Abstract
Vascular malformations (VMs) are a wide vascular or lymphatic group of lesions common on the head and neck. The objective of this study was to assess the efficacy and morbidity of sclerotherapy for the treatment of VMs in the oral and perioral area. Special attention was given to factors that may contribute to minimizing postoperative morbidity. Data from 25 patients (32 lesions) with oral VMs submitted to sclerotherapy with monoethanolamine oleate (EAO) were included. A structured form was used to collect data. An arbitrary score was determined to evaluate postoperative morbidity. Each of the following signs or symptoms received one point: pain, swelling, hematoma, ulceration, erythema, transient numbness, and transient itching. Pain and swelling were further divided into mild to moderate (1 point) and severe (2 points). Theoretically, the score was in the range of 0–9. Calculated scores ranged 0–4. The patients were further divided into two groups with scores of 0–1 denoting minimal morbidity (MIN) and 2–4 denoting significant morbidity (SIG). The number of lesions in each morbidity-score group were comparable (MIN 17and SIG 15). There were no statistically significant differences between the groups regarding age, number of applications, or average injection volume per mm lesion. Statistically significant differences were noted regarding gender (p = 0.05), lesion diameter (p = 0.030), total volume of first (p = 0.007) and second application (p = 0.05), and total injected volume (p = 0.03). Factors contributing to the risk for significant morbidity included being male, lesion diameter > 5 mm, volume > 0.3 mL per application, and total injected volume > 0.3 mL. A waiting time of 12 weeks prior to additional EAO application was required in 12 out of 29 lesions for clinical observation of complete regression. It was concluded that sclerotherapy with EAO as monotherapy is easy to apply, safe, and effective within a small number of sessions. Application of <0.3 mL EAO per session, and a waiting time of 12 weeks prior to the second application, would significantly minimize morbidity.
1. Introduction
Vascular malformations (VMs) are a wide vascular- or lymphatic-system heterogeneous group of lesions common in the head and neck [1]. Pathogenesis is associated with the disrupted morphogenesis of the endothelium (e.g., proliferation, migration, adhesion, differentiation, and survival) [2]. In order to promote standard communication between practitioners, in 1996 the International Society for the Study of Vascular Anomalies (ISSVA) classified vascular anomalies as “vascular tumors” (hemangiomas) and “vascular malformations” according to the pathology’s biological characteristics. Vascular malformations (VMs) were further classified as simple, combined, of major named vessels, associated with other anomalies, and high- or slow-flow lesions [3,4]. VMs can be divided into low-flow (venous, capillary, or lymphatic component) and high-flow (arterial or arteriovenous component) [5]. The majority of VMs in the oral cavity are venous and slow-flow in nature. The main affected areas are the lips, tongue, buccal mucosa, and palate. VMs in the oral cavity may lead to esthetic disorders, pain, and bleeding [6,7,8]. Spontaneous regression is rarely observed. Moreover, VMs can expand and are either single or multiple [8]. Signs and symptoms include pain, ulcerations, bleeding, discomfort, and cosmetic disturbance [6,9,10]. Trauma, pregnancy, or hormonal factors were implied as promoters [4]. Treatment is thought to be necessary in the presence of clinical symptoms, personal discomfort, or cosmetic disturbance. Different treatment modalities were proposed, including surgery, laser, embolization, cryotherapy, sclerotherapy, and corticosteroids [8,11,12].
Recent studies pointed out the potential role of mediators, e.g., vitamins and asymmetric dimethylarginine (ADMA), in vascular malformations [13,14]. Analysis of over 2100 developed drugs identified cholecalciferol (vitamin D3) as a potential therapeutic option [15].
Sclerotherapy is an efficient and conservative method for the treatment of VMs. It is a simple procedure that consists of intralesional injection with a low recurrence rate, good esthetic results, and reasonable morbidity [12]. Among the various available sclerosing agents, 5% monoethanolamine oleate (EAO) is characterized by high efficacy and a low toxic effect. The efficiency and safety of sclerotherapy using EAO for the treatment of VMs in various regions outside the oral cavity is well-established [1,16,17,18].
The objective of the present study was to assess efficacy and morbidity following sclerotherapy (intralesional injection of EAO as monotherapy) of VMs in the oral and perioral areas. Special attention was given to factors that may contribute to minimizing postoperative morbidity. The null hypothesis was that postoperative morbidity is dose-dependent.
2. Materials and Methods
2.1. Characterization and Data Collection
This was a retrospective descriptive study based on data collection from patients with oral VMs submitted to sclerotherapy with EAO at the Department of Oral and Maxilofacial Surgery, Rabin Medical Center, Petah-Tikva, Israel. An electronic and manual database search was performed to locate patients diagnosed with VM in the oral cavity treated by sclerotherapy. The electronic search used the terms “vascular”, “vascular malformations” and “vascular lesions” from 2013 to 2017, resulting in 25 cases that were included in the present study. The hospital ethics committee approved the study in accordance with the Declaration of Helsinki (protocol no. 14-0190).
A structured form was used to collect demographic data (age, gender), diagnostic resources used, location and size (greatest diameter) of the VMs, dosage and number of intralesional applications of EAO, interval between treatment sessions and follow-up sessions, intra- and postoperative complications, response to treatment, morbidity, patients’ satisfaction, recurrence, and total follow-up period. Morbidity was further classified as minor-to-moderate or severe according to intensity and duration of symptoms, patients’ return to the clinic, and the need for further treatment (medications or other).
2.2. Inclusion Criteria
- Clinical observation of painless purplish vesicles or bullae with soft consistency on palpation.
- Diascopy showing changes in coloring, intralesional ischemia, and decrease or alteration in e shape.
Lesions were diagnosed as low-flow VM on the basis of a patient’s anamnesis, and the clinical evaluation of the color, consistency, and size of the lesion. Diascopy (performed by applying pressure and observing color changes), the diagnostic injection of a vasoconstrictor agent (observing size and color changes), and aspiratory puncture and auscultation were also performed as needed.
2.3. Exclusion Criteria
- High-flow VMs,
- inadequate available data, and
- Patient’s medication interfering with wound healing (e.g., steroids, bisphosphonates, anticoagulants) or specific states preventing the use of EAO (e.g., pregnancy, lactation).
2.4. Sclerotherapy
Application of the sclerosing solution followed manufacturer’s instructions Figure 1. Local anesthesia was provided using lidocaine 2% adrenalin 1:100,000 using a block technique away from the lesion to avoid access of the local anesthetic to the lesion. EAO was not diluted to allow maximal volume injection of the active EAO. The injection was applied in the central region of the VM with introduction of the needle to a depth that included half the volume of the VM. Before injecting the sclerosing solution, positive blood aspiration was verified. Blanching and/or progressive increase in the pressure for injection and/or leakage from the lesion surface were used as criteria for interrupting the procedure.
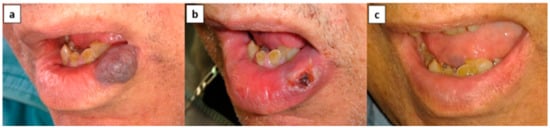
Figure 1.
Patient no. 2. (a) Lower-lip vascular malformation (VM) before treatment, (b) ulceration following monoethanolamine oleate (EAO) sclerotherapy, and (c) complete resolution.
2.5. Morbidity Score
An arbitrary score was determined to evaluate postoperative morbidity. Each of the following signs or symptoms received one point: pain, swelling, hematoma, ulceration, erythema, transient numbness, and transient itching. Pain and swelling were further divided into mild-to-moderate (1 point) and severe 2 (points). A morbidity score was calculated for each patient. Theoretically, the score could be in the range of 0–9. Calculated scores ranged 0–4. The patients were further divided into two groups, 0–1 for minimal morbidity (MIN) and 2–4 for significant morbidity (SIG).
2.6. Statistical Analysis
Data were collected using a Microsoft Excel 2016 spreadsheet (Microsoft Corp, Redmond, WA, USA). Statistical analyses were performed using SPSS Statistics for Window and version 22.0 (IBM Corp, Armonk, NY, USA). Kolmogorov–Smirnov tests were conducted to test normal distribution. Measurements showed normal distribution (p > 0.05). Descriptive statistics were produced, and means (M) and standard deviations (SD) were calculated for all continuous measurements. Categorical data were expressed as fractions (number of occurrences divided by the total dataset), and fractions were also expressed as percentages. Statistical analyses of differences between the groups were assessed using Student’s t-test for continuous parameters and the chi-squared test for categorical data. p values lower than 5% were considered statistically significant.
3. Results
Twenty-five patients, 15 females (60%) and 10 males (40%), with a total of 32 VM lesions were included in the study. Table 1 summarizes the main demographic data and the clinical characteristics. Mean age was 56 ± 17 years. Fourteen patients were below the age of 60.

Table 1.
Patient data.
The most frequently involved area (Figure 2) was the tongue (13 lesions, 41%) followed by the lower lip (8 lesions, 25%), upper lip (5 lesions, 16%), buccal mucosa (5 lesions, 16%), and floor of mouth (1 lesion, 3%).
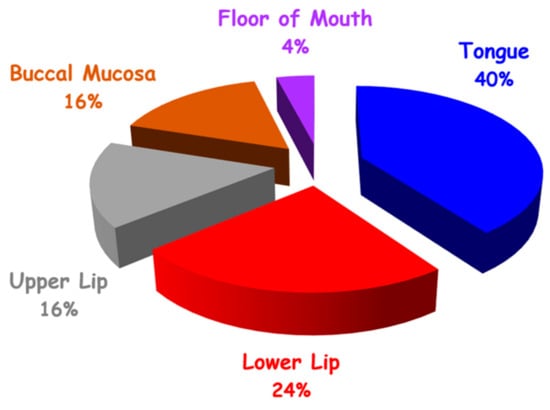
Figure 2.
Distribution of VM in oral cavity by site (N = 32).
Lesion diameter was in the range of 3–35 mm (Figure 2). The main reason for intervention was esthetic disturbance (14 patients, 56%), followed by discomfort (9 patients, 36%), bleeding (7 patients, 28%), and pain (3 patients, 12%).
The volume of injected EAO (Figure 3) ranged from 0.1 to 4 mL with an average of 0.06 mL per 1 mm of lesion diameter.
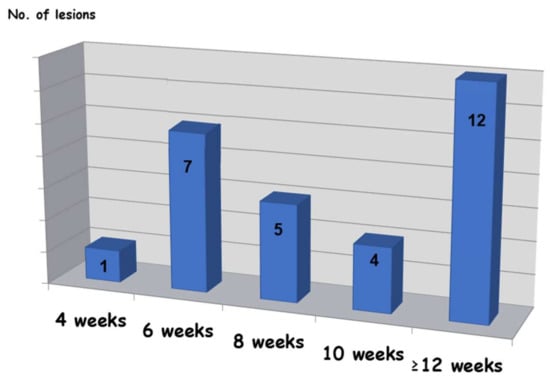
Figure 3.
Time required for complete resolution.
The majority of lesions were treated by a single application of EAO (25, 78%), while 6 (19%) underwent two treatment sessions and one case (3%) needed three treatment sessions. Among the seven cases that underwent more than one treatment, four sessions were held within a 1–2 week interval from the previous session. Follow-up sessions were conducted with a 1–3 week interval and until a total resolution of the lesion was observed.
Twenty-nine lesions (~91%) showed total clinical regression (Figure 3), two lesions only achieved partial resolution, and 1 could not be determined because the patient was lost from the follow-up sessions. Among two cases with partial response, the outcome was sufficient for patients’ satisfaction because symptoms of pain, discomfort, and esthetics were improved, and they did not want to continue with the treatment.
In most cases (28/29 lesions, 97%), healing was still in process after 1–3 weeks, and the response for treatment could not be determined at that time. In 97% of the cases (28/29 lesions), more than dix weeks were needed for complete resolution, while in 72% (21/29 lesions), complete healing was observed after eight weeks or more. In 41% of the cases (12/29 lesions), the required waiting time to resolution was 12 weeks.
Most of the patients (19, 76%) experienced side effects to some degree. Common side effects included pain (11, 44%), swelling (7, 28%), hematoma (6, 24%), and ulceration (5, 20%). Less common side effects included erythema (2, 8%), transient numbness of the lower lip (1, 4%), and transient itching sensation of the lower lip (1, 4%). One patient developed necrotic tissue on the anterior part of the tongue (Figure 4), requiring intervention for debridement, after which complete healing was achieved.
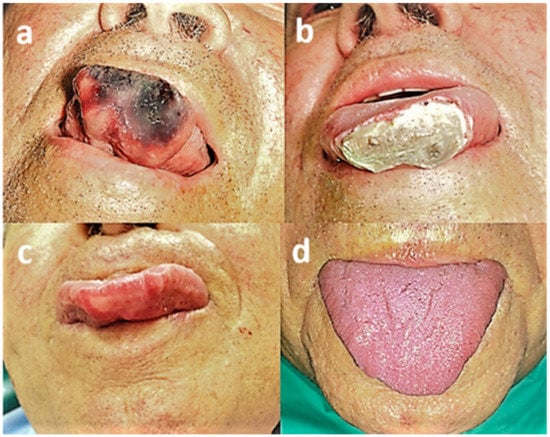
Figure 4.
Patient no. 8. (a) Clinical view prior to treatment; (b) necrotic tissue on anterior tongue part following sclerotherapy; (c) clinical view following debridement; (d) complete healing.
The number of lesions in each morbidity-score group were close (MIN-17; SIG-15). There were no statistically significant differences between groups regarding age, number of applications, or average volume per mm lesion (Table 2). Statistically significant differences were noted regarding gender (p = 0.05), lesion diameter (p = 0.030), volume of first application (p = 0.007), volume of second application (p = 0.05), and total injected volume (p = 0.03). Patients of male gender with lesion diameter > 5 mm, volume > 0.3 mL per application, and total volume > 0.3 mL showed higher morbidity.

Table 2.
Morbidity score. MIN, minimal morbidity; SIG, significant morbidity.
4. Discussion
The subjects included in the present study were adults with a mean age of 59 ± 18 years. Other studies reported a higher prevalence of VMs in the young [19,20,21].
Sixty-one percent of VMs were in women, as reported in other studies [8,21]. However, in the MIN group, women’s prevalence was 76%, while in the SIG group, there was male predominance (53%). Increased morbidity in males was reported in this study for the first time. Future studies are required to further investigate this correlation.
VMs were most frequently found on the tongue, whereas in most of the literature, the lower lip is the most frequent site [6,7,20]. One explanation may be the performance of this study in a maxillofacial department. Patients with lip lesions approach plastic surgeons or dermatologists more frequently than patients with lesions in other areas.
All the VMs were venous with slow blood flow. Success rate in such cases is in the range of 70–100% [1,20,22,23,24,25,26,27,28]. It was previously reported that, for smaller lesions, there is no need for complementary therapies [22]. In the present study, lesions ranging up to 35 mm did not require any additional therapy. Consequently, it could be deduced that sclerotherapy with EAO is effective with low toxicity [7,16]. In addition, postoperative morbidity is minimal following less invasive than surgery [1,28].
Ideal EAO concentration and dosing for treatment is still debatable [1,8,19,20]. A previous suggestion was <1 mL per session [29]. Hyodoh et al. (2005) reported that a single application was sufficient for total resolution in smaller vascular lesions (≤30 mm) [17]. On average, 1.25 sessions were needed for resolution, 1.29 in the MIN group and 1.2 in the SIG group. Results are comparable to those in previous studies [19,20,28]. We suggest that a 5% concentration is effective compared to an average of 3.7 sessions with concentrations of 1.5% and 2.5% as reported by Johann (2005) [1].
Complications in sclerotherapy are dose-dependent [19,30,31,32]. Excessive EAO amount or high pressure may be responsible [28,32]. In the present study, light pressure and positive aspiration were used to minimize morbidity. Patients undergoing hematopoietic stem-cell transplantation receive high doses of chemotherapy and radiotherapy that cause severe immunosuppression. Mucositis accounts for an increased morbidity score and the potential risk of clinical complications [33,34,35]. These complications resemble those seen in the present study. The morbidity score in the present study enabled assessment of additional factors contributing to morbidity. Unlike total injection volume, gender and lesion diameter were not under operator control. The total morbidity of both groups was not high. However, to further minimize morbidity without endangering efficacy, it is suggested that a volume not exceeding 0.3 mL should be applied per session. A longer waiting time between sessions is also suggested.
The present study demonstrated that, in most cases (72%), complete healing was observed after 8–12 weeks, while in 41% of the cases, the required waiting time to resolution was 12 weeks. Consequently, no additional applications were required. In the present study, the required interval between treatment sessions and the waiting time necessary for complete healing were longer than previously reported [20]. A total application volume not exceeding 0.3 mL was associated with increased morbidity. Therefore, one application of 0.3 mL EAO might be sufficient in most cases, provided there is also a longer waiting time between sessions.
There are no standard treatment algorithms available for VMs, and the disorder has significant unmet clinical needs. Improved understanding of the pathogenesis of vascular anomalies may provide insights to the development of new targeted therapies [2]. Recently, two factors emerged associated with epithelial dysfunction [13,14]. Vitamin D and asymmetric dimethylarginine (ADMA) play a crucial role in endothelial function, and may be links for the known interaction of chronic periodontitis (CP) and coronary heart disease (CHD). Patients with CP and CP + CHD had significantly lower serum levels of vitamin D compared to those with CHD and healthy controls. Moreover, the presence of CP negatively influenced serum vitamin D levels [13]. Patients with CHD and CP + CHD had higher levels of salivary and serum ADMA compared to healthy subjects and CP patients [14]. Future therapies targeted for increasing vitamin D [15] or decreasing ADMA might be alternatives for the medical treatment of VMs.
Taking into consideration the limitations of the study, such as the reduced number of samples (32 lesions) and the fact that it was a retrospective study, the application of EAO as a monotherapy was an easy, simple, and quick treatment because it was performed as an outpatient procedure with a limited number of sessions. It was well-tolerated by patients and resulted in low morbidity. The data showed that this is an effective treatment in 100% of the cases without the need for surgery (Figure 1).
5. Conclusions
Sclerotherapy with EAO as a monotherapy is easy to apply, safe, and effective within a small number of sessions. Application of <0.3 mL EAO per session with a waiting time of 8–12 weeks before the second application significantly minimizes morbidity.
Author Contributions
Conceptualization, I.Z., G.C., M.A., and L.C.; data curation, I.Z.; formal analysis, I.Z., G.C., M.A., and L.C.; methodology, I.Z., G.C., M.A., and L.C.; validation, L.C.; writing—original draft, I.Z., G.C., M.A., and L.C.; writing—review and editing, I.Z., G.C., M.A., and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Markovic, J.N.; Nag, U.; Shortell, C.K. Safety and efficacy of foam sclerotherapy for treatment of low-flow vascular malformations in children. J. Vasc. Surg. Venous Lymphat. Disord. 2020. [Google Scholar] [CrossRef]
- Zúñiga-Castillo, M.; Teng, C.L.; Teng, J.M.C. Genetics of vascular malformation and therapeutic implications. Curr. Opin. Pediatr. 2019, 31, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, P.; Wójcicka, K. Epidemiology, diagnostics and treat-ment of vascular tumours and malformations. Adv. Clin. Exp. Med. 2014, 23, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Clemens, R.K.; Pfammatter, T.; Meier, T.O.; Alomari, A.I.; Amann-Vesti, B.R. Vascular malformations revisited. Vasa 2015, 44, 5–22. [Google Scholar] [CrossRef] [PubMed]
- De Maria, L.; De Sanctis, P.; Balakrishnan, K.; Tollefson, M.; Brinjikji, W. Sclerotherapy for venous malformations of head and neck: Systematic review and meta-analysis. Neurointervention 2020, 15, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.A.F.; Ito, F.A.; Lima, H.G.; Novais, J.D.; Novais, J.B.; Dallazen, E.; Takahama, A., Jr. Sclerotherapy for Extensive Vascular Malformation in the Tongue. J. Craniofac. Surg. 2019, 30, e796–e799. [Google Scholar] [CrossRef] [PubMed]
- Buckmiller, L.M. Update on hemangiomas and vascular malformations. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 476–487. [Google Scholar] [CrossRef]
- Hoque, S.; Das, B.K. Treatment of venous malformations with ethanolamine oleate: A descriptive study of 83 cases. Pediatr. Surg. Int. 2011, 27, 527–531. [Google Scholar] [CrossRef]
- Dubois, J.; Soulez, G.; Oliva, V.L.; Berthiaume, M.J.; Lapierre, C.; Therasse, E. Soft-tissue venous malformations in adult patients: Imaging and therapeutic issues. Radiographics 2001, 21, 1519–1531. [Google Scholar] [CrossRef]
- García, E.D.; Jiménez, I.F.; Carrera, M.T.; González, F.S. Hemangiomas y malformaciones vasculares. ¿Qué se puede hacer? Bol. Pediatr. 2001, 41, 137–143. [Google Scholar]
- Zheng, J.W.; Mai, H.M.; Zhang, L.; Wang, Y.A.; Fan, X.D.; Su, L.X.; Qin, Z.P.; Yang, Y.W.; Jiang, Y.H.; Zhao, Y.F.; et al. Guidelines for the treatment of head and neck venous malformations. Int. J. Clin. Exp. Med. 2013, 6, 377–389. [Google Scholar]
- Ogawa, Y.; Inoue, K. Electrothrombosis as a treatment of cirsoid angioma in the face and scalp and varicosis of the leg. Plast. Reconstr. Surg. 1982, 70, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.C.; Zhu, W.; Davis, C.T.; Bowman-Kirigin, J.A.; Chan, A.C.; Ling, J.; Walker, A.E.; Goitre, L.; Delle Monache, S.; Retta, S.F.; et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation 2015, 131, 289–299. [Google Scholar] [CrossRef]
- Horbach, S.E.; Lokhorst, M.M.; Saeed, P.; de Goüyon Matignon de Pon-touraude, C.M.; Rothová, A.; van der Horst, C.M. Sclerotherapy for low-flow vascular malformations of the head and neck: A systematic review of sclerosing agents. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 295–304. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Grossmann, S.D.M.C.; do Amaral, M.B.F.; de Castro, W.H.; Navarro, T.P.; Procopio, R.J.; da Silva, T.A.; de Oliveira, C.d.N.A.; Mesquita, R.A. Effectiveness and safety of foam sclerotherapy with 5% ethanolamine oleate in the treatment of low-flow venous malformations in the head and neck region: A case series. Int. J. Oral Maxillofac. Surg. 2018, 47, 900–907. [Google Scholar] [CrossRef]
- Hyodoh, H.; Hori, M.; Akiba, H.; Tamakawa, M.; Hyodoh, K.; Hareyama, M. Peripheral vascular malformations: Imaging, treatment approaches, and therapeutic issues. Radiographics 2005, 25 (Suppl. 1), S159–S171. [Google Scholar] [CrossRef]
- Manzano, B.R.; Premoli, A.M.; Santaella, N.G.; Ikuta, C.R.; Rubira, C.M.; da Silva Santos, P.S. Sclerotherapy as an esthetic indication in oral vascular malformations: A case series. An. Bras. Dermatol. 2019, 94, 521–526. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, M.H.; Kwon, O.K.; Cha, S.H.; Chang, K.H. Craniofacial cavernous venous malformations: Percutaneous sclerotherapy with use of ethanolamine oleate. J. Vasc. Interv. Radiol. 2002, 13, 475–482. [Google Scholar] [CrossRef]
- Costa, J.R.; Torriani, M.A.; Hosni, E.S.; D’Avila, O.P.; de Figueiredo, P.J. Sclerotherapy for vascular malformations in the oral and max-illofacial region: Treatment and follow-up of 66 lesions. J. Oral Maxillofac. Surg. 2011, 69, e88–e92. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, I.; Al-Hadad, I.; Rehman, K.; McCafferty, I.; Monaghan, A. The use of foam sclerotherapy to treat low-flow vascular malformations of the head and neck. J. Surg. Case Rep. 2014, 2014, rju095. [Google Scholar] [CrossRef] [PubMed]
- Buckmiller, L.M.; Richter, G.T.; Suen, J.Y. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010, 16, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Fowell, C.; Verea Linares, C.; Jones, R.; Nishikawa, H.; Monaghan, A. Venous malformations of the head and neck: Current concepts in management. Br. J. Oral Maxillofac. Surg. 2017, 55, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.; Damm, D.D.; Allen, C.; Bouquot, J. Oral and Maxillofacial Pathology, 3rd ed.; Saunders: Philadelphia, PA, USA, 2002; p. 984. [Google Scholar]
- Cabrera, J.; Redondo, P. Sclerosing treatment of vascular malformations. An. Sist. Sanit. Navar. 2004, 27 (Suppl. 1), 117–126. [Google Scholar]
- Deveikis, J.P. Percutaneous ethanol sclerotherapy for vascular malformations in the head and neck. Arch. Facial Plast. Surg. 2005, 7, 322–325. [Google Scholar] [CrossRef]
- Kim, K.H.; Sung, M.W.; Roh, J.L.; Han, M.H. Sclerotherapy for congenital lesions in the head and neck. Otolaryngol. Head Neck Surg. 2004, 131, 307–316. [Google Scholar] [CrossRef]
- Hong, S.K.; Lee, H.J.; Seo, J.K.; Lee, D.; Hwang, S.W.; Sung, H.S. Reactive vascular lesions treated using ethanolamine oleate sclerotherapy. Dermatol. Surg. 2010, 36, 1148–1152. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakanishi, H.; Koizumi, Y.; Seike, T.; Kanda, I.; Kubo, Y. Sclerotherapy of hemangioma with late involution. Dermatol. Surg. 2003, 29, 668–671. [Google Scholar]
- Yakes, W.F.; Luethke, J.M.; Parker, S.H.; Stavros, A.T.; Rak, K.M.; Hopper, K.D.; Dreisbach, J.N.; Griffin, D.J.; Seibert, C.E.; Carter, T.E. Ethanol embolization of vascular malformations. Radiographics 1990, 10, 787–796. [Google Scholar] [CrossRef]
- O’Donovan, J.C.; Donaldson, J.S.; Morello, F.P.; Pensler, J.M.; Vogelzang, R.L.; Bauer, B. Symptomatic hemangiomas and venous malformations in infants, children, and young adults: Treatment with percutaneous injection of sodium tetradecyl sulfate. Am. J. Roentgenol. 1997, 169, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.S.; Weiss, R.A. Complications of sclerotherapy. Semin. Cutan. Med. Surg. 2007, 26, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Barrach, R.H.; de Souza, M.P.; da Silva, D.P.; Lopez, P.S.; Montovani, J.C. Oral changes in individuals undergoing hematopoietic stem cell transplantation. Braz. J. Otorhinolaryngol. 2015, 81, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bollero, P.; Passarelli, P.C.; D’Addona, A.; Pasquantonio, G.; Mancini, M.; Condò, R.; Cerroni, L. Oral management of adult patients undergoing hematopoietic stem cell transplantation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 876–887. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).