M.; De Leacy, R.; Chatterjee, R.; Rai, A.; Dumont, T.; Kan, P.; et al. Impact of procedure time on outcomes of thrombectomy for stroke. J. Am. Coll. Cardiol. 2019, 73, 879–890. [Google Scholar] [CrossRef]
- Flottmann, F.; Leischner, H.; Broocks, G.; Nawabi, J.; Bernhardt, M.; Faizy, T.D.; Deb-Chatterji, M.; Thomalla, G.; Fiehler, J.; Brekenfeld, C. Recanalization rate per retrieval attempt in mechanical thrombectomy for acute ischemic stroke. Stroke 2018, 49, 2523–2525. [Google Scholar] [CrossRef]
- Iwata, T.; Mori, T.; Tajiri, H.; Miyazaki, Y.; Nakazaki, M.; Mizokami, K. Initial experience of a novel sheath guide for transbrachial coil embolization of cerebral aneurysms in the anterior cerebral circulation. Neurosurgery 2013, 72, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Alakbarzade, V.; Pereira, A.C. Cerebral catheter angiography and its complications. Pract. Neurol. 2018, 18, 393–398. [Google Scholar] [CrossRef]
- Bracard, S.; Ducrocq, X.; Mas, J.L.; Soudant, M.; Oppenheim, C.; Moulin, T.; Guillemin, F. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol. 2016, 15, 1138–1147. [Google Scholar] [CrossRef]
- Coutinho, J.M.; Liebeskind, D.S.; Slater, L.A.; Nogueira, R.G.; Clark, W.; Dávalos, A.; Bonafé, A.; Jahan, R.; Fischer, U.; Gralla, J.; et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: A pooled analysis of the SWIFT and STAR studies. JAMA Neurol. 2017, 74, 268–274. [Google Scholar] [CrossRef]
- Albayram, S.; Selcuk, D. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: Evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am. J. Neuroradiol. 2004, 25, 159–160. [Google Scholar]
- Soeda, A.; Sakai, N.; Murao, K.; Sakai, H.; Ihara, K.; Yamada, N.; Imakita, S.; Nagata, I. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am. J. Neuroradiol. 2003, 24, 2035–2038. [Google Scholar] [PubMed]
- Yi, H.J.; Sung, J.H.; Lee, D.H. Bridging intravenous thrombolysis before mechanical thrombectomy for large artery occlusion may be detrimental with thrombus fragmentation. Curr. Neurovascular Res. 2020, 17, 18–26. [Google Scholar] [CrossRef]
- Kim, B.; Kim, K.; Jeon, P.; Kim, S.; Kim, H.; Byun, H.; Cha, J.; Hong, S.; Jo, K. Thromboembolic complications in patients with clopidogrel resistance after coil embolization for unruptured intracranial aneurysms. AJNR Am. J. Neuroradiol. 2014, 35, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, C.D.; Goldstein, L.B. Diagnostic testing for coagulopathies in patients with ischemic stroke. Stroke 2000, 31, 3067–3078. [Google Scholar] [CrossRef]
| Included Patients (n = 78) | Excluded Patients (n = 61) | p-Value | ||
|---|---|---|---|---|
| Baseline characteristics | Age (years) (mean ± SD) | 68.2 ± 10.9 | 67.1 ± 14.9 | 0.597 |
| Sex (male) | 51 (65.4%) | 41 (67.2%) | 0.858 | |
| Atrial fibrillation | 28 (35.9%) | 25 (41.0%) | 0.599 | |
| Diabetes Mellitus | 16 (20.5%) | 14 (23.0%) | 0.836 | |
| Hypertension | 45 (57.7%) | 34 (55.7%) | 0.864 | |
| Previous cerebral infarction | 9 (11.5%) | 10 (16.4%) | 0.461 | |
| Smoking | 28 (35.9%) | 22 (36.1%) | 1.000 | |
| Stroke etiology | 0.602 | |||
| Cardioembolic | 30 | 27 | ||
| Non-cardioembolic | 48 | 34 | ||
| Occlusion site (anterior circulation) | 73 (93.6%) | 54 (88.5%) | 0.366 | |
| Tandem occlusion | 10 (12.8%) | 6 (9.8%) | 0.790 | |
| NIHSS score on admission (mean ± SD) | 11.3 ± 5.88 | 12.6 ± 7.65 | 0.240 | |
| The time from the LNT to the puncture (mean ± SD) | 371.0 ± 246.2 | 343.7 ± 230.3 | 0.505 | |
| Treatment details | IV thrombolysis | 37 (47.4%) | 20 (32.8%) | 0.086 |
| Antiplatelet preparation | 17 (21.8%) | 20 (32.8%) | 0.177 | |
| Heparinization | 41 (52.6%) | 41 (67.2%) | 0.086 | |
| Balloon-guided catheter | 15 (19.2%) | 20 (32.8%) | 0.078 | |
| Intermediate catheter | 69 (88.5%) | 46 (75.4%) | 0.069 | |
| Total number of MT attempts (mean ± SD) | 2.91 ± 2.35 | 2.43 ± 1.64 | 0.174 | |
| Procedural time (mean ± SD) | 69.8 ± 35.0 | 58.2 ± 31.2 | 0.043 | |
| Rescue treatment | 9 (11.5%) | 6 (9.8%) | 0.790 | |
| Procedural Thromboembolism | p-Value | |||
|---|---|---|---|---|
| Yes (n = 16) | No (n = 62) | |||
| Baseline characteristics | Age (years) (mean ± SD) * | 73.8 ± 8.18 | 66.8 ± 11.2 | 0.021 |
| Sex (male) | 10 (62.5%) | 41 (66.1%) | 0.776 | |
| Atrial fibrillation | 8 (50.0%) | 20 (32.3%) | 0.245 | |
| Diabetes Mellitus | 4 (25.0%) | 12 (19.4%) | 0.729 | |
| Hypertension | 10 (62.5%) | 35 (56.5%) | 0.780 | |
| Previous cerebral infarction | 0 | 9 (14.5%) | 0.191 | |
| Smoking | 3 (18.8%) | 25 (40.3%) | 0.147 | |
| Stroke etiology | 0.149 | |||
| Cardioembolic | 9 | 21 | ||
| Non-cardioembolic | 7 | 41 | ||
| Occlusion site (anterior circulation) | 16 (100%) | 57 (91.9%) | 0.577 | |
| Tandem occlusion | 3 (18.8%) | 7 (11.3%) | 0.420 | |
| NIHSS score on admission (mean ± SD) | 11.5 ± 5.32 | 11.2 ± 6.05 | 0.861 | |
| The time from the LNT to the puncture (mean ± SD) | 305.0 ± 223.7 | 388.1 ± 250.6 | 0.231 | |
| Treatment details | IV thrombolysis * | 12 (75.0%) | 25 (40.3%) | 0.023 |
| Antiplatelet preparation | 2 (12.5%) | 15 (24.2%) | 0.499 | |
| Heparinization * | 4 (25.0%) | 37 (59.7%) | 0.023 | |
| Balloon-guided catheter | 1 (6.3%) | 14 (22.6%) | 0.175 | |
| Intermediate catheter | 16 (100%) | 53 (85.5%) | 0.191 | |
| Total number of MT attempts (mean ± SD) | 3.38 ± 2.78 | 2.79 ± 2.24 | 0.379 | |
| Procedural time (mean ± SD) * | 90.9 ± 35.6 | 64.4 ± 33.0 | 0.006 | |
| Rescue treatment | 2 (12.5%) | 7 (11.3%) | 1.000 | |
| Clinical and radiological outcomes | Good clinical outcome | 11 (68.8%) | 36 (58.1%) | 0.570 |
| Successful recanalization | 14 (87.5%) | 49 (79.0%) | 0.723 | |
| Postprocedural hemorrhage | 9 (56.3%) | 11 (17.7%) | 0.003 | |
| Adjusted OR | Adjusted 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.084 | 0.992–1.185 | 0.076 |
| Procedural time | 1.020 | 1.002–1.039 | 0.030 |
| IV thrombolysis | 4.697 | 1.223–18.042 | 0.024 |
| AUC | 95% CI | p-Value | |
|---|---|---|---|
| Procedural time | 0.711 | 0.570–0.851 | 0.010 |
| IV thrombolysis | 0.634 | 0.483–0.785 | 0.077 |
| Procedural time and IV thrombolysis | 0.795 | 0.677–0.914 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, T.M.; Jang, J.H.; Kim, Y.Z.; Kim, K.H.; Kim, S.H. Factors Associated with Procedural Thromboembolisms after Mechanical Thrombectomy for Acute Ischemic Stroke. Medicina 2020, 56, 353. https://doi.org/10.3390/medicina56070353
Nam TM, Jang JH, Kim YZ, Kim KH, Kim SH. Factors Associated with Procedural Thromboembolisms after Mechanical Thrombectomy for Acute Ischemic Stroke. Medicina. 2020; 56(7):353. https://doi.org/10.3390/medicina56070353
Chicago/Turabian StyleNam, Taek Min, Ji Hwan Jang, Young Zoon Kim, Kyu Hong Kim, and Seung Hwan Kim. 2020. "Factors Associated with Procedural Thromboembolisms after Mechanical Thrombectomy for Acute Ischemic Stroke" Medicina 56, no. 7: 353. https://doi.org/10.3390/medicina56070353
APA StyleNam, T. M., Jang, J. H., Kim, Y. Z., Kim, K. H., & Kim, S. H. (2020). Factors Associated with Procedural Thromboembolisms after Mechanical Thrombectomy for Acute Ischemic Stroke. Medicina, 56(7), 353. https://doi.org/10.3390/medicina56070353






