Cognitive Health of Nonagenarians in Southern Italy: A Descriptive Analysis from a Cross-Sectional, Home-Based Pilot Study of Exceptional Longevity (Cilento Initiative on Aging Outcomes or CIAO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Patients and Methods
2.3. Lifestyle Assessment
2.4. Neurological and Psychometric Assessments
- ADAS-Non-Cog (non-cognitive) is used to describe a subject’s behavior problems;
- ADAS-Cog evaluates specific cognitive sufficiency characteristics sensitive to the process of deterioration from primary degenerative dementia.
2.5. Laboratory Blood Testing
2.6. Statistical Analysis
3. Results
3.1. Lifestyle Assessment
3.2. Cognitive and Neurological Assessment
3.3. Functional Assessment
3.4. Laboratory Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galluzzo, L.; Gandin, C.; Ghirini, S.; Scafato, E. L’invecchiamento della popolazione: Opportunità o sfida? Not Ist Super Sanità 2012, 25, iii–vi. [Google Scholar]
- Kinsella, K.; He, W. An Aging World: 2008; (International Population Reports, P95/09-1); US Government Printing Office: Washington, DC, USA, 2009. [Google Scholar]
- World Health Organization. World Health Day 2012—Ageing and health—Toolkit for Event Organizers; WHO: Geneva, Switzerland, 2012; Available online: http://whqlibdoc.who.int/hq/2012/WHO_DCO_WHD_2012.1_eng.pdf (accessed on 1 May 2020).
- Franceschi, C.; Bonafè, M. Centenarians as a model for healthy aging. Biochem. Soc. Trans. 2003, 31, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Ranberg, K.; Vasegaard, L.; Jeune, B. Dementia is not inevitable: A population-based study of Danish centenarians. J. Gerontol. 2001, 56, P152–P1592. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Motta, L.; Valensin, S.; Rapisarda, R.; Franzone, A.; Berardelli, M.; Motta, M.; Monti, D.; Bonafe, M.; Ferrucci, L.; et al. Do men and women follow different trajectories to reach extreme longevity? Italian Multicenter Study on Centenarians (IMUSCE). Aging Clin. Exp. Res. 2000, 12, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Scelzo, A.; Di Somma, S.; Antonini, P.; Montross, L.P.; Schork, N.; Brenner, D.; Jeste, D.V. Mixed-methods quantitative–qualitative study of 29 nonagenarians and centenarians in rural Southern Italy: Focus on positive psychological traits. Int. Psychogeriatrics 2017, 30, 31–38. [Google Scholar] [CrossRef]
- Daniels, L.B.; Antonini, P.; Marino, R.; Rizzo, M.; Navarin, S.; Lucibello, S.G.; Maisel, A.S.; Pizza, V.; Brenner, D.A.; Jeste, D.V.; et al. Cardiovascular health of nonagenarians in southern Italy: A cross-sectional, home-based pilot study of longevity. J. Cardiovasc. Med. 2020, 21, 89–98. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survuval in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-Mental State Examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- McDonald, R.S. Assessing treatment effect: Behavior rating scales. In Handbook for Clinical Memory Assessment for Older Adults; Poon, L.W., Ed.; American Psychological Association: Washington, DC, USA, 1986; pp. 129–138. [Google Scholar]
- Zec, R.F.; Landreth, E.S.; Vicari, S.K.; Feldman, E.; Belman, J.; Andrise, A.; Robbs, R.; Kumar, V.; Becker, R. Alzheimer disease assessment scale: Useful for both early detection and staging of dementia of the Alzheimer type. Alzheimer Dis. Assoc. Disord. 1992, 6, 89–102. [Google Scholar] [CrossRef]
- Fioravanti, M.; Fionda, A.; Vitale, B.; Ferrario, E.; Varetto, O. L’ADAS—Alzheimer’s Disease Assessment Scale nella versione italiana. Criteri di scelta dei vocaboli delle liste di memorizzazione e stabilità nel lungo termine dei punteggi di soggetti anziani normali. Boll. Psicol. Appl. 1995, 215, 27–35. [Google Scholar]
- Podhorna, J.; Krahnke, T.; Shear, M.; Harrison, J.E.; Initiative, A.D.N. Alzheimer’s Disease Assessment Scale-Cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: Change over time and the effect of enrichment strategies. Alzheimer’s Res. Ther. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Grotz, R.C.; Downs, T.D.; Cash, H.R. Progress in Development of the Index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Activities of Daily Living Evaluation. Encyclopedia of Nursing & Allied Health; Enotes Nursing Encyclopedia MedicineNet.com Medical Dictionary; Krapp, K., Ed.; Gale Group, Inc.: Farmington Hills, MI, USA, 2002; Available online: eNotes.com (accessed on 11 October 2007).
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Musicco, M.; Caltagirone, C.; Sorbi, S.; Bonavita, V. Linee Guida per la Diagnosi di Malattia di Alzheimer e di Demenza; Gruppo di Studio per le Demenze della Società Italiana di Neurologia: Segrate, Italy, 2004. [Google Scholar]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N, N-diethyl-paraphenylenediamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar]
- Lindschinger, M.; Nadlinger, K.; Adelwöhrer, N.; Holweg, K.; Wögerbauer, M.; Birkmayer, J.; Smolle, K.H.; Wonisch, W. Oxidative stress: Potential of distinct peroxide determination systems. Clin. Chem. Lab. Med. 2004, 42. [Google Scholar] [CrossRef]
- Colombo, C.; Muti, P.; Pala, V.; Cavalleri, A.; Venturelli, E.; Locardi, M.; Berrino, F.; Secreto, G. Plant-based diet, serum fatty acid profile, and free radicals in postmenopausal women: The diet and androgens (DIANA) randomized trial. Int. J. Boil. Markers 2005, 20, 169–176. [Google Scholar] [CrossRef]
- Iamele, L.; Fiocchi, R.; Vernocchi, A. Evaluation of an Automated Spectrophotometric Assay for Reactive Oxygen Metabolites in Serum. Clin. Chem. Lab. Med. 2002, 40. [Google Scholar] [CrossRef]
- Pantelidis, P.; Lambert-Hammill, M.; Wierzbicki, A.S. Simple Sequence-specific-Primer-PCR Method to Identify the Three Main Apolipoprotein E Haplotypes. Clin. Chem. 2003, 49, 1945–1948. [Google Scholar] [CrossRef]
- Small, G.W. What we need to know about age related memory loss. BMJ 2002, 324, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Brayne, C.; Calloway, P. The Association of Education and Socioeconomic Status with the Mini Mental State Examination and the Clinical Diagnosis of Dementia in Elderly People. Age Ageing 1990, 19, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.F.; Wilcox, M.; McCormick, M.A.; Lawler, E.; Perls, T. Cardiovascular Advantages Among the Offspring of Centenarians. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M425–M431. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.F.; Wilcox, M.A.; McCormick, M.A.; Perls, T. Cardiovascular disease delay in centenarian offspring. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, M385–M389. [Google Scholar] [CrossRef]
- Terry, D.F.; Wilcox, M.A.; McCormick, M.A.; Pennington, J.Y.; Schoenhofen, E.A.; Andersen, S.; Perls, T. Lower All-Cause, Cardiovascular, and Cancer Mortality in Centenarians’ Offspring. J. Am. Geriatr. Soc. 2004, 52, 2074–2076. [Google Scholar] [CrossRef]
- Perls, T.; Kunkel, L.M.; Puca, A.A. The genetics of exceptional human longevity. J. Mol. Neurosci. 2002, 19, 233–238, Erratum in: J. Mol. Neurosci. 2002, 12, 488. [Google Scholar] [CrossRef]
- Atzmon, G.; Schechter, C.; Greiner, W.; Davidson, D.; Rennert, G.; Barzilai, N. Clinical phenotype of families with longevity. J. Am. Geriatr. Soc. 2004, 52, 274–277. [Google Scholar] [CrossRef]
- Karasik, D.; Hannan, M.T.; Cupples, L.A.; Felson, D.; Kiel, D.P. Genetic contribution to biological aging: The Framingham Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, 218–226. [Google Scholar] [CrossRef]
- Ikeda, A.; Iso, H.; Toyoshima, H.; Kondo, T.; Mizoue, T.; Koizumi, A.; Inaba, Y.; Tamakoshi, A. JACC Study Group Parental longevity and mortality amongst Japanese men and women: The JACC Study. J. Intern. Med. 2006, 259, 285–295. [Google Scholar] [CrossRef]
- Willcox, B.J.; Willcox, D.C.; He, Q.; Curb, J.D.; Suzuki, M. Siblings of Okinawan centenarians share lifelong mortality advantages. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2006, 61, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, M.; De Craen, A.J.M.; Meijer, P.H.E.M.D.; Beekman, M.; Blauw, G.J.; Slagboom, P.E.; Westendorp, R.G.J. Evidence of genetic enrichment for exceptional survival using a family approach: The Leiden Longevity Study. Eur. J. Hum. Genet. 2005, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Perls, T.; Kohler, I.V.; Andersen, S.; Schoenhofen, E.; Pennington, J.; Young, R.; Terry, D.F.; Elo, I.T. Survival of parents and siblings of supercentenarians. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2007, 62, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.R.; Nolan, V.G.; Andersen, S.; Perls, T.; Terry, D.F. Centenarian offspring: Start healthier and stay healthier. J. Am. Geriatr. Soc. 2008, 56, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, R.G.; Van Heemst, D.; Rozing, M.P.; Frölich, M.; Mooijaart, S.P.; Blauw, G.; Beekman, M.; Heijmans, B.T.; De Craen, A.J.; Slagboom, P.E.; et al. Nonagenarian Siblings and Their Offspring Display Lower Risk of Mortality and Morbidity than Sporadic Nonagenarians: The Leiden Longevity Study. J. Am. Geriatr. Soc. 2009, 57, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Atzmon, G.; Schechter, C.; Schaefer, E.J.; Cupples, L.A.; Lipton, R.; Cheng, S.; Shuldiner, A.R. Unique Lipoprotein Phenotype and Genotype Associated with Exceptional Longevity. JAMA 2003, 290, 2030–2040. [Google Scholar] [CrossRef]
- Atzmon, G.; Rincon, M.; Schechter, C.B.; Shuldiner, A.R.; Lipton, R.B.; Bergman, A.; Barzilai, N. Lipoprotein genotype and conservedpathway for exceptional longevity in humans. PLoS Biol. 2006, 4, e113. [Google Scholar] [CrossRef]
- Caselli, G.; Lapucci, E.; Lipsi, R.M.; Pozzi, L.; Baggio, G.; Carru, C.; Deiana, L.; Franceschi, C.; Vaupel, J.W. Maternal longevity is associated with lower infant mortality. Demogr. Res. 2014, 31, 1275–1296. [Google Scholar] [CrossRef]
- Bucci, L.; Ostan, R.; Cevenini, E.; Pini, E.; Scurti, M.; Vitale, G.; Mari, D.; Caruso, C.; Sansoni, P.; Fanelli, F.; et al. Centenarians’ offspring as a model of healthy aging: A reappraisal of the data on Italian subjects and a comprehensive overview. Aging 2016, 8, 510–519. [Google Scholar] [CrossRef]
- Manini, T.; Beavers, D.P.; Pahor, M.; Guralnik, J.M.; Spring, B.; Church, T.S.; King, A.C.; Folta, S.C.; Glynn, N.W.; Marsh, A.P.; et al. Effect of Physical Activity on Self-Reported Disability in Older Adults: Results from the LIFE Study. J. Am. Geriatr. Soc. 2017, 65, 980–988. [Google Scholar] [CrossRef]
- Cevenini, E.; Caruso, C.; Candore, G.; Capri, M.; Nuzzo, D.; Duro, G.; Rizzo, C.; Colonna-Romano, G.; Lio, D.; Carlo, D.; et al. Age-Related Inflammation: The Contribution of Different Organs, Tissues and Systems. How to Face it for Therapeutic Approaches. Curr. Pharm. Des. 2010, 16, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bonafè, M.; Olivieri, F.; Mari, D.; Baggio, G.; Mattace, R.; Berardelli, M.; Sansoni, P.; De Benedictis, G.; De Luca, M.; Marchegiani, F.; et al. P53 codon 72 polymorphism and longevity: Additional data on centenarians from continental Italy and Sardinia. Am. J. Hum. Genet. 1999, 65, 1782–1785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertram, L. Alzheimer’s disease: One disorder, too many genes? Hum. Mol. Genet. 2004, 13, 135R–141R. [Google Scholar] [CrossRef] [PubMed]
- Roses, A.D.; Saunders, A.M.; Alberts, M.A.; Strittmatter, W.J.; Schmechel, D.; Gorder, E.; Pericak-Vance, M.A. Apolipoprotein E E4 allele and risk of dementia. JAMA 1995, 273, 374–375. [Google Scholar] [CrossRef]
- Schellenberg, G.D. Genetic dissection of Alzheimer disease, a heterogeneous disorder. Proc. Natl. Acad. Sci. USA 1995, 92, 8552–8559. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Couzin, J. Genetics. Once shunned, test for Alzheimer’s risk headed to market. Science 2008, 319, 1022–1023. [Google Scholar] [CrossRef]
- Coon, K.D.; Myers, A.J.; Craig, D.W.; Webster, J.A.; Pearson, J.V.; Lince, D.H.; Zismann, V.L.; Beach, T.G.; Leung, D.; Bryden, L.; et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J. Clin. Psychiatry 2007, 68, 613–618. [Google Scholar] [CrossRef]
- Bird, T.D. Genetic aspects of Alzheimer disease. Genet. Med. 2008, 10, 231–239. [Google Scholar] [CrossRef]
- Chai, C.K. The genetics of Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2007, 22, 37–41. [Google Scholar] [CrossRef]
- Daw, E.W.; Payami, H.; Nemens, E.J.; Nochlin, D.; Bird, T.D.; Schellenberg, G.D.; Wijsman, E.M. The Number of Trait Loci in Late-Onset Alzheimer Disease. Am. J. Hum. Genet. 1999, 66, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Arrighi, H.M.; Michels, S.; Cedarbaum, J.M. Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimer’s Dement. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Lipnicki, D.M.; Kochan, N.A.; Crawford, J.D.; Thalamuthu, A.; Andrews, G.; Brayne, C.; Matthews, F.E.; Stephan, B.C.M.; Lipton, R.B.; et al. The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PLoS ONE 2015, 10, e0142388. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr. Opin. drug Discov. Dev. 2006, 9. [Google Scholar]
- Hirsch, E.; Breidert, T.; Rousselet, E.; Hunot, S.; Hartmann, A.; Michel, P.P. The role of glial reaction and inflammation in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 214–228. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Fuente, M.; Miquel, J. An Update of the Oxidation-Inflammation Theory of Aging: The Involvement of the Immune System in Oxi-Inflamm-Aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Treichler, E.B.H.; Jeste, D.V. Cognitive decline in older adults: Applying multiple perspectives to develop novel prevention strategies. Int. Psychogeriatrics 2019, 31, 913–916. [Google Scholar] [CrossRef]
- Pizza, V.; Agresta, A.; Iorio, E.L.; Capasso, A. Oxidative Stress and Aging. Curr. Neurobiol. 2013, 4, 93–98. [Google Scholar]
- Treichler, E.B.H.; Glorioso, D.; Lee, E.E.; Wu, T.-C.; Tu, X.M.; Daly, R.; O’Brien, C.; Smith, J.L.; Jeste, D.V. A pragmatic trial of a group intervention in senior housing communities to increase resilience. Int. Psychogeriatrics 2020, 32, 173–182. [Google Scholar] [CrossRef] [PubMed]
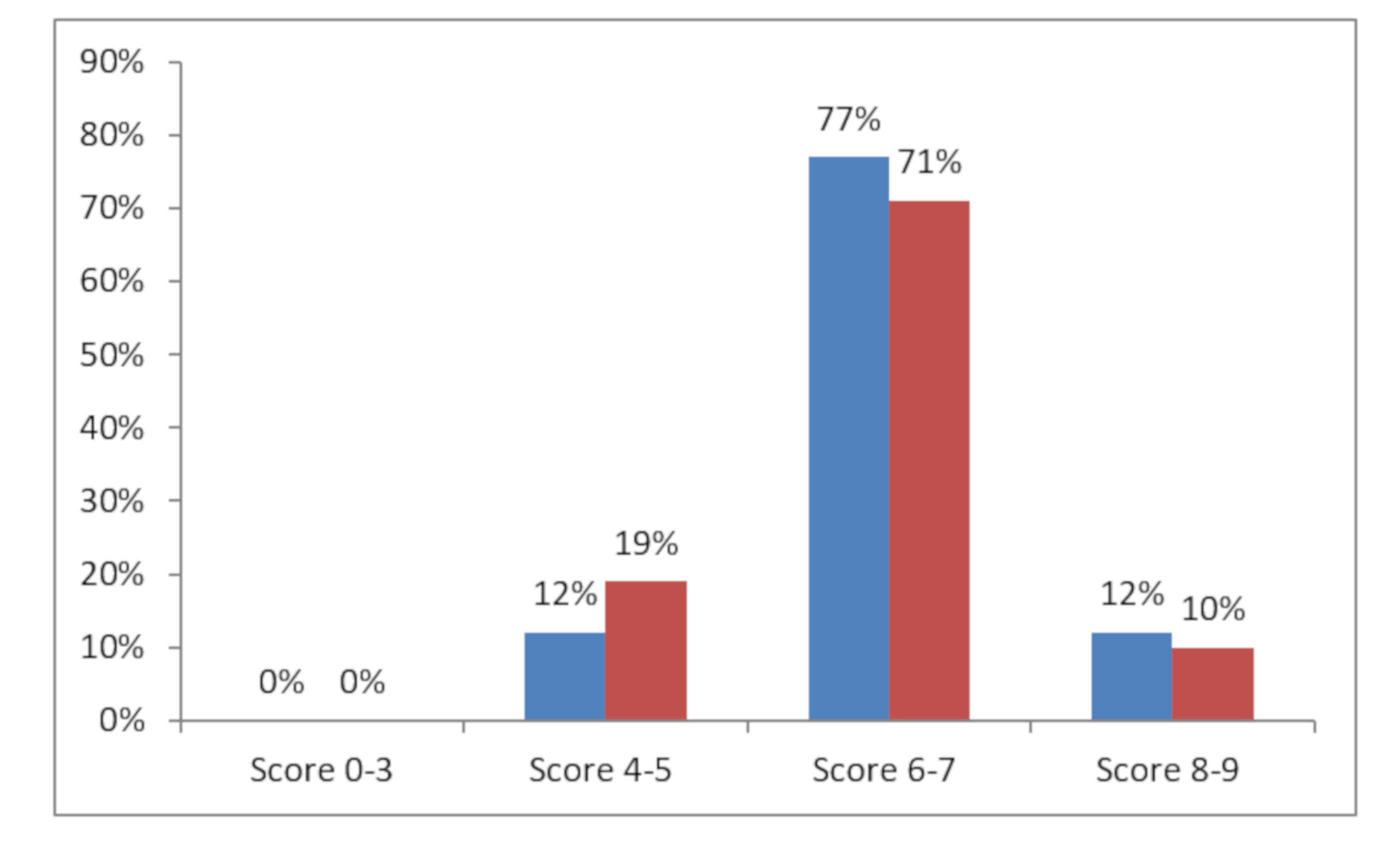
| Cohabitants (N = 49) | NCs (N = 29) | |
|---|---|---|
| Age (years)mean +/− SD | 61.67 +/− 5.52 | 93.69 +/− 3.34 |
| % Female | 53.1 | 62.1 |
| MMSE mean +/− SD (NV 24–30) | 26.08 +/− 1.56 | 21.80 +/− 4.33 |
| ADL mean +/− SD (NV 4–6) | NT | 4 +/− 2.07 |
| IADL mean +/− SD (NV 6–8) | NT | 3.38 +/− 2.27 |
| BAP mean +/− (NV >2200 mcmol/L) | 2088.92 +/− 129.98 | 2079.47 +/− 117.15 |
| d-ROMs mean +/− SD (NV 200–300 uCARR) | 371.16 +/− 65.85 | 386.17 +/− 65.85 |
| APOE4 (% positive patients) | 10.42 | 10.34 |
| Lifestyle/Habits | ||
| Current smoking (% of patients) | 29.2 | 0 |
| Daily Alcohol consumption (% of patients) | 47.9 | 76.9 |
| Regular physical activity (% of patients) | 30.6 | 15.4 |
| Score | NCs | Cohabitants | ||||
|---|---|---|---|---|---|---|
| Total (#) | Females (#) | Males (#) | Total (#) | Females (#) | Males (#) | |
| 29 | 19 | 10 | 49 | 26 | 23 | |
| Unknown | 1 | 0 | 1 | - | - | - |
| 0–10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10–19 | 12 | 8 | 4 | 0 | 0 | 0 |
| 20–23 | 0 | 0 | 0 | 1 | 1 | 0 |
| 24–30 | 16 | 11 | 5 | 48 | 25 | 23 |
| Score | Total of Subjects (#) | Females (#) | Males (#) |
|---|---|---|---|
| 49 | 26 | 23 | |
| NT | 1 | 1 | 0 |
| Subtest 1 (+) | 15 | 7 | 8 |
| Subtest 7 (+) | 8 | 4 | 4 |
| Subtest 1 e 7 (+) (−) Subtest 1 and/or 7 | 3 22 | 2 12 | 1 10 |
| Score | ADL | IADL | ||||
|---|---|---|---|---|---|---|
| Total (#) | Females (#) | Males (#) | Total (#) | Females (#) | Males (#) | |
| 29 | 19 | 10 | ||||
| 0–2 | 8 | 7 | 1 | 12 | 7 | 5 |
| 3–4 | 5 | 3 | 2 | 6 | 3 | 3 |
| 5–8 | 16 | 9 | 7 | 11 | 9 | 2 |
| Patient Code | Age | Degree of Kinship | MMSE | ADAS-Cog | ADL | IADL | BAP (NV >2200 mcmol/L) | d-ROMs_Test (NV 200–300 uCARR) | APOE4 [Positive or Negative] |
|---|---|---|---|---|---|---|---|---|---|
| 1000 | 93 | 18.4 | 3 | 1 | 2115.00 | 376 | negative | ||
| 1001 | 91 | Sister | 24.2 | 4 | 6 | 1910.72 | 354 | negative | |
| 1002 | 54 | grandson | 27.2 | 3.2 | 1937.73 | 400 | negative | ||
| 1003 | 54 | grandson | 27.2 | 4.9 | 1964.71 | 366 | negative | ||
| 2001 | 101 | 13.2 | 0 | 0 | 2166.00 | 346 | negative | ||
| 2003 | 72 | daughter-in-law | 24.7 | 4.9 | 2148.53 | 454 | negative | ||
| 2002 | 74 | son | 24.7 | 9.3 | 2019.00 | 306 | negative | ||
| 2081 | 95 | 15.2 | 5 | 2 | 2011.00 | 429 | negative | ||
| 2082 | 67 | Sister | 25 | 5.3 | 2190.00 | 413 | negative | ||
| 2083 | 74 | Son | 25.3 | 3.6 | 2139.00 | 431 | negative | ||
| 4011 | 91 | 17.2 | 6 | 1 | 1909.95 | 457 | negative | ||
| 4012 | 56 | Son | 26.2 | 4.6 | 1885.65 | 266 | negative | ||
| 4013 | 57 | daughter-in-law | 28 | 3.2 | 2001.59 | 335 | negative |
| Patient Code | Age | Degree of Kinship | MMSE | ADAS-Cog | ADL | IADL | BAP (NV >2200 mcmol/L) | D_ROMS_Test (NV 200–300 uCARR) | APOE4 [Positive or Negative] |
|---|---|---|---|---|---|---|---|---|---|
| 2091 PN | 93 | 24.4 | 5 | 4 | 2165.00 | 471 | negative | ||
| 2092 MR | 59 | son-in-law | 25.2 | 5.9 | 2176.00 | 345 | negative | ||
| 2093 IM | 61 | daughter | 27.2 | 6.9 | 1981.65 | 393 | positive | ||
| 2111 AG | 90 | 24.2 | 5 | 7 | 2109.15 | 425 | negative | ||
| 2112 FS | 62 | son-in-law | 28 | 3.6 | 2277.34 | 353 | positive | ||
| 2113 MD | 58 | daughter | 25.2 | 2.2 | 2282.40 | 310 | negative | ||
| 2061 AG | 91 | 24.2 | 6 | 6 | 2174.00 | 273 | negative | ||
| 2062 FI | 64 | son-in-law | 25.9 | 11.6 | 2122.00 | 460 | negative | ||
| 2063 EM | 64 | daughter | 24.9 | 6.2 | 1947.65 | 448 | negative | ||
| 2031 EC | 92 | 24.2 | 6 | 5 | 1985.86 | 484 | positive | ||
| 2032 EC | 60 | son | 24.9 | 3.3 | 2092.73 | 380 | positive | ||
| 2033 TS | 58 | daughter-in-law | 24.9 | 1.6 | 1994.39 | 335 | negative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizza, V.; Antonini, P.; Marino, R.; D’Arena, G.; Lucibello, S.G.; Rizzo, M.; Brenner, D.A.; Jeste, D.V.; Di Somma, S. Cognitive Health of Nonagenarians in Southern Italy: A Descriptive Analysis from a Cross-Sectional, Home-Based Pilot Study of Exceptional Longevity (Cilento Initiative on Aging Outcomes or CIAO). Medicina 2020, 56, 218. https://doi.org/10.3390/medicina56050218
Pizza V, Antonini P, Marino R, D’Arena G, Lucibello SG, Rizzo M, Brenner DA, Jeste DV, Di Somma S. Cognitive Health of Nonagenarians in Southern Italy: A Descriptive Analysis from a Cross-Sectional, Home-Based Pilot Study of Exceptional Longevity (Cilento Initiative on Aging Outcomes or CIAO). Medicina. 2020; 56(5):218. https://doi.org/10.3390/medicina56050218
Chicago/Turabian StylePizza, Vincenzo, Paola Antonini, Rossella Marino, Giovanni D’Arena, Serena Grazia Lucibello, Marianna Rizzo, David A. Brenner, Dilip V. Jeste, and Salvatore Di Somma. 2020. "Cognitive Health of Nonagenarians in Southern Italy: A Descriptive Analysis from a Cross-Sectional, Home-Based Pilot Study of Exceptional Longevity (Cilento Initiative on Aging Outcomes or CIAO)" Medicina 56, no. 5: 218. https://doi.org/10.3390/medicina56050218
APA StylePizza, V., Antonini, P., Marino, R., D’Arena, G., Lucibello, S. G., Rizzo, M., Brenner, D. A., Jeste, D. V., & Di Somma, S. (2020). Cognitive Health of Nonagenarians in Southern Italy: A Descriptive Analysis from a Cross-Sectional, Home-Based Pilot Study of Exceptional Longevity (Cilento Initiative on Aging Outcomes or CIAO). Medicina, 56(5), 218. https://doi.org/10.3390/medicina56050218





