COVID-19 and Pulmonary Hypertension in Children: What Do We Know So Far?
Abstract
1. Introduction
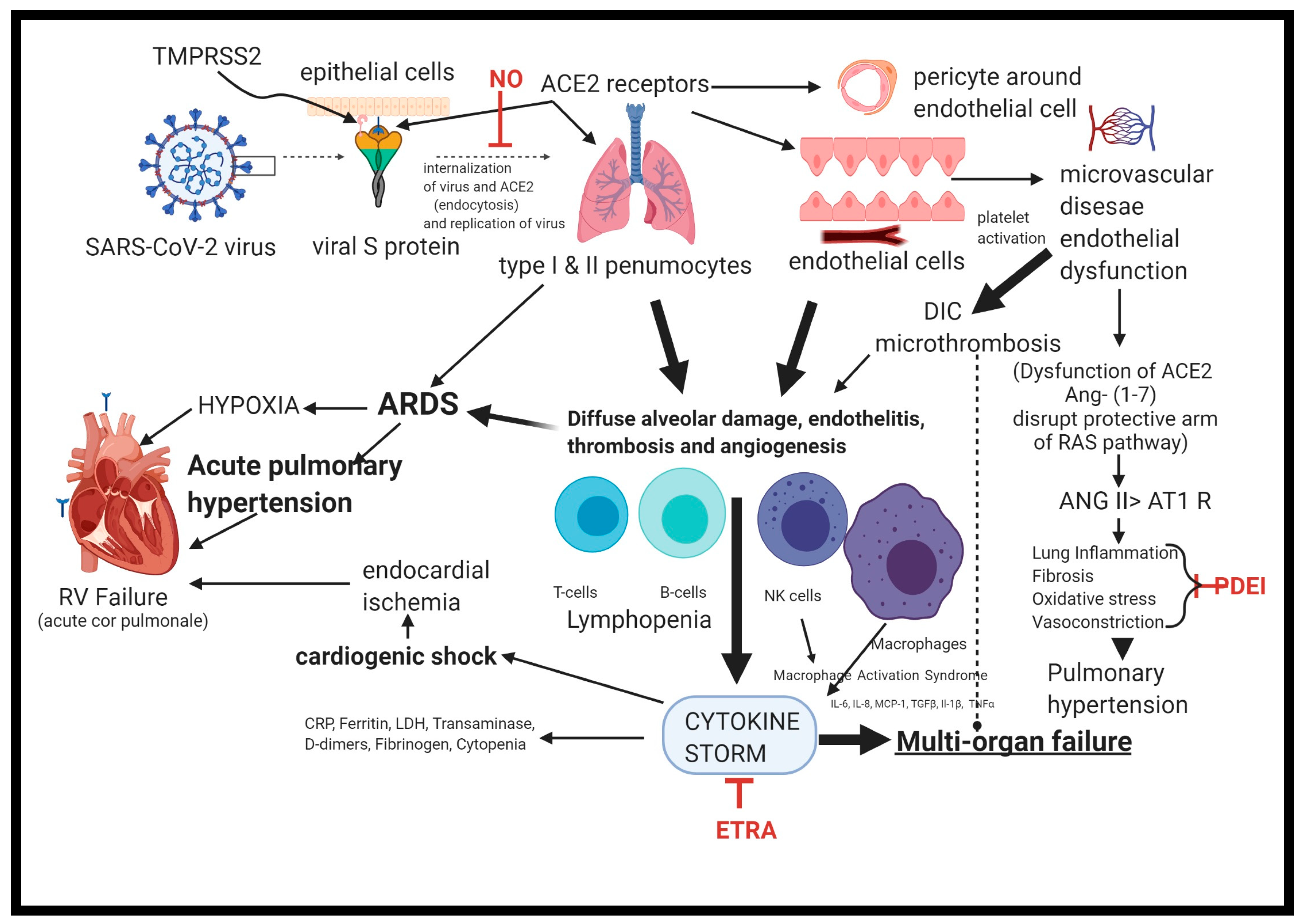
2. Pathophysiology of PH Occurring with COVID-19
3. Differences between Children and Adults in COVID-19
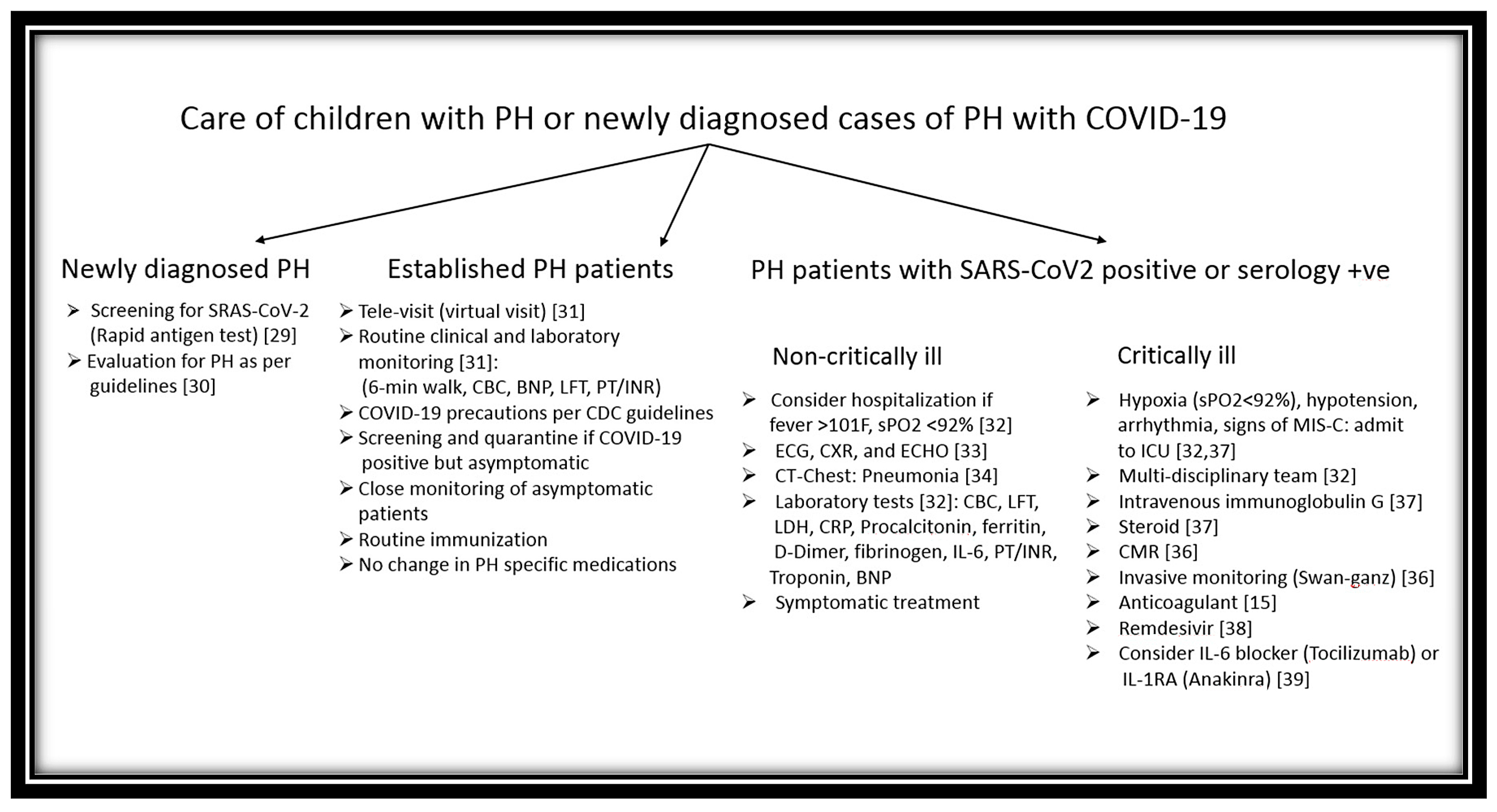
4. Management of Preexisting or New-Onset PH Occurring with COVID-19
5. Impact of COVID-19 on PH Care in Children
6. Conclusions
Funding
Conflicts of Interest
References
- COVID-19 Map. John Hopkins University Coronavirus Resource Center. Available online: https://coronavirus.jhus.edu/map.html (accessed on 27 November 2020).
- Leeb, R.T.; Price, S.; Sliwa, S.; Kimball, A.; Szucs, L.; Caruso, E.; Godfred-Cato, S.; Lozier, M. COVID-19 trends among school-aged children-United States, 1 March–19 September 2020. MMWR 2020, 69, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J. The Impact of COVID-19 on Pulmonary Hypertension. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/08/35/the-impact-covid-19-on-pulmonary-hypertension (accessed on 28 September 2020).
- Olfe, J.; Grafmann, M.; Kozlik-Feldman, R. A teenager with a congenital heart defect and Covid-19. Cardiol. Young 2020, 30, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, M.; Rodriguez-Campy, P.; Sanchez-Codez, M.; Guiterrez-Rosa, I.; Castellano-Martinez, A.; Rodriguez-Benitez, A. New-onset right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol. Young 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J. Virol. 2019, 93, e01815-18. [Google Scholar] [CrossRef] [PubMed]
- Offringa, A.; Montijn, R.; Singh, S.; Paul, M.; Pinto, Y.M.; Pinto-Sietsma, S.-J. The mechanistic overview of SARS-CoV-2 using angiotensin-converting enzyme 2 to enter the cell for replication: Possible treatment options related to the renin-angiotensin system Eur Heart J Cardiovasc. Pharmacotherapy 2020, 6, 317–325. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult in-patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 54–62. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, S.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.-H.; Healy, A.I.; Hamid, Q.; Halwani, R. Airway expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endothelitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackerman, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelitis, thrombosis, and angiogenesis in COVID-19. NEJM 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Hope, K.; Fremed, M.; Misra, N.; Altman, C.; Glickstein, J.; Sanchez-De-Toledo, J.; Fraisse, A.; Miller, J.; Snyder, C.; et al. Role of pediatric cardiologist in the COVID-19 pandemic. Pediatr. Cardiol. 2020, 1–17, (Online ahead of print). [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Church, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Burger, C.D.; Delossantos, B.D.; Grinnan, D.; Ralph, D.; Rayner, S.G.; Ryan, J.J.; Safdar, Z.; Ventetuolo, C.E.; Zamanian, R.T.; et al. A survey-based estimate of COVID-19 incidence and outcomes among patients with PAH or CTEPH and impact on the process of care. Ann. Am. Thorac. Soc. 2020, 17, 1576–1582. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Xia, T.; Fu, J.; Feng, X.; Mu, D.; Feng, Q.; Hei, M.; Hu, X.; Li, Z.; et al. Chinese expert consensus on perinatal and neonatal management for the prevention and control of the 2019 novel corona virus infection (first edition). Am. Transl. Med. 2020, 8, 47. [Google Scholar] [CrossRef]
- Akerstrom, S.; Mousavi-Jazi, M.; Klingstrom, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef]
- Isidori, A.M.; Giannetta, E.; Pofi, R.; Venneri, M.A.; Gianfrilli, D.; Campolo, F.; Mastroianni, C.M.; Lenzi, A.; d’Ettorre, G. Targeting the NO-cGMP-PDE5 pathways in COVID-19 patients. The DEADLO project. Andrology 2020. (online ahead of print). [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kleniewska, P.; Kolodziesjczyk, M.; Skibska, B.; Gorica, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. [Google Scholar] [CrossRef]
- Araz, O. Current pharmacological approach to ARDS: The place of bosentan. Eurasian J. Med. 2020, 52, 81–85. [Google Scholar] [CrossRef]
- Badagliacca, R.; Sciomer, S.; Petrosillo, N. Endothelin receptor antagonists for pulmonary hypertension and COVID-19: Friend or foe. JHLT 2020, 39, 729–730. [Google Scholar]
- Calcaianu, G.; Calcaianu, K.; Gschwend, A.; Canuel, M.; Meziani, F.; Kessier, R. Hemodynamic profile of pulmonary hypertension in ARDS. Pulm. Circ. 2018, 8, 2045893217753415. [Google Scholar] [CrossRef] [PubMed]
- Nuche, J.; de la Cal, T.S.; Guarch, C.J.L.; Lopez-Medrano, F.; Delgado, C.P.O.; Ynsaurriaga, F.A.; Delgado, J.F.; Ibáñez, B.; Oliver, E.; Subías, P.E. Effects of coronavirus 2019 in pulmonary circulation. The particular scenario of precapillary pulmonary hypertension. Diagnostics 2020, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Zepol, W.M.; Snider, M.T. Pulmonary hypertension in severe acute respiratory failure. NEJM 1977, 296, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Baldetti, L.; Beneduce, A.; Calvo, F.; Gramenga, M.; Pazzanese, V.; Ingallina, G.; Napolano, A.; Finazzi, R.; Ruggeri, A.; et al. Pulmonary hypertension and right ventricular involvement in hospitalized patients with COVID-19. Heart 2020, 106, 1324–1331. [Google Scholar] [CrossRef]
- Source. Available online: https://www.clinicaltrials.gov/ (accessed on 5 November 2020).
- Kobayashi, J.; Murata, I. Nitric oxide inhalation as interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann. Intensive Care 2020, 10, 1–2. [Google Scholar] [CrossRef]
- Definitions of Diagnostic, Screening, and Surveillance Testing for SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 5 October 2020).
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef]
- Ryan, J.J.; Melendres-Groves, L.; Zamanian, R.T.; Oudiz, R.J.; Chakinala, M.; Rosenweig, E.B.; Gomberg-Maitland, M. Care of patient with pulmonary arterial hypertension during the coronavirus (COVID-19) pandemic. Pulm. Circ. 2020, 10, 2045894020920153. [Google Scholar] [CrossRef]
- Kache, S.; Chisti, M.J.; Gumbo, F.; Mupere, E.; Zhi, X.; Nallasamy, K.; Nakagawa, S.; Lee, J.H.; Di Nardo, M.; De La Oliva, P.; et al. COVID-19 PICU Guidelines: For high and limited-resource settings. Pediatr. Res. 2020, 88, 705–716. [Google Scholar] [CrossRef]
- Lazzeri, C.; Bonizzoli, M.; Batacchi, S.; Peris, A. Echocardiographic assessment of the right ventricle in COVID-19 related acute respiratory syndrome. Intern. Emerg. Med. 2020, 1–5, Online ahead of print. [Google Scholar] [CrossRef]
- Xia, W.; Shao, J.; Gio, Y.; Peng, X.; Li, Z.; Hu, D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr. Pulmonol. 2020, 55, 1169–1174. [Google Scholar] [CrossRef]
- Lins, M.; Vandevenne, J.; Thillai, M.; Lavon, B.R.; Lanclus, M.; Bonte, S.; Godon, R.; Kendall, I.; De Backer, J.; De Backer, W. Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad. Radiol. 2020, 27, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- The European Society of Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. Available online: https://www.escardio.org/Education/VOVID-19-and-Cardiology/ESC-COVID-19-Guidance (accessed on 28 September 2020).
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem inflammatory syndrome in US children and adolescents. NEJM 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of the COVID-19-preliminary report. NEJM 2020, 383, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Khou, V.; Anderson, J.J.; Strange, G.; Corrigan, C.; Collins, N.; Celermajer, D.S.; Dwyer, N.; Feenstra, J.; Horrigan, M.; Keating, D.; et al. Diagnostic delay in pulmonary arterial hypertension: Insights from the Australian and New Zeeland pulmonary hypertension registry. Respirology 2020, 25, 863–871. [Google Scholar] [CrossRef]
- John, W.S.; Arachchillage, D.J.; McCabe, C.; Price, L.C. Covid-19 pneumonia and pulmonary vascular disease: A UK center perspective Respiratory Medicine and Research. Respir. Med. Res. 2020, 78, 100781. [Google Scholar]
- Yogeswaran, A.; Gall, H.; Tello, K.; Grunig, E.; Xanthouli, P.; Ewert, R.; Kamp, J.C.; Olsson, K.M.; Wißmüller, M.; Rosenkranz, S.; et al. Impact of SARS-CoV-2 pandemic on pulmonary hypertension out-patient clinics in Germany: A multi-center study. Pulm. Circ. 2020, 10, 2045894020941682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B. COVID-19 and Pulmonary Hypertension in Children: What Do We Know So Far? Medicina 2020, 56, 716. https://doi.org/10.3390/medicina56120716
Das BB. COVID-19 and Pulmonary Hypertension in Children: What Do We Know So Far? Medicina. 2020; 56(12):716. https://doi.org/10.3390/medicina56120716
Chicago/Turabian StyleDas, Bibhuti B. 2020. "COVID-19 and Pulmonary Hypertension in Children: What Do We Know So Far?" Medicina 56, no. 12: 716. https://doi.org/10.3390/medicina56120716
APA StyleDas, B. B. (2020). COVID-19 and Pulmonary Hypertension in Children: What Do We Know So Far? Medicina, 56(12), 716. https://doi.org/10.3390/medicina56120716




