Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analyses
3. Results
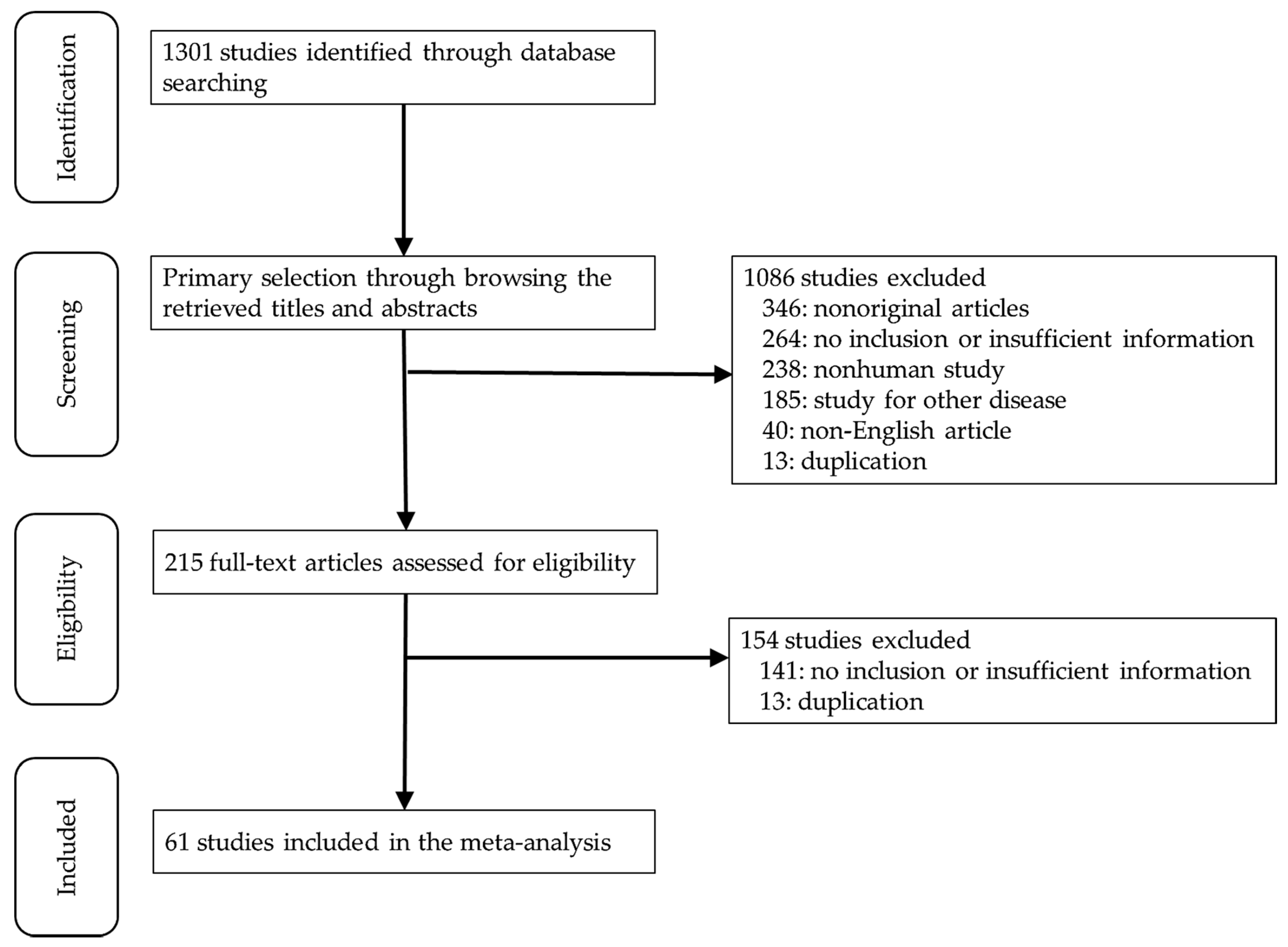
3.1. Selection and Characteristics of the Studies
3.2. Epstein–Barr virus (EBV) Infected Rates of Gastric Carcinomas (GCs)
3.3. Correlations Between Epstein–Barr virus (EBV) Infection and Clinicopathological Characteristics in Gastric Carcinomas (GCs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burke, A.P.; Yen, T.S.; Shekitka, K.M.; Sobin, L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 1990, 3, 377–380. [Google Scholar]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef] [PubMed]
- Chen, J.N.; Ding, Y.G.; Feng, Z.Y.; Li, H.G.; He, D.; Du, H.; Wu, B.; Shao, C.K. Association of distinctive Epstein-Barr virus variants with gastric carcinoma in Guangzhou, southern China. J. Med. Virol. 2010, 82, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Shibata, D.; Weiss, L.M. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 1992, 140, 769–774. [Google Scholar]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, T.H.; Yi, S.Y.; Jung, W.H.; Park, K.H. Relationship between Epstein-Barr virus-encoded RNA expression, apoptosis and lymphocytic infiltration in gastric carcinoma with lymphoid-rich stroma. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2004, 13, 353–360. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, S.J.; Kim, Y.; Kim, A.; Shin, N.; Choi, K.U.; Lee, C.H.; Huh, G.Y.; Kim, K.M.; Setia, N.; et al. High-throughput Protein and mRNA Expression-based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications. Am. J. Surg. Pathol. 2017, 41, 106–115. [Google Scholar] [CrossRef]
- Baek, D.W.; Kang, B.W.; Kim, J.G. The Predictive Value of Epstein-Barr Virus-Positivity in Patients Undergoing Gastrectomy Followed by Adjuvant Chemotherapy. Chonnam Med. J. 2018, 54, 173–177. [Google Scholar] [CrossRef][Green Version]
- Birkman, E.M.; Mansuri, N.; Kurki, S.; Ålgars, A.; Lintunen, M.; Ristamäki, R.; Sundström, J.; Carpén, O. Gastric cancer: Immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch. Int. J. Pathol. 2018, 472, 369–382. [Google Scholar] [CrossRef]
- Böger, C.; Krüger, S.; Behrens, H.M.; Bock, S.; Haag, J.; Kalthoff, H.; Röcken, C. Epstein-Barr virus-associated gastric cancer reveals intratumoral heterogeneity of PIK3CA mutations. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1005–1014. [Google Scholar] [CrossRef]
- Bösch, F.; Todorova, R.; Link, H.; Westphalen, C.B.; Boeck, S.; Heinemann, V.; Werner, J.; Kirchner, T.; Angele, M.K.; Neumann, J. Molecular subtyping of gastric cancer with respect to the growth pattern of lymph-node metastases. J. Cancer Res. Clin. Oncol. 2019, 145, 2689–2697. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Castillo, M.; Chavez, I.; Barreda, F.; Suarez, N.; Nieves, J.; Bernabe, L.A.; Valdivia, D.; Ruiz, E.; Dias-Neto, E.; et al. Prevalence of Helicobacter pylori Infection, Its Virulent Genotypes, and Epstein-Barr Virus in Peruvian Patients With Chronic Gastritis and Gastric Cancer. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chapel, F.; Fabiani, B.; Davi, F.; Raphael, M.; Tepper, M.; Champault, G.; Guettier, C. Epstein-Barr virus and gastric carcinoma in Western patients: Comparison of pathological parameters and p53 expression in EBV-positive and negative tumours. Histopathology 2000, 36, 252–261. [Google Scholar] [CrossRef]
- de Lima, M.A.; Ferreira, M.V.; Barros, M.A.; Pardini, M.I.; Ferrasi, A.C.; Rabenhorst, S.H. Epstein-Barr virus-associated gastric carcinoma in Brazil: Comparison between in situ hybridization and polymerase chain reaction detection. Braz. J. Microbiol. [Publ. Braz. Soc. Microbiol.] 2012, 43, 393–404. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Sahnane, N.; Tibiletti, M.G.; Magnoli, F.; Vanoli, A.; Sessa, F.; Chiaravalli, A.M. EBV⁺ and MSI Gastric Cancers Harbor High PD-L1/PD-1 Expression and High CD8⁺ Intratumoral Lymphocytes. Cancers 2018, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.R.; de Oliveira, K.S.; Ferraz, J.J.; Leal, M.F.; Calcagno, D.Q.; Seabra, A.D.; Khayat, A.S.; Montenegro, R.C.; Alves, A.P.; Assumpção, P.P.; et al. Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol. 2014, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.R.T.; Almeida, M.C.A.; Khayat, A.S.; da Silva, E.L.; Soares, P.C.; Chaves, L.C.; Burbano, R.M.R. Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas. World J. Gastroenterol. 2018, 24, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, H.Y.; Zhao, X.X.; Chen, J.N.; Zhang, Y.W.; Huang, Y.; Xue, L.; Li, H.G.; Du, H.; Wu, X.Y.; et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Hum. Pathol. 2016, 53, 25–34. [Google Scholar] [CrossRef]
- Gasenko, E.; Isajevs, S.; Camargo, M.C.; Offerhaus, G.J.A.; Polaka, I.; Gulley, M.L.; Skapars, R.; Sivins, A.; Kojalo, I.; Kirsners, A.; et al. Clinicopathological characteristics of Epstein-Barr virus-positive gastric cancer in Latvia. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1328–1333. [Google Scholar] [CrossRef]
- Grogg, K.L.; Lohse, C.M.; Pankratz, V.S.; Halling, K.C.; Smyrk, T.C. Lymphocyte-rich gastric cancer: Associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2003, 16, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wei, J.; Scott, R.S.; Chen, Y.; Chen, Z.; Zhao, W.; Zhang, C.; Wang, B.; Chai, C.; Dai, G.; et al. Prevalence and characteristics of Epstein-Barr virus associated gastric carcinoma in Gansu Province, Northwest China with mRNA expression of glycoprotein BMRF2. J. Med. Virol. 2020, 92, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Kim, M.A.; Lee, H.S.; Kim, W.H. Loss of ARID1A Expression is Related to Gastric Cancer Progression, Epstein-Barr Virus Infection, and Mismatch Repair Deficiency. Appl. Immunohistochem. Mol. Morphol. Aimm 2016, 24, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Ng, K.F.; Chen, K.H.; Hsu, J.T.; Liu, K.H.; Yeh, T.S.; Chen, T.C. Prognostic factors in Epstein-Barr virus-associated stage I-III gastric carcinoma: Implications for a unique type of carcinogenesis. Oncol. Rep. 2014, 32, 530–538. [Google Scholar] [CrossRef]
- Huang, S.C.; Ng, K.F.; Yeh, T.S.; Cheng, C.T.; Lin, J.S.; Liu, Y.J.; Chuang, H.C.; Chen, T.C. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: Combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int. J. Cancer 2019, 145, 3218–3230. [Google Scholar] [CrossRef]
- Irkkan, C.; Balci, S.; Güler Tezel, G.; Akinci, B.; Yalcin, B.; Güler, G. Comparison of Clinicopathologic Parameters and Survivals Between Epstein-Barr Virus-positive and Her2-positive Gastric Cancers. Appl. Immunohistochem. Mol. Morphol. Aimm 2017, 25, 609–614. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuwata, T.; Kuboki, Y.; Shitara, K.; Nagatsuma, A.K.; Aizawa, M.; Yoshino, T.; Doi, T.; Ohtsu, A.; Ochiai, A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2017, 20, 407–415. [Google Scholar] [CrossRef]
- Kawazoe, A.; Shitara, K.; Kuboki, Y.; Bando, H.; Kojima, T.; Yoshino, T.; Ohtsu, A.; Ochiai, A.; Togashi, Y.; Nishikawa, H.; et al. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 69–76. [Google Scholar] [CrossRef]
- Kijima, Y.; Ishigami, S.; Hokita, S.; Koriyama, C.; Akiba, S.; Eizuru, Y.; Aikou, T. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett. 2003, 200, 33–40. [Google Scholar] [CrossRef]
- Kim, Y.B.; Ahn, J.M.; Bae, W.J.; Sung, C.O.; Lee, D. Functional loss of ARID1A is tightly associated with high PD-L1 expression in gastric cancer. Int. J. Cancer 2019, 145, 916–926. [Google Scholar] [CrossRef]
- Kim, T.S.; da Silva, E.; Coit, D.G.; Tang, L.H. Intratumoral Immune Response to Gastric Cancer Varies by Molecular and Histologic Subtype. Am. J. Surg. Pathol. 2019, 43, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Koriyama, C.; Akiba, S.; Itoh, T.; Sueyoshi, K.; Minakami, Y.; Corvalan, A.; Yonezawa, S.; Eizuru, Y. E-cadherin and beta-catenin expression in Epstein-Barr virus-associated gastric carcinoma and their prognostic significance. World J. Gastroenterol. 2007, 13, 3925–3931. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, K.C.; Nam, E.S.; Cho, S.J.; Park, H.R.; Min, S.K.; Seo, J.; Choe, J.Y.; Lee, H.K.; Kang, H.S.; et al. Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection. Oncotarget 2017, 8, 82399–82414. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.Y.; Yuen, S.T.; Chung, L.P.; Chu, K.M.; Wong, M.P.; Branicki, F.J.; Ho, J.C. Microsatellite instability, Epstein-Barr virus, mutation of type II transforming growth factor beta receptor and BAX in gastric carcinomas in Hong Kong Chinese. Br. J. Cancer 1999, 79, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lai, Y.; Sun, L.; Zhang, X.; Liu, R.; Feng, G.; Zhou, L.; Jia, L.; Huang, X.; Kang, Q.; et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum. Pathol. 2016, 55, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, I.S.; Lee, J.H.; Park, Y.S.; Kang, H.J.; Na, H.K.; Ahn, J.Y.; Kim, D.H.; Choi, K.D.; Song, H.J.; et al. Clinical application of early gastric carcinoma with lymphoid stroma based on lymph node metastasis status. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2017, 20, 793–801. [Google Scholar] [CrossRef]
- Ma, C.; Patel, K.; Singhi, A.D.; Ren, B.; Zhu, B.; Shaikh, F.; Sun, W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am. J. Surg. Pathol. 2016, 40, 1496–1506. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Hao, Y.; Nie, Y.; Li, Z.; Qian, M.; Liang, Q.; Yu, J.; Zeng, M.; Wu, K. Differentiated tumor immune microenvironment of Epstein-Barr virus-associated and negative gastric cancer: Implication in prognosis and immunotherapy. Oncotarget 2017, 8, 67094–67103. [Google Scholar] [CrossRef]
- Martinez-Ciarpaglini, C.; Fleitas-Kanonnikoff, T.; Gambardella, V.; Llorca, M.; Mongort, C.; Mengual, R.; Nieto, G.; Navarro, L.; Huerta, M.; Rosello, S.; et al. Assessing molecular subtypes of gastric cancer: Microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications. ESMO Open 2019, 4, e000470. [Google Scholar] [CrossRef]
- Min, B.H.; Tae, C.H.; Ahn, S.M.; Kang, S.Y.; Woo, S.Y.; Kim, S.; Kim, K.M. Epstein-Barr virus infection serves as an independent predictor of survival in patients with lymphoepithelioma-like gastric carcinoma. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 852–859. [Google Scholar] [CrossRef]
- Nogueira, C.; Mota, M.; Gradiz, R.; Cipriano, M.A.; Caramelo, F.; Cruz, H.; Alarcão, A.; FC, E.S.; Oliveira, F.; Martinho, F.; et al. Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Portugal. Infect. Agents Cancer 2017, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.J.; Kim, J.H.; Eom, D.W. Prognostic Significance of Categorizing Gastric Carcinoma by PD-L1 Expression and Tumor Infiltrating Lymphocytes. Ann. Clin. Lab. Sci. 2018, 48, 695–706. [Google Scholar] [PubMed]
- Osumi, H.; Kawachi, H.; Yoshio, T.; Ida, S.; Yamamoto, N.; Horiuchi, Y.; Ishiyama, A.; Hirasawa, T.; Tsuchida, T.; Hiki, N.; et al. Epstein-Barr virus status is a promising biomarker for endoscopic resection in early gastric cancer: Proposal of a novel therapeutic strategy. J. Gastroenterol. 2019, 54, 774–783. [Google Scholar] [CrossRef]
- Pereira, M.A.; Ramos, M.; Faraj, S.F.; Dias, A.R.; Yagi, O.K.; Zilberstein, B.; Cecconello, I.; Alves, V.A.F.; de Mello, E.S.; Ribeiro, U., Jr. Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. J. Surg. Oncol. 2018, 117, 829–839. [Google Scholar] [CrossRef]
- Ramos, M.; Pereira, M.A.; Amorim, L.C.; de Mello, E.S.; Faraj, S.F.; Ribeiro, U.; Hoff, P.M.G.; Cecconello, I.; de Castria, T.B. Gastric cancer molecular classification and adjuvant therapy: Is there a different benefit according to the subtype? J. Surg. Oncol. 2020, 121, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Oliveira, A.; Malta, M.; Oliveira, C.; Silva, F.; Galaghar, A.; Afonso, L.P.; Neves, M.C.; Medeiros, R.; Pimentel-Nunes, P.; et al. Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World J. Gastroenterol. 2017, 23, 7292–7302. [Google Scholar] [CrossRef]
- Roh, C.K.; Choi, Y.Y.; Choi, S.; Seo, W.J.; Cho, M.; Jang, E.; Son, T.; Kim, H.I.; Kim, H.; Hyung, W.J.; et al. Single Patient Classifier Assay, Microsatellite Instability, and Epstein-Barr Virus Status Predict Clinical Outcomes in Stage II/III Gastric Cancer: Results from CLASSIC Trial. Yonsei Med. J. 2019, 60, 132–139. [Google Scholar] [CrossRef]
- Saito, R.; Abe, H.; Kunita, A.; Yamashita, H.; Seto, Y.; Fukayama, M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: The prognostic implications. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2017, 30, 427–439. [Google Scholar] [CrossRef]
- Setia, N.; Ahn, S.; Han, H.S.; Park, D.Y.; Lauwers, G.Y. Predictive value of WHO classification for PD-L1 and Her2/Neu expression and distinct associations with protein expression based classification in gastric carcinoma. Hum. Pathol. 2019, 94, 64–70. [Google Scholar] [CrossRef]
- Shen, H.; Zhong, M.; Wang, W.; Liao, P.; Yin, X.; Rotroff, D.; Knepper, T.C.; McLeod, H.L.; Zhou, C.; Xie, S.; et al. EBV infection and MSI status significantly influence the clinical outcomes of gastric cancer patients. Clin. Chim. Acta; Int. J. Clin. Chem. 2017, 471, 216–221. [Google Scholar] [CrossRef]
- Shibata, D.; Hawes, D.; Stemmermann, G.N.; Weiss, L.M. Epstein-Barr virus-associated gastric adenocarcinoma among Japanese Americans in Hawaii. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1993, 2, 213–217. [Google Scholar]
- Shinozaki, A.; Ushiku, T.; Morikawa, T.; Hino, R.; Sakatani, T.; Uozaki, H.; Fukayama, M. Epstein-Barr virus-associated gastric carcinoma: A distinct carcinoma of gastric phenotype by claudin expression profiling. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2009, 57, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, W.; Guan, W.; Cai, L.; Qiao, M.; Zheng, L.; Jiang, R.; Wang, R.; Wang, L. Integrated assessment of PD-L1 expression and molecular classification facilitates therapy selection and prognosis prediction in gastric cancer. Cancer Manag. Res. 2019, 11, 6397–6410. [Google Scholar] [CrossRef]
- Trimeche, M.; Ksiâa, F.; Ziadi, S.; Mestiri, S.; Hachana, M.; Gacem, R.B.; Sriha, B.; Korbi, S. Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Tunisia. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.D.; Feng, W.; Li, W.; Khoury, T.; Li, Q.; Alrawi, S.; Yu, Y.; Xie, K.; Yao, J.; Tan, D. Characteristics of Epstein-Barr virus-associated gastric cancer: A study of 235 cases at a comprehensive cancer center in USA. J. Exp. Clin. Cancer Res. CR 2009, 28, 14. [Google Scholar] [CrossRef]
- Valentini, A.M.; Di Pinto, F.; Coletta, S.; Guerra, V.; Armentano, R.; Caruso, M.L. Tumor microenvironment immune types in gastric cancer are associated with mismatch repair however, not HER2 status. Oncol. Lett. 2019, 18, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.; zur Hausen, A.; Klein Kranenbarg, E.; van de Velde, C.J.; Middeldorp, J.M.; van den Brule, A.J.; Meijer, C.J.; Bloemena, E. EBV-positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 664–670. [Google Scholar] [CrossRef]
- Vo, Q.N.; Geradts, J.; Gulley, M.L.; Boudreau, D.A.; Bravo, J.C.; Schneider, B.G. Epstein-Barr virus in gastric adenocarcinomas: Association with ethnicity and CDKN2A promoter methylation. J. Clin. Pathol. 2002, 55, 669–675. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, B.; Yan, L.P.; Huang, B.H.; Zhao, P. Relationship between Epstein-Barr virus-encoded proteins with cell proliferation, apoptosis, and apoptosis-related proteins in gastric carcinoma. World J. Gastroenterol. 2005, 11, 3234–3239. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, D.; Qu, L.; Cao, X.; Jia, Z.; Zhao, T.; Wang, Q.; Jiang, J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget 2017, 8, 64066–64082. [Google Scholar] [CrossRef]
- Xing, X.; Guo, J.; Ding, G.; Li, B.; Dong, B.; Feng, Q.; Li, S.; Zhang, J.; Ying, X.; Cheng, X.; et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology 2018, 7, e1356144. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, A.; Nishikawa, J.; Shimokuri, K.; Shuto, T.; Takagi, T.; Takagi, F.; Kobayashi, Y.; Yamamoto, M.; Miura, O.; Yanai, H.; et al. Clinicopathologic Characteristics of Epstein-Barr Virus-Associated Gastric Cancer Over the Past Decade in Japan. Microorganisms 2019, 7, 305. [Google Scholar] [CrossRef]
- Yen, R.L.; Telisinghe, P.U.; Cunningham, A.; Abdullah, M.S.; Chong, C.F.; Chong, V.H. Profiles of Epstein-Barr virus associated gastric carcinomas in Brunei Darussalam. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 10489–10493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, J.Y.; Sy, K.; Brezden-Masley, C.; Streutker, C.J. Histo- and immunohistochemistry-based estimation of the TCGA and ACRG molecular subtypes for gastric carcinoma and their prognostic significance: A single-institution study. PLoS ONE 2019, 14, e0224812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.N.; Dong, M.; Zhang, Z.G.; Zhang, Y.W.; Wu, J.Y.; Du, H.; Li, H.G.; Huang, Y.; Shao, C.K. Clinical significance of spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia in Epstein-Barr virus-associated and Epstein-Barr virus-negative gastric cancer. Hum. Pathol. 2017, 63, 128–138. [Google Scholar] [CrossRef]
- Zhang, Y.W.; He, D.; Tan, C.; Dong, M.; Zhou, L.; Shao, C.K. Differential expression of HER2 and downstream proteins in prediction of advanced tumor phenotypes and overall survival of patients with Epstein-Barr virus-positive vs. negative gastric cancers. Pathol. Res. Pract. 2019, 215, 152675. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, S.; Li, H.; Lin, X. Expression and significance of EBV, ARID1A and PIK3CA in gastric carcinoma. Mol. Med. Rep. 2019, 19, 2125–2136. [Google Scholar] [CrossRef]
- Farahmand, M.; Monavari, S.H.; Shoja, Z.; Ghaffari, H.; Tavakoli, M.; Tavakoli, A. Epstein-Barr virus and risk of breast cancer: A systematic review and meta-analysis. Future Oncol. 2019, 15, 2873–2885. [Google Scholar] [CrossRef]
- de Lima, M.A.P.; Neto, P.J.N.; Lima, L.P.M.; Gonçalves Júnior, J.; Teixeira Junior, A.G.; Teodoro, I.P.P.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr virus (EBV) and cervical carcinoma: A meta-analysis. Gynecol. Oncol. 2018, 148, 317–328. [Google Scholar] [CrossRef]
- de Lima, M.A.P.; Teodoro, I.P.P.; Galiza, L.E.; Filho, P.; Marques, F.M.; Pinheiro Junior, R.F.F.; Macedo, G.E.C.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr Virus and Oral Carcinoma: A Systematic Review with Meta-Analysis. Crit. Rev. Oncog. 2019, 24, 349–368. [Google Scholar] [CrossRef]
- Chen, X.Z.; Chen, H.; Castro, F.A.; Hu, J.K.; Brenner, H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine 2015, 94, e792. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009, 137, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Marano, L.; Marrelli, D.; De Luca, R.; Roviello, G.; Savelli, V.; Tan, P.; Roviello, F. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br. J. Surg. 2018, 105, 159–167. [Google Scholar] [CrossRef]
- Li, S.; Du, H.; Wang, Z.; Zhou, L.; Zhao, X.; Zeng, Y. Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci. China. Life Sci. 2010, 53, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.W.; Zhao, X.Q.; Liu, J.; Yang, W.J. Clinicopathological features of Epstein-Barr virus-associated gastric carcinoma: A systematic review and meta-analysis. J. B.U.ON. Off. J. Balk. Union Oncol. 2019, 24, 1092–1099. [Google Scholar]
- Lee, J.H.; Kim, S.H.; Han, S.H.; An, J.S.; Lee, E.S.; Kim, Y.S. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 2009, 24, 354–365. [Google Scholar] [CrossRef]
- Chiaravalli, A.M.; Cornaggia, M.; Furlan, D.; Capella, C.; Fiocca, R.; Tagliabue, G.; Klersy, C.; Solcia, E. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch. Int. J. Pathol. 2001, 439, 158–169. [Google Scholar] [CrossRef]
- Elsaleh, H.; Powell, B.; Soontrapornchai, P.; Joseph, D.; Goria, F.; Spry, N.; Iacopetta, B. p53 gene mutation, microsatellite instability and adjuvant chemotherapy: Impact on survival of 388 patients with Dukes’ C colon carcinoma. Oncology 2000, 58, 52–59. [Google Scholar] [CrossRef]
- Tokunaga, M.; Land, C.E.; Uemura, Y.; Tokudome, T.; Tanaka, S.; Sato, E. Epstein-Barr virus in gastric carcinoma. Am. J. Pathol. 1993, 143, 1250–1254. [Google Scholar]
- Fang, W.L.; Chen, M.H.; Huang, K.H.; Lin, C.H.; Chao, Y.; Lo, S.S.; Li, A.F.; Wu, C.W.; Shyr, Y.M. The Clinicopathological Features and Genetic Alterations in Epstein-Barr Virus-Associated Gastric Cancer Patients after Curative Surgery. Cancers 2020, 12, 1517. [Google Scholar] [CrossRef]
- Dasari, V.; Sinha, D.; Neller, M.A.; Smith, C.; Khanna, R. Prophylactic and therapeutic strategies for Epstein-Barr virus-associated diseases: Emerging strategies for clinical development. Expert Rev. Vaccines 2019, 18, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Rodriquenz, M.G.; Roviello, G.; D’Angelo, A.; Lavacchi, D.; Roviello, F.; Polom, K. MSI and EBV Positive Gastric Cancer’s Subgroups and Their Link With Novel Immunotherapy. J. Clin. Med. 2020, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
| Study | Location | Number of Patients | EBV | Study | Location | Number of Patients | EBV | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||||
| Ahn 2017 | Korea | 349 | 26 | 323 | Ma 2017 | China | 571 | 31 | 540 |
| Castaneda 2019 | Peru | 375 | 72 | 303 | Martinez- Ciarpaglini 2019 | Spain | 209 | 13 | 196 |
| Birkman 2018 | Finland | 238 | 17 | 221 | Min 2016 | Korea | 145 | 124 | 21 |
| Böger 2017 | Germany | 484 | 22 | 462 | Nogueira 2017 | Portugal | 82 | 9 | 73 |
| Bösch 2019 | Germany | 189 | 11 | 178 | Noh 2018 | Korea | 449 | 36 | 413 |
| Baek 2018 | Korea | 276 | 59 | 217 | Osumi 2019 | Japan | 898 | 71 | 827 |
| Chapel 2000 | France | 56 | 7 | 49 | Pereira 2018 | Brazil | 286 | 30 | 256 |
| Cho 2004 | Korea | 24 | 19 | 5 | Ramos 2019 | Brazil | 178 | 18 | 160 |
| de Lima 2012 | Brazil | 160 | 11 | 149 | Ribeiro 2017 | Portugal | 179 | 15 | 164 |
| De Rosa 2018 | Italy | 169 | 33 | 136 | Roh 2019 | Korea | 582 | 41 | 541 |
| de Souza 2014 | Brazil | 125 | 12 | 113 | Saito 2017 | Japan | 232 | 96 | 136 |
| de Souza 2018 | Brazil | 302 | 62 | 240 | Setia 2019 | USA/Korea | 486 | 33 | 453 |
| Dong 2016 | China | 855 | 59 | 796 | Shen 2017 | China | 202 | 42 | 160 |
| Gasenko 2019 | Latvia | 302 | 26 | 276 | Shibata 1993 | USA | 187 | 19 | 168 |
| Grogg 2003 | USA | 110 | 7 | 103 | Shinozaki 2009 | Japan | 111 | 43 | 68 |
| Guo 2019 | China | 270 | 18 | 252 | Sun 2019 | China | 165 | 2 | 163 |
| Han 2016 | Korea | 410 | 30 | 380 | Trimeche 2009 | Tunisia | 96 | 4 | 92 |
| Huang 2014 | Taiwan | 1020 | 52 | 968 | Truong 2009 | USA | 235 | 12 | 223 |
| Huang 2019 | Taiwan | 1248 | 65 | 1183 | Valentini 2019 | Italy | 70 | 2 | 68 |
| Irkkan 2017 | Turkey | 105 | 8 | 97 | van Beek 2004 | Netherlands | 566 | 41 | 525 |
| Kawazoe 2017 | Japan | 487 | 25 | 462 | Vo 2002 | USA | 108 | 11 | 97 |
| Kawazoe 2019 | Japan | 225 | 14 | 211 | Wang 2005 | China | 58 | 13 | 45 |
| Kijima 2003 | Japan | 420 | 28 | 392 | Wu 2017 | China | 340 | 17 | 323 |
| Kim 2019 (a) | Korea | 273 | 25 | 248 | Xing 2017 | China | 967 | 34 | 933 |
| Kim 2019 (b) | USA | 43 | 6 | 37 | Yanagi 2019 | Japan | 1067 | 69 | 998 |
| Koriyama 2007 | Japan | 149 | 49 | 100 | Zhang 2017 | China | 218 | 64 | 154 |
| Kwon 2017 | Korea | 394 | 26 | 368 | Yoon 2019 | USA | 107 | 3 | 104 |
| Leung 1999 | China (Hong Kong) | 79 | 18 | 61 | Yen 2014 | Brunei Darussalam | 81 | 25 | 56 |
| Li 2016 | China | 137 | 30 | 107 | Zhang 2019 | China | 1013 | 58 | 955 |
| Lim 2017 | Korea | 241 | 215 | 26 | Zhou 2019 | China | 300 | 28 | 272 |
| Ma 2016 | USA | 44 | 7 | 37 | |||||
| Number of Subsets | Fixed Effect (95% CI) | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) | |
|---|---|---|---|---|---|
| EBV positive rate | 61 | 0.116 (0.111, 0.121) | <0.001 | 0.113 (0.088, 0.143) | 0.912 |
| Asia | 34 | 0.121 (0.115, 0.128) | <0.001 | 0.138 (0.096, 0.194) | 0.238 |
| America | 13 | 0.132 (0.118, 0.148) | <0.001 | 0.103 (0.077, 0.137) | 0.002 |
| Europe | 12 | 0.083 (0.073, 0.095) | <0.001 | 0.080 (0.061, 0.106) | 0.558 |
| Africa | 1 | 0.042 (0.016, 0.106) | 1.000 | 0.042 (0.016, 0.106) | - |
| Number of Subsets | Fixed Effect (95% CI) | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) | MRT (p-Value) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| EBV-positive | 20 | 61.848 (61.115, 62.581) | <0.001 | 62.161 (60.126, 64.197) | 0.693 | 0.568 |
| EBV-negative | 16 | 63.532 (63.219, 63.846) | <0.001 | 63.519 (60.349, 66.690) | 0.788 | |
| Male ratio | ||||||
| EBV-positive | 44 | 0.823 (0.802, 0.843) | 0.063 | 0.824 (0.796, 0.849) | 0.189 | <0.001 |
| EBV-negative | 40 | 0.638 (0.629, 0.647) | <0.001 | 0.639 (0.620, 0.658) | 0.945 | |
| Size (cm) | ||||||
| EBV-positive | 12 | 3.840 (3.666, 4.015) | <0.001 | 4.890 (4.223, 5.556) | <0.001 | 0.918 |
| EBV-negative | 7 | 4.595 (4.507, 4.683) | <0.001 | 4.588 (4.354, 4.823) | 0.957 | |
| Tumor differentiation, poorly | ||||||
| EBV-positive | 20 | 0.674 (0.630, 0.716) | 0.004 | 0.682 (0.611, 0.745) | 0.514 | 0.112 |
| EBV-negative | 20 | 0.608 (0.595, 0.622) | <0.001 | 0.597 (0.525, 0.665) | 0.761 | |
| Lymphatic invasion | ||||||
| EBV-positive | 7 | 0.487 (0.429, 0.546) | <0.001 | 0.476 (0.299, 0.659) | 0.843 | 0.523 |
| EBV-negative | 7 | 0.498 (0.483, 0.513) | <0.001 | 0.522 (0.454, 0.588) | 0.583 | |
| Vascular invasion | ||||||
| EBV-positive | 7 | 0.297 (0.249, 0.350) | <0.001 | 0.286 (0.189, 0.408) | 0.636 | 0.890 |
| EBV-negative | 7 | 0.276 (0.263, 0.290) | <0.001 | 0.297 (0.202, 0.413) | 0.875 | |
| Perineural invasion | ||||||
| EBV-positive | 8 | 0.415 (0.350, 0.482) | <0.001 | 0.399 (0.213, 0.619) | 0.807 | 0.094 |
| EBV-negative | 8 | 0.517 (0.498, 0.535) | <0.001 | 0.521 (0.458, 0.584) | 0.875 | |
| Low pT stage (pT1/T2) | ||||||
| EBV-positive | 33 | 0.435 (0.401, 0.471) | <0.001 | 0.366 (0.274, 0.469) | 0.066 | 0.670 |
| EBV-negative | 31 | 0.413 (0.402, 0.424) | <0.001 | 0.350 (0.283, 0.422) | 0.141 | |
| Lymph node metastasis | ||||||
| EBV-positive | 40 | 0.493 (0.461, 0.526) | <0.001 | 0.595 (0.496, 0.686) | 0.014 | 0.127 |
| EBV-negative | 37 | 0.593 (0.583, 0.604) | <0.001 | 0.655 (0.595, 0.711) | 0.064 | |
| pTNM stage | ||||||
| EBV-positive | 25 | 0.507 (0.469, 0.544) | <0.001 | 0.500 (0.419, 0.580) | 0.738 | 0.236 |
| EBV-negative | 25 | 0.451 (0.439, 0.463) | <0.001 | 0.460 (0.425, 0.496) | 0.411 | |
| Histologic Type | Number of Subsets | Fixed Effect (95% CI) | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) |
|---|---|---|---|---|---|
| Tubular adenocarcinoma | 6 | 0.152 (0.132, 0.174) | <0.001 | 0.174 (0.086, 0.320) | 0.531 |
| Poorly cohesive carcinoma | 8 | 0.102 (0.063, 0.160) | 0.038 | 0.078 (0.033, 0.173) | 0.263 |
| Mixed carcinoma | 4 | 0.043 (0.016, 0.109) | 0.306 | 0.039 (0.013, 0.113) | 0.054 |
| Papillary carcinoma | 2 | 0.022 (0.004, 0.101) | 0.530 | 0.022 (0.004, 0.101) | - |
| Mucinous carcinoma | 4 | 0.053 (0.013, 0.190) | 0.688 | 0.053 (0.013, 0.190) | 0.042 |
| GCLS | 5 | 0.576 (0.468, 0.676) | 0.203 | 0.573 (0.428, 0.706) | 0.748 |
| Solid carcinoma | 2 | 0.130 (0.046, 0.316) | 0.828 | 0.130 (0.046, 0.316) | - |
| Undifferentiated carcinoma | 1 | 0.111 (0.015, 0.500) | 1.000 | 0.111 (0.015, 0.500) | - |
| Markers | Number of Subsets | Fixed Effect (95% CI) | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) | MRT (p-Value) |
|---|---|---|---|---|---|---|
| PD-L1 in tumor cells | ||||||
| EBV-positive | 14 | 0.500 (0.447, 0.554) | <0.001 | 0.573 (0.449, 0.688) | 0.047 | <0.001 |
| EBV-negative | 14 | 0.337 (0.323, 0.352) | <0.001 | 0.183 (0.118, 0.272) | 0.008 | |
| PD-L1 in immune cells | ||||||
| EBV-positive | 8 | 0.610 (0.531, 0.683) | <0.001 | 0.832 (0.630, 0.935) | 0.007 | 0.002 |
| EBV-negative | 8 | 0.572 (0.552, 0.592) | <0.001 | 0.487 (0.357, 0.619) | 0.081 | |
| p53 overexpression | ||||||
| EBV-positive | 5 | 0.359 (0.256, 0.477) | 0.223 | 0.194 (0.067, 0.446) | 0.023 | 0.090 |
| EBV-negative | 4 | 0.464 (0.418, 0.511) | <0.001 | 0.439 (0.314, 0.572) | 0.502 | |
| ARID1A | ||||||
| EBV-positive | 4 | 0.295 (0.206, 0.403) | 0.309 | 0.295 (0.196, 0.418) | 0.519 | 0.021 |
| EBV-negative | 4 | 0.176 (0.153, 0.201) | 0.055 | 0.170 (0.134, 0.214) | 0.530 | |
| HER2 | ||||||
| EBV-positive | 8 | 0.048 (0.024, 0.093) | 0.723 | 0.048 (0.024, 0.093) | 0.167 | 0.051 |
| EBV-negative | 8 | 0.101 (0.088, 0.115) | <0.001 | 0.104 (0.070, 0.152) | 0.739 | |
| Microsatellite instability | ||||||
| EBV-positive | 5 | 0.087 (0.040, 0.179) | 0.240 | 0.077 (0.028, 0.190) | 0.230 | 0.536 |
| EBV-negative | 5 | 0.104 (0.089, 0.121) | <0.001 | 0.108 (0.069, 0.166) | 0.637 | |
| CD8+ TILs | ||||||
| EBV-positive | 4 | 0.705 (0.584, 0.802) | 0.100 | 0.761 (0.547, 0.894) | 0.163 | 0.001 |
| EBV-negative | 4 | 0.307 (0.275, 0.341) | <0.001 | 0.269 (0.141, 0.450) | 0.851 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, J.-S.; Kim, N.-Y.; Kang, D.-W. Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis. Medicina 2020, 56, 345. https://doi.org/10.3390/medicina56070345
Pyo J-S, Kim N-Y, Kang D-W. Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis. Medicina. 2020; 56(7):345. https://doi.org/10.3390/medicina56070345
Chicago/Turabian StylePyo, Jung-Soo, Nae-Yu Kim, and Dong-Wook Kang. 2020. "Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis" Medicina 56, no. 7: 345. https://doi.org/10.3390/medicina56070345
APA StylePyo, J.-S., Kim, N.-Y., & Kang, D.-W. (2020). Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis. Medicina, 56(7), 345. https://doi.org/10.3390/medicina56070345






