Sinus Augmentation with Simultaneous, Non-Submerged, Implant Placement Using a Minimally Invasive Hydraulic Technique
Abstract
1. Introduction
2. Materials and Methods
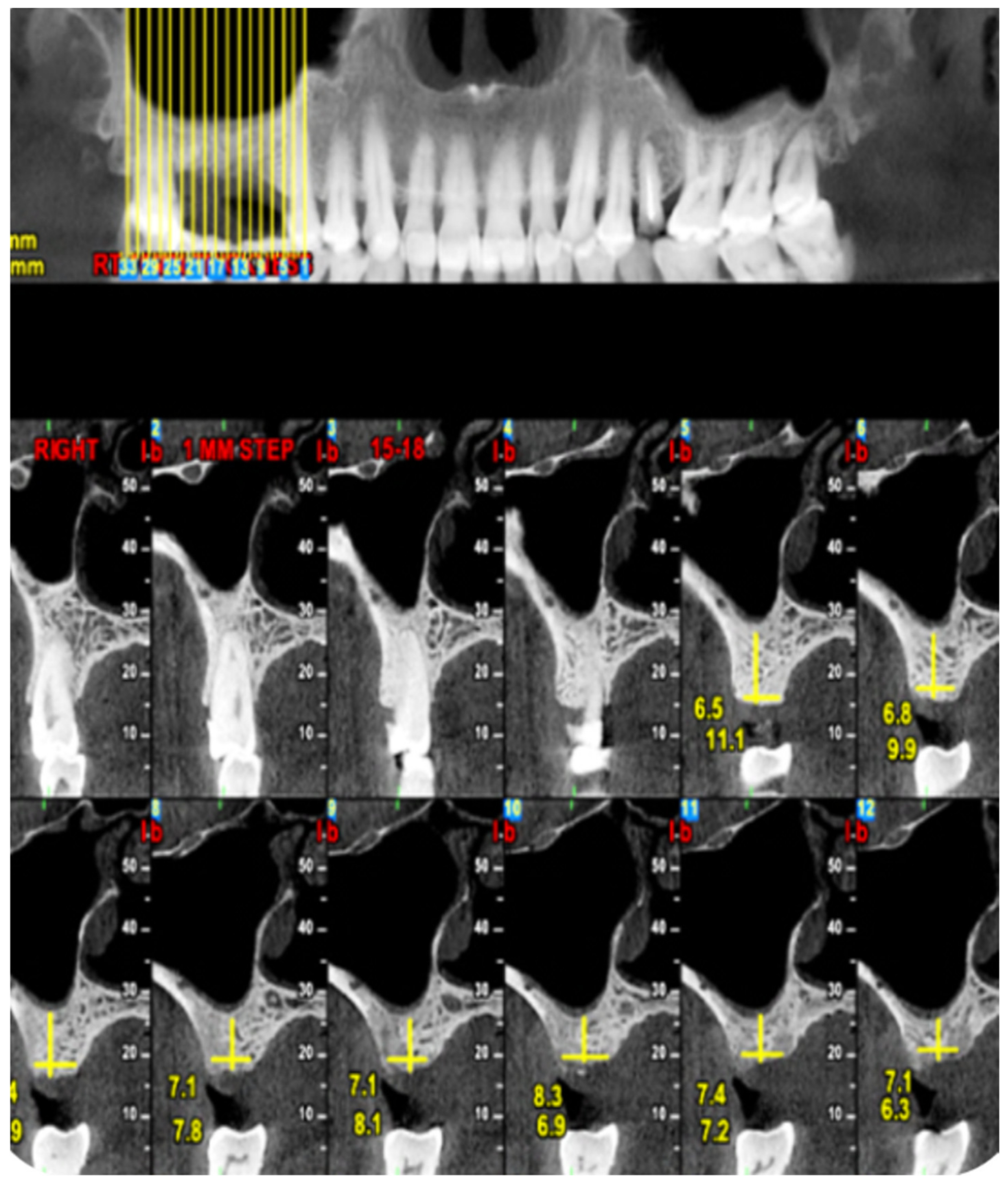
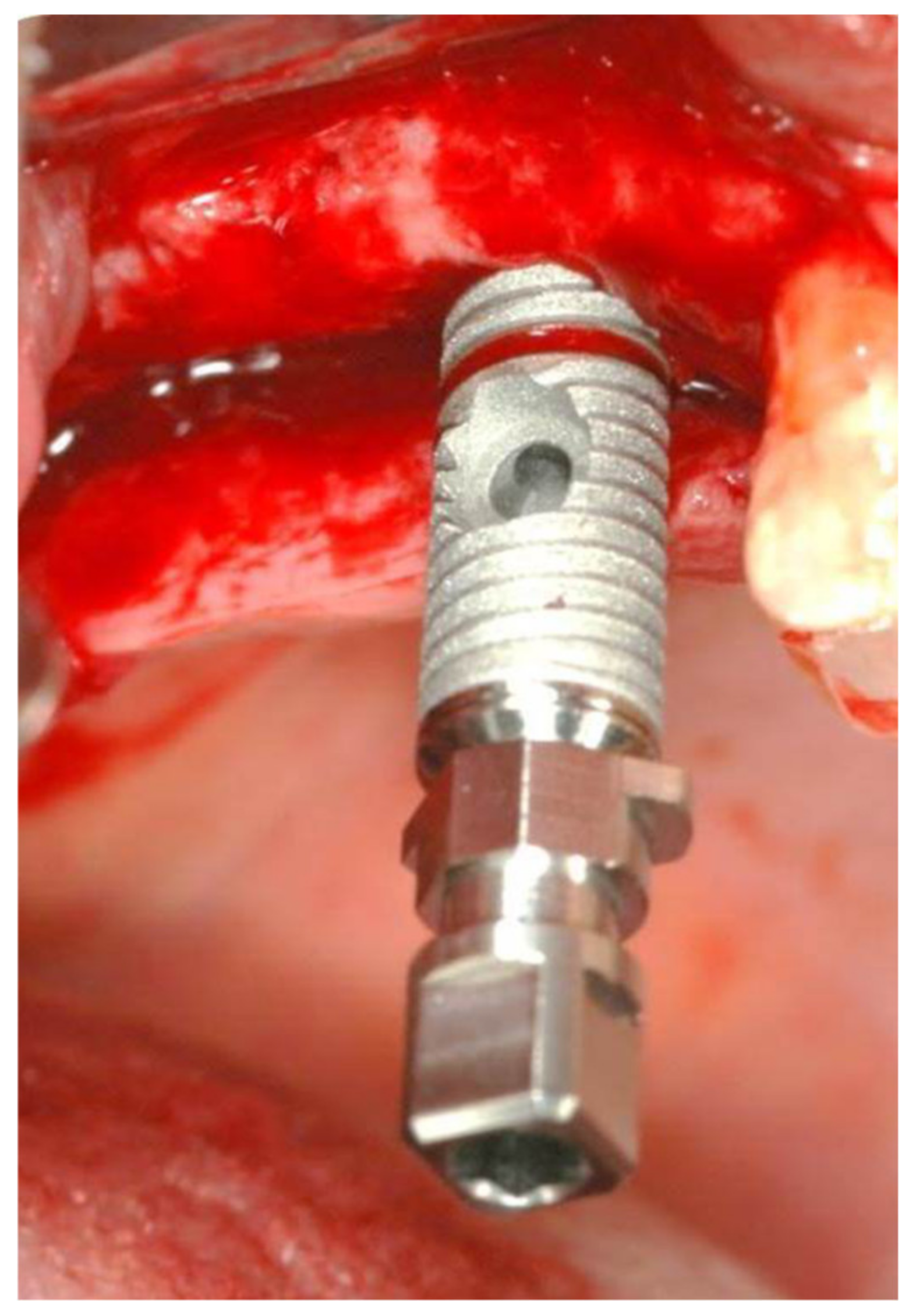
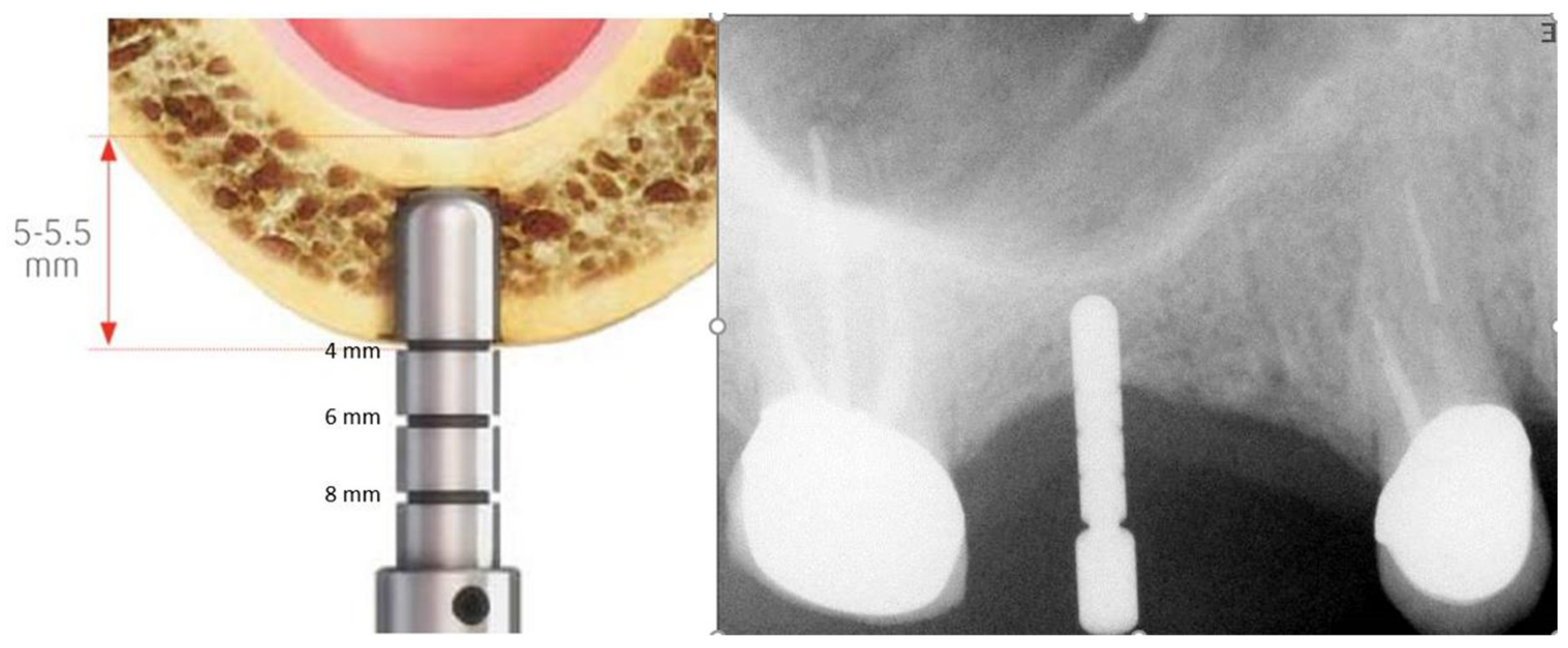
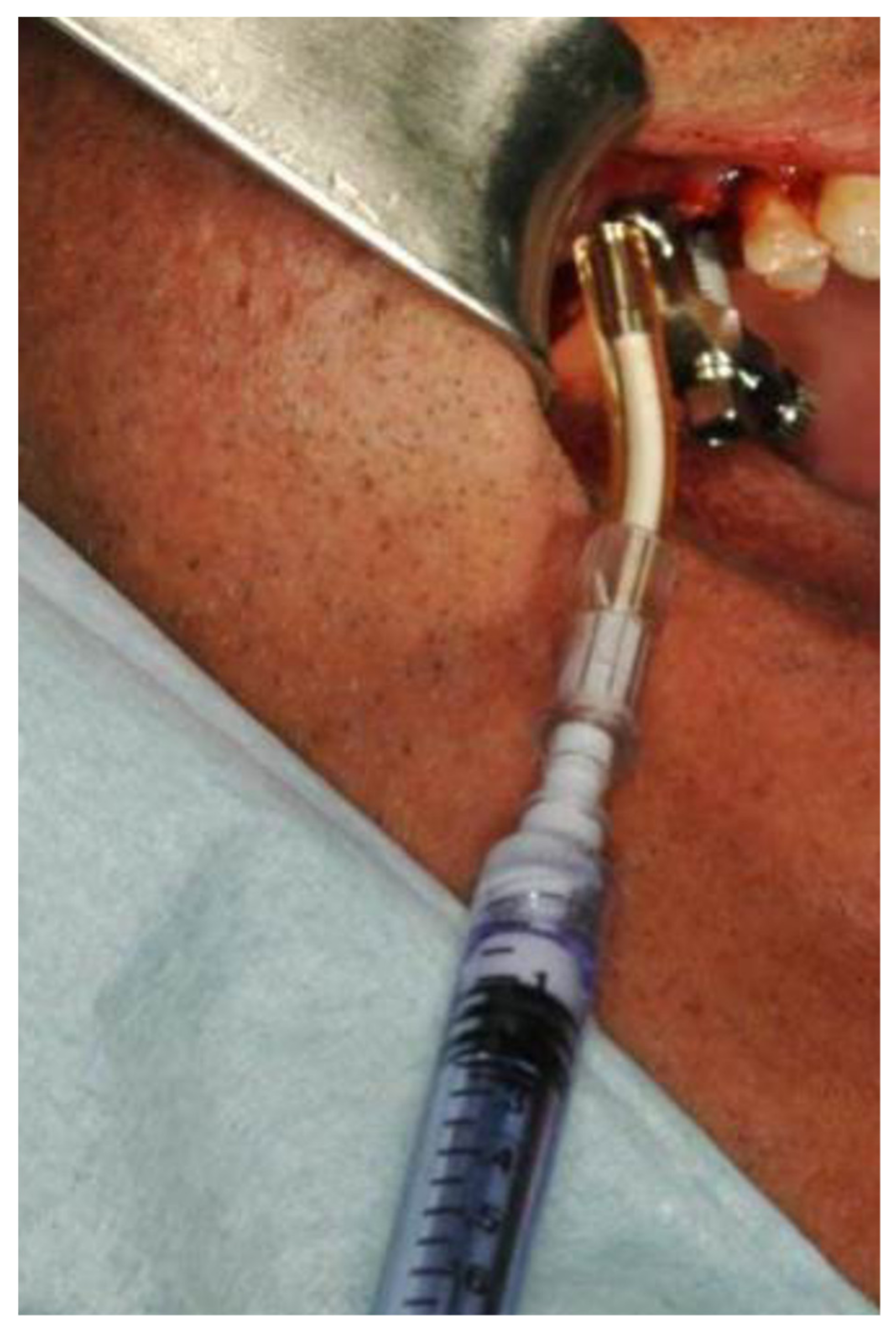
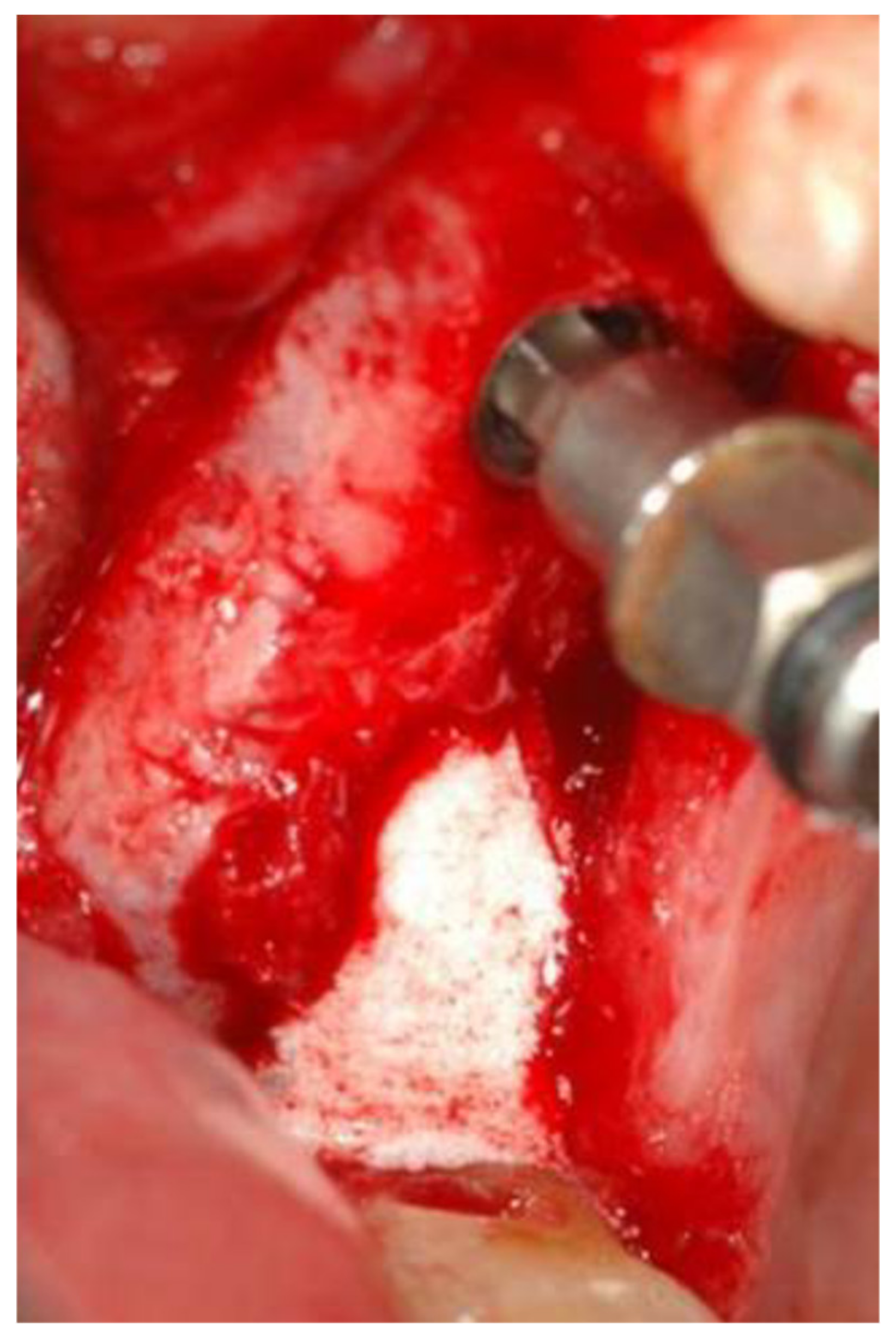
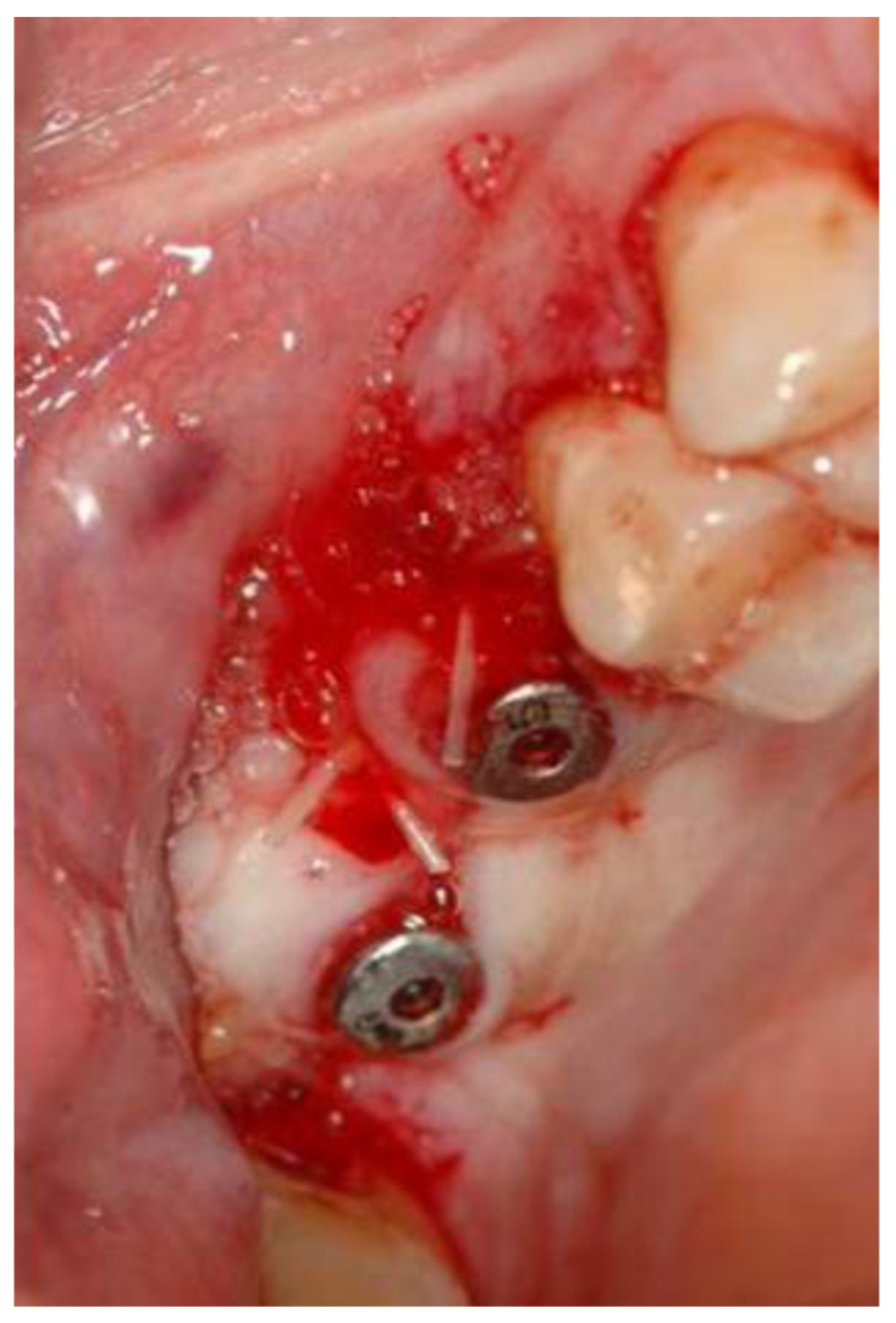
2.1. Standard Surgical Procedure
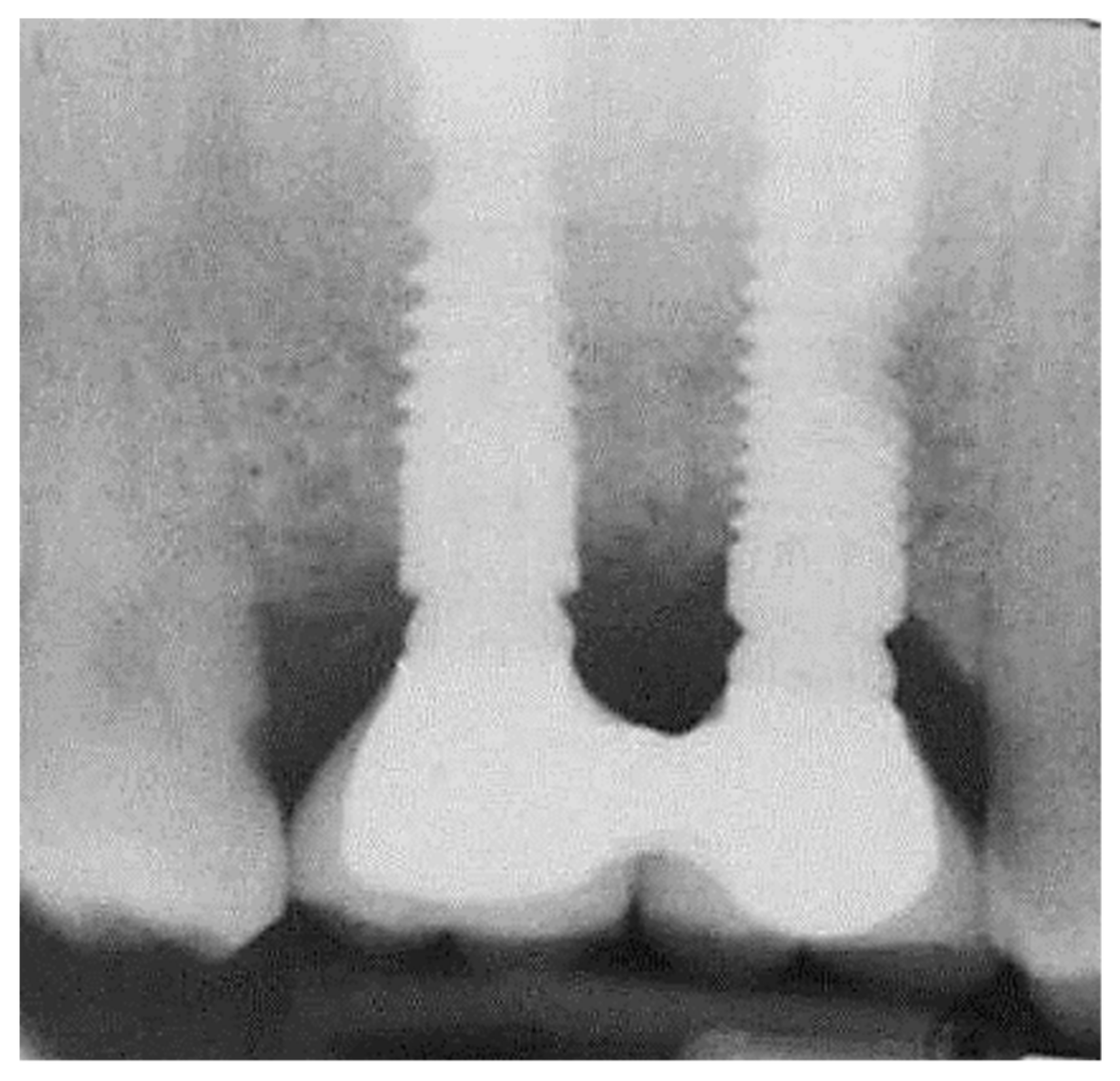
2.2. Marginal Bone Loss
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, C.M. Origin and Uses of Primum Non Nocere-Above All, Do No Harm! J. Clin. Pharmacol. 2005, 45, 371–377. [Google Scholar] [CrossRef]
- Rocci, A.; Martignoni, M.; Gottlow, J. Immediate loading in the maxilla using flapless surgery, implants placed in predetermined positions, and prefabricated provisional restorations: a retrospective 3-year clinical study. Clin. Implant. Dent. Relat. Res. 2003, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Arısan, V.; Karabuda, C.Z.; Özdemir, T. Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin. Oral Implant. Res. 2010, 21, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, R.V.; Sapthagiri, E. Flapless implant surgery: a 2-year follow-up study of 40 implants. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e237–e243. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Chew, Y.S.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: 1- versus 2-stage implant placement. Cochrane Database Syst. Rev. 2009, 8, CD006698. [Google Scholar] [CrossRef]
- Troiano, G.; Russo, L.L.; Canullo, L.; Ciavarella, M.; Muzio, L.L.; Laino, L. Early and late implant failure of submerged versus non-submerged implant healing: A systematic review, meta-analysis and trial sequential analysis. J. Clin. Periodontol. 2018, 45, 613–623. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabilitation 2014, 41, 443–476. [Google Scholar] [CrossRef]
- Xavier, S.P.; Dias, R.R.; Sehn, F.P.; Kahn, A.; Chaushu, L.; Chaushu, G. Maxillary sinus grafting with autograft vs. fresh frozen allograft: a split-mouth histomorphometric study. Clin. Oral. Implants Res. 2015, 26, 1080–1085. [Google Scholar] [CrossRef]
- Xavier, S.P.; Silva, E.R.; Kahn, A.; Chaushu, L.; Chaushu, G. Maxillary Sinus Grafting with Autograft Versus Fresh-Frozen Allograft: A Split-Mouth Evaluation of Bone Volume Dynamics. Int. J. Oral Maxillofac. Implant. 2015, 30, 1137–1142. [Google Scholar] [CrossRef]
- Sehn, F.P.; Dias, R.R.; de Santana Santos, T.; Silva, E.R.; Salata, L.A.; Chaushu, G.; Xavier, S.P. Fresh-frozen allografts combined with bovine bone mineral enhance bone formation in sinus augmentation. J. Biomater. Appl. 2015, 29, 1003–1013. [Google Scholar] [CrossRef]
- Better, H.; Slavescu, D.; Barbu, H.; Cochran, D.L.; Chaushu, G. Patients perceptions of recovery after maxillary sinus augmentation with a minimally invasive implant device. Quintessence Int. 2014, 45, 779–787. [Google Scholar]
- Better, H.; Slavescu, D.; Barbu, H.; Cochran, D.L.; Chaushu, G. Minimally invasive sinus lift implant device: a multicenter safety and efficacy trial preliminary results. Clin. Implant Dent. Relat. Res. 2014, 16, 520–526. [Google Scholar] [CrossRef]
- Mardinger, O.; Chaushu, G.; Sigalov, S.; Herzberg, R.; Shlomi, B.; Schwartz-Arad, D. Factors affecting changes in sinus graft height between and above the placed implants. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, e6–e11. [Google Scholar] [CrossRef]
- Mardinger, O.; Moses, O.; Chaushu, G.; Manor, Y.; Tulchinsky, Z.; Nissan, J. Challenges associated with reentry maxillary sinus augmentation. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 287–291. [Google Scholar] [CrossRef]
- Chaushu, G.; Vered, M.; Mardinger, O.; Nissan, J. Histomorphometric Analysis After Maxillary Sinus Floor Augmentation Using Cancellous Bone–Block Allograft. J. Periodontol. 2010, 81, 1147–1152. [Google Scholar] [CrossRef]
- Manor, Y.; Mardinger, O.; Bietlitum, I.; Nashef, A.; Nissan, J.; Chaushu, G. Late signs and symptoms of maxillary sinusitis after sinus augmentation. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, e1–e4. [Google Scholar] [CrossRef]
- Chaushu, G.; Mardinger, O.; Calderon, S.; Moses, O.; Nissan, J. The Use of Cancellous Block Allograft for Sinus Floor Augmentation With Simultaneous Implant Placement in the Posterior Atrophic Maxilla. J. Periodontol. 2009, 80, 422–428. [Google Scholar] [CrossRef]
- Mardinger, O.; Nissan, J.; Chaushu, G. Sinus Floor Augmentation With Simultaneous Implant Placement in the Severely Atrophic Maxilla: Technical Problems and Complications. J. Periodontol. 2007, 78, 1872–1877. [Google Scholar] [CrossRef]
- Shlomi, B.; Horowitz, I.; Kahn, A.; Dobriyan, A.; Chaushu, G. The effect of sinus membrane perforation and repair with Lambone on the outcome of maxillary sinus floor augmentation: a radiographic assessment. Int. J. Oral Maxillofac. Implant. 2004, 19, 559–562. [Google Scholar]
- Mazor, Z.; Peleg, M.; Garg, A.K.; Chaushu, G. The Use of Hydroxyapatite Bone Cement for Sinus Floor Augmentation With Simultaneous Implant Placement in the Atrophic Maxilla. A Report of 10 Cases. J. Periodontol. 2000, 71, 1187–1194. [Google Scholar] [CrossRef]
- Peleg, M.; Chaushu, G.; Mazor, Z.; Ardekian, L.; Bakoon, M. Radiological Findings of the Post-Sinus Lift Maxillary Sinus: A Computerized Tomography Follow-Up. J. Periodontol. 1999, 70, 1564–1573. [Google Scholar] [CrossRef]
- Peleg, M.; Mazor, Z.; Chaushu, G.; Garg, A.K. Sinus Floor Augmentation With Simultaneous Implant Placement in the Severely Atrophic Maxilla. J. Periodontol. 1998, 69, 1397–1403. [Google Scholar] [CrossRef]
- Mardinger, O.; Poliakov, H.; Beitlitum, I.; Nissan, J.; Chaushu, G. The Patient’s Perception of Recovery After Maxillary Sinus Augmentation: A Prospective Study. J. Periodontol. 2009, 80, 572–576. [Google Scholar] [CrossRef]
- Tatum, H. Maxillary and sinus implant reconstructions. Dent. Clin. North Am. 1986, 30, 207–229. [Google Scholar]
- Summers, R.B. A new concept in maxillary implant surgery: the osteotome technique. Compendium 1994, 15, 154–156. [Google Scholar]
- Chen, L.; Cha, J. An 8-Year Retrospective Study: 1,100 Patients Receiving 1,557 Implants Using the Minimally Invasive Hydraulic Sinus Condensing Technique. J. Periodontol. 2005, 76, 482–491. [Google Scholar] [CrossRef]
- Tepper, G.; Haas, R.; Zechner, W.; Krach, W.; Watzek, G. Three-dimensional finite element analysis of implant stability in the atrophic posterior maxilla: a mathematical study of the sinus floor augmentation. Clin. Oral Implant. Res. 2002, 13, 657–665. [Google Scholar] [CrossRef]
- Abramhoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- De Smet, E.; Jacobs, R.; Gijbels, F.; Naert, I. The accuracy and reliability of radiographic methods for the assessment of marginal bone level around oral implants. Dentomaxillofac. Radiol. 2002, 31, 176–181. [Google Scholar] [CrossRef]
- Hof, M.; Tepper, G.; Semo, B.; Arnhart, C.; Watzek, G.; Pommer, B. Patients’ perspectives on dental implant and bone graft surgery: questionnaire-based interview survey. Clin. Oral Implants Res. 2014, 25, 42–45. [Google Scholar] [CrossRef]
- Santoro, M.; Pippi, R. Intrasinus Bone Gain with the Osteotome Sinus Floor Elevation Technique: A Review of the Literature. Int. J. Oral Maxillofac. Implant. 2018, 33, 995–1002. [Google Scholar] [CrossRef]
- Torkzaban, P.; Arabi, S.R.; Roshanaei, G.; Rostami, M.; Soheilifar, S. A Comparative Study of Clinical Parameters in Submerged and Non submerged Implants. J. Clin. Diagn. Res. 2015, 9, ZC26–ZC29. [Google Scholar] [CrossRef]
- Nemli, S.; Güngör, M.; Aydın, C.; Yılmaz, H.; Türkcan, I.; Demirköprülü, H.; Aydin, C.; Yilmaz, H. Clinical evaluation of submerged and non-submerged implants for posterior single-tooth replacements: a randomized split-mouth clinical trial. Int. J. Oral Maxillofac. Surg. 2014, 43, 1484–1492. [Google Scholar] [CrossRef]
- Gulati, M.; Govila, V.; Verma, S.; Rajkumar, B.; Anand, V.; Aggarwal, A.; Jain, N. In Vivo evaluation of two-piece implants placed following one-stage and two-stage surgical protocol in posterior Mandibular region. Assessment of alterations in Crestal bone level. Clin. Implant Dent. Rel. Res. 2015, 17, 854–861. [Google Scholar] [CrossRef]
- Tallarico, M.; Vaccarella, A.; Marzi, G.C. Clinical and radiological outcomes of 1- versus 2-stage implant placement: 1-year results of a randomized clinical trial. Eur. J. Oral Implantol. 2011, 4, 13–20. [Google Scholar]
- Enkling, N.; Jöhren, P.; Klimberg, T.; Mericske-Stern, R.; Jervøe-Storm, P.-M.; Bayer, S.; Gülden, N.; Jepsen, S. Open or submerged healing of implants with platform switching: a randomized, controlled clinical trial. J. Clin. Periodontol. 2011, 38, 374–384. [Google Scholar] [CrossRef]
- Bruckmoser, E.; Gruber, R.; Steinmassl, O.; Eder, K.; Watzinger, F.; Bayerle-Eder, M.; Jesch, P. Crestal Sinus Floor Augmentation Using Hydraulic Pressure and Vibrations: A Retrospective Single Cohort Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 1149–1154. [Google Scholar] [CrossRef]
- Better, H.; Chaushu, L.; Nissan, J.; Xavier, S.; Talarico, M.; Chaushu, G. The Feasibility of Flapless Approach to Sinus Augmentation Using an Implant Device Designed According to Residual Alveolar Ridge Height. Int. J. Periodontics Restor. Dent. 2018, 38, 601–606. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Mazor, Z. A simplified approach to the minimally invasive antral membrane elevation technique utilizing a viscoelastic medium for hydraulic sinus floor elevation. Oral. Maxillofac. Surg. 2015, 19, 97–101. [Google Scholar] [CrossRef]
- Lopez, M.A.; Andreasi Bassi, M.; Confalone, L.; Carinci, F. Maxillary sinus floor elevation via crestal approach: the evolution of the hydraulic pressure technique. J. Craniofac. Surg. 2014, 25, 127–132. [Google Scholar] [CrossRef]
- Jesch, P.; Bruckmoser, E.; Bayerle, A.; Eder, K.; Bayerle-Eder, M.; Watzinger, F. A pilot-study of a minimally invasive technique to elevate the sinus floor membrane and place graft for augmentation using high hydraulic pressure: 18-month follow-up of 20 cases. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 293–300. [Google Scholar] [CrossRef]
- Kim, J.M.; Sohn, D.S.; Heo, J.U.; Park, J.S.; Jung, H.S.; Moon, J.W.; Lee, J.H.; Park, I.S. Minimally invasive sinus augmentation using ultrasonic piezoelectric vibration and hydraulic pressure: a multicenter retrospective study. Implant Dent. 2012, 21, 536–542. [Google Scholar] [CrossRef]
- Bensaha, T. Outcomes of flapless crestal maxillary sinus elevation under hydraulic pressure. Int. J. Oral Maxillofac. Implant. 2012, 27, 1223–1229. [Google Scholar]
- Lin, T.H.S.; Chen, L.; Cha, J.; Jeffcoat, M.; Kao, D.W.K.; Nevins, M.; Fiorellini, J.P. The effect of cigarette smoking and native bone height on dental implants placed immediately in sinuses grafted by hydraulic condensation. Int. J. Periodontics Restor. Dent. 2012, 32, 255–261. [Google Scholar]
- Kao, D.W.K.; DeHaven, H.A. Controlled Hydrostatic Sinus Elevation: A Novel Method of Elevating the Sinus Membrane. Implant. Dent. 2011, 20, 425–429. [Google Scholar] [CrossRef]
- Kim, D.Y.; Itoh, Y.; Kang, T.H. Evaluation of the effectiveness of a water lift system in the sinus membrane-lifting operation as a sinus surgical instrument. Clin. Implant Dent. Relat. Res. 2012, 14, 585–594. [Google Scholar] [CrossRef]
- Chen, L.; Cha, J.; Chen, H.-C. Two different clinical indications using hydraulic sinus condensing (HSC) technique: ten years follow-up. Dent. Implant. Updat. 2009, 20, 33–38. [Google Scholar]
- Kher, U.; Ioannou, A.L.; Kumar, T.; Siormpas, K.; Mitsias, M.E.; Mazor, Z.; Kotsakis, G.A. A minimally invasive antral membrane elevation technique in the posterior maxilla. J. Craniomaxillofac. Surg. 2014, 42, 1942–1947. [Google Scholar] [CrossRef]
- Tallarico, M.; Better, H.; De Riu, G.; Meloni, S.M. A novel implant system dedicate to hydraulic Schneiderian membrane elevation and simultaneously bone graft augmentation: An up-to 45 months retrospective clinical study. J. Cranio-Maxillofacial Surg. 2016, 44, 1089–1094. [Google Scholar] [CrossRef]
- Gatti, F.; Gatti, C.; Tallarico, M.; Tommasato, G.; Meloni, S.; Chiapasco, M. Maxillary Sinus Membrane Elevation Using a Special Drilling System and Hydraulic Pressure: A 2-Year Prospective Cohort Study. Int. J. Periodontics Restor. Dent. 2018, 38, 593–599. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaushu, L.; Chaushu, G.; Better, H.; Naishlos, S.; Kolerman, R.; Aragoneses, J.M.; Calvo-Guirado, J.L.; Nissan, J. Sinus Augmentation with Simultaneous, Non-Submerged, Implant Placement Using a Minimally Invasive Hydraulic Technique. Medicina 2020, 56, 75. https://doi.org/10.3390/medicina56020075
Chaushu L, Chaushu G, Better H, Naishlos S, Kolerman R, Aragoneses JM, Calvo-Guirado JL, Nissan J. Sinus Augmentation with Simultaneous, Non-Submerged, Implant Placement Using a Minimally Invasive Hydraulic Technique. Medicina. 2020; 56(2):75. https://doi.org/10.3390/medicina56020075
Chicago/Turabian StyleChaushu, Liat, Gavriel Chaushu, Hadar Better, Sarit Naishlos, Roni Kolerman, Juan Manuel Aragoneses, José Luis Calvo-Guirado, and Joseph Nissan. 2020. "Sinus Augmentation with Simultaneous, Non-Submerged, Implant Placement Using a Minimally Invasive Hydraulic Technique" Medicina 56, no. 2: 75. https://doi.org/10.3390/medicina56020075
APA StyleChaushu, L., Chaushu, G., Better, H., Naishlos, S., Kolerman, R., Aragoneses, J. M., Calvo-Guirado, J. L., & Nissan, J. (2020). Sinus Augmentation with Simultaneous, Non-Submerged, Implant Placement Using a Minimally Invasive Hydraulic Technique. Medicina, 56(2), 75. https://doi.org/10.3390/medicina56020075








