Association of Asymmetric Dimethylarginine and Nitric Oxide with Cardiovascular Risk in Patients with End-Stage Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Etiology and Severity of ESLD
3.2. Cardiovascular Risk
3.3. Associations between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zarrinpar, A.; Busuttil, R.W. Liver transplantation: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Raval, Z.; Harinstein, M.E.; Skaro, A.I.; Erdogan, A.; DeWolf, A.M.; Shah, S.J.; Fix, O.K.; Kay, N.; Abecassis, M.I.; Gheorghiade, M.; et al. Cardiovascular Risk Assessment of the Liver Transplant Candidate. J. Am. Coll. Cardiol. 2011, 58, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Costa, S.P.; Weir, M.R.; Robb, J.F.; Fleisher, L.A.; Kasiske, B.L.; Carithers, R.L.; Ragosta, M.; Bolton, K.; Auerbach, A.D.; et al. Cardiac Disease Evaluation and Management Among Kidney and Liver Transplantation Candidates: A Scientific Statement From the American Heart Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2012, 60, 434–480. [Google Scholar] [CrossRef] [PubMed]
- Lluch, P.; Segarra, G.; Medina, P. Asymmetric dimethylarginine as a mediator of vascular dysfunction in cirrhosis. World J. Gastroenterol. 2015, 21, 9466. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease: Mechanisms of Endothelial Dysfunction and Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef]
- Fiedler, L. The DDAH/ADMA pathway is a critical regulator of NO signaling in vascular homeostasis. Cell Adhes. Migr. 2008, 2, 149–150. [Google Scholar] [CrossRef][Green Version]
- Sibal, L.; Agarwal, S.C.; Schwedhelm, E.; Lüneburg, N.; Böger, R.H.; Home, P.D. A study of endothelial function and circulating asymmetric dimethylarginine levels in people with Type 1 diabetes without macrovascular disease or microalbuminuria. Cardiovasc. Diabetol. 2009, 8, 27. [Google Scholar] [CrossRef]
- Boöger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 2009, 119, 1592–1600. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Mehta, G.; Balasubramaniyan, V.; Mohamed, F.E.Z.; Davies, N.; Sharma, V.; Iwakiri, Y.; Jalan, R. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J. Hepatol. 2015, 62, 325–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, L.S.; George, J.; Wang, J.H. Current concepts on the role of nitric oxide in portal hypertension. World J. Gastroenterol. 2013, 19, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Teerlink, T.; Van Leeuwen, P.A.M. The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin. Nutr. 2003, 22, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.; Milewski, K.; Zielińska, M. Asymmetric Dimethylarginine and Hepatic Encephalopathy: Cause, Effect, or Association? Neurochem. Res. 2017, 42, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Siroen, M.P.C.; Wiest, R.; Richir, M.C.; Teerlink, T.; A Rauwerda, J.; Drescher, F.T.; Zorger, N.; Van Leeuwen, P.A.M. Transjugular intrahepatic portosystemic shunt-placement increases arginine/asymmetric dimethylarginine ratio in cirrhotic patients. World J. Gastroenterol. 2008, 14, 7214–7219. [Google Scholar] [CrossRef]
- Siroen, M.P.C.; Warlé, M.C.; Teerlink, T.; Nijveldt, R.J.; Kuipers, E.J.; Metselaar, H.J.; Tilanus, H.W.; Kuik, D.J.; Van Der Sijp, J.R.; Meijer, S.; et al. The transplanted liver graft is capable of clearing asymmetric dimethylarginine. Liver Transpl. 2004, 10, 1524–1530. [Google Scholar] [CrossRef]
- Wnuk, Z.; Kokot, F.; Kunsdorf-Wnuk, A. Diagnostic value of plasma asymmetric and symmetric dimethylarginine levels in liver transplant recipients. Pol. Arch. Med. Wewn. 2012, 122, 367–373. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Ž.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Kim, W.R. Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Trgo, G.; Zaja, I.; Bogut, A.; Vicic, V.K.; Meter, I.; Lovrencic, M.V.; Radman, M. Association of Asymmetric Dimethylarginine With Acute Pancreatitis–Induced Hyperglycemia. Pancreas 2016, 45, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Erice, E.; Gilabert, R.; Reverter, E.; Abraldes, J.G.; Garcia-Pagan, J.-C.J.-C.; Bosch, J. Cardiovascular Risk Factors and Systemic Endothelial Function in Patients with Cirrhosis. Am. J. Gastroenterol. 2013, 108, 75–82. [Google Scholar] [CrossRef]
- Willeit, P.; Freitag, D.F.; Laukkanen, J.A.; Chowdhury, S.; Gobin, R.; Mayr, M.; Di Angelantonio, E.; Chowdhury, R. Asymmetric dimethylarginine and cardiovascular risk: Systematic review and meta-analysis of 22 prospective studies. J. Am. Heart Assoc. 2015, 4, e001833. [Google Scholar] [CrossRef]
- Sheen, J.-M.; Chen, Y.-C.; Tain, Y.-L.; Huang, L.-T. Increased Circulatory Asymmetric Dimethylarginine and Multiple Organ Failure: Bile Duct Ligation in Rat as a Model. Int. J. Mol. Sci. 2014, 15, 3989–4006. [Google Scholar] [CrossRef]
- Lluch, P.; Torondel, B.; Medina, P.; Segarra, G.; Del Olmo, J.A.; Serra, M.A.; Rodrigo, J.M. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J. Hepatol. 2004, 41, 55–59. [Google Scholar] [CrossRef]
- Kasumov, T.; Edmison, J.M.; Dasarathy, S.; Bennett, C.; Lopez, R.; Kalhan, S.C. Plasma levels of asymmetric dimethylarginine in patients with biopsy-proven nonalcoholic fatty liver disease. Metabolism 2011, 60, 776–781. [Google Scholar] [CrossRef]
- Boga, S.; Alkim, H.; Koksal, A.R.; Bayram, M.; Ozguven, M.B.Y.; Ergun, M.; Neijmann, S.T.; Ozgon, G.; Alkim, C. Increased Plasma Levels of Asymmetric Dimethylarginine in Nonalcoholic Fatty Liver Disease. J. Investig. Med. 2015, 63, 871–877. [Google Scholar] [CrossRef]
- Karakecili, F.; Cikman, A.; Aydin, M.; Gulhan, B. Asymmetrical Dimethylarginine Levels in Hepatitis B Virus-Positive Patients. Ann. Lab. Med. 2018, 38, 446–449. [Google Scholar] [CrossRef]
- Lluch, P.; Cortina, B.; Vila, J.M.; Segarra, G.; Mauricio, M.D.; Del Olmo, J.A.; Serra, M.A.; Lluch, S.; Rodrigo, J.M. Unchanged plasma levels of dimethylarginines and nitric oxide in chronic hepatitis C. Scand. J. Gastroenterol. 2009, 44, 224–228. [Google Scholar] [CrossRef]
- Lluch, P.; Cortina, B.; Vila, J.M.; Segarra, G.; Mauricio, M.D.; Del Olmo, J.A.; Serra, M.A.; Lluch, S.; Rodrigo, J.M. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007, 46, 62–71. [Google Scholar] [CrossRef]
- Møller, S.; Bendtsen, F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018, 38, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012, 32, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.-T.; Stichtenoth, D.O.; Frölich, J.C.; Manns, M.P.; Böker, K.-H.W. Elevated Nitric Oxide Levels in Patients with Chronic Liver Disease and Cirrhosis Correlate with Disease Stage and Parameters of Hyperdynamic Circulation. Z. Gastroenterol. 2002, 40, 907–913. [Google Scholar] [CrossRef]
- Sarela, A.I.; Mihaimeed, F.M.A.; Batten, J.J.; Davidson, B.R.; Mathie, R.T. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut 1999, 44, 749–753. [Google Scholar] [CrossRef]
- Elsing, C.; Harenberg, S.; Stremmel, W.; Herrmann, T. Serum levels of soluble Fas, nitric oxide and cytokines in acute decompensated cirrhotic patients. World J. Gastroenterol. 2007, 13, 421–425. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Z.; Lee, S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct–ligated rats. Gastroenterology 2002, 118, 937–944. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Maruyama, H.; Kobayashi, K.; Kiyono, S.; Yokosuka, O. Interrelationship between insulin resistance and portal haemodynamic abnormality in cirrhosis. Int. J. Med. Sci. 2017, 14, 240–245. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, L.; Supronowicz, L.; Panasiuk, A.; Cylwik, B.; Gruszewska, E.; Flisiak, R. The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clin. Exp. Med. 2014, 14, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Iakobishvili, Z.; Weisz, G. Associated Risk of Malignancy in Patients with Cardiovascular Disease: Evidence and Possible Mechanism. Am. J. Med. 2017, 130, 780–785. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (N = 160) | MELD < 18 (N = 100) | MELD ≥ 18 (N = 60) | P (MELD < 18 vs. ≥ 18) |
|---|---|---|---|---|
| ADMA (µmol/L) | 0.75 (0.59–0.94) | 0.70 (0.58–0.91) | 0.84 (0.64–0.97) | 0.013 |
| NO (µmol/L) | 22.4 (14.2–31.8) | 19.9 (13.0–27.9) | 27.1 (18.7–47.3) | <0.001 |
| Age (years) | 60 (52–64) | 60 (54–64) | 59 (51–64) | 0.971 |
| Male (%) | 71 | 72 | 68 | 0.623 |
| Hypertension (%) | 29 | 36 | 18 | 0.018 |
| Previous CVD (%) | 7 | 5 | 10 | 0.324 |
| Diabetes (%) | 6 | 6 | 5 | 0.791 |
| HCC (%) | 28 | 39 | 10 | <0.001 |
| SCORE-based CV Risk | ||||
| Low (%) | 54 | 57 | 50 | 0.391 |
| High (%) | 46 | 43 | 50 | |
| BMI (kg/m2) | 25.9 (23.3–29.0) | 26.3 (23.6–29.1) | 25.5 (23.0–28.7) | 0.388 |
| CRP (mg/L) | 7.9 (2.9–18.7) | 5.2 (2.7–12.4) | 11.7 (6.6–25.2) | <0.001 |
| Total cholesterol (mmol/L) | 3.35 (2.50–4.50) | 3.80 (2.90–4.90) | 2.60 (91.60–3.50) | <0.001 |
| HDL cholesterol (mmol/L) | 0.87 (0.60–1.20) | 0.97 (0.70–1.29) | 0.69 (0.35–1.00) | <0.001 |
| LDL cholesterol (mmol/L) | 2.00 (1.40–2.98) | 2.35 (1.60–2.95) | 1.70 (1.00–3.24) | 0.010 |
| Triglycerides (mmol/L) | 0.80 (0.60–1.20) | 0.91 (0.68–1.23) | 0.60 (0.51–1.88) | <0.001 |
| FPG (mmol/L) | 5.4 (4.9–6.2) | 5.4 (5.0–6.2) | 5.4 (4.8–6.1) | 0.564 |
| Insulin (pmol/L) | 117 (81–188) | 123 (87–188) | 108 (78–195) | 0.456 |
| HOMA2-B (%) | 171 (127–234) | 174 (139–231) | 159 (110–245) | 0.542 |
| HOMA2-IS (%) | 40 (25–58) | 37 (25–55) | 42 (24–60) | 0.408 |
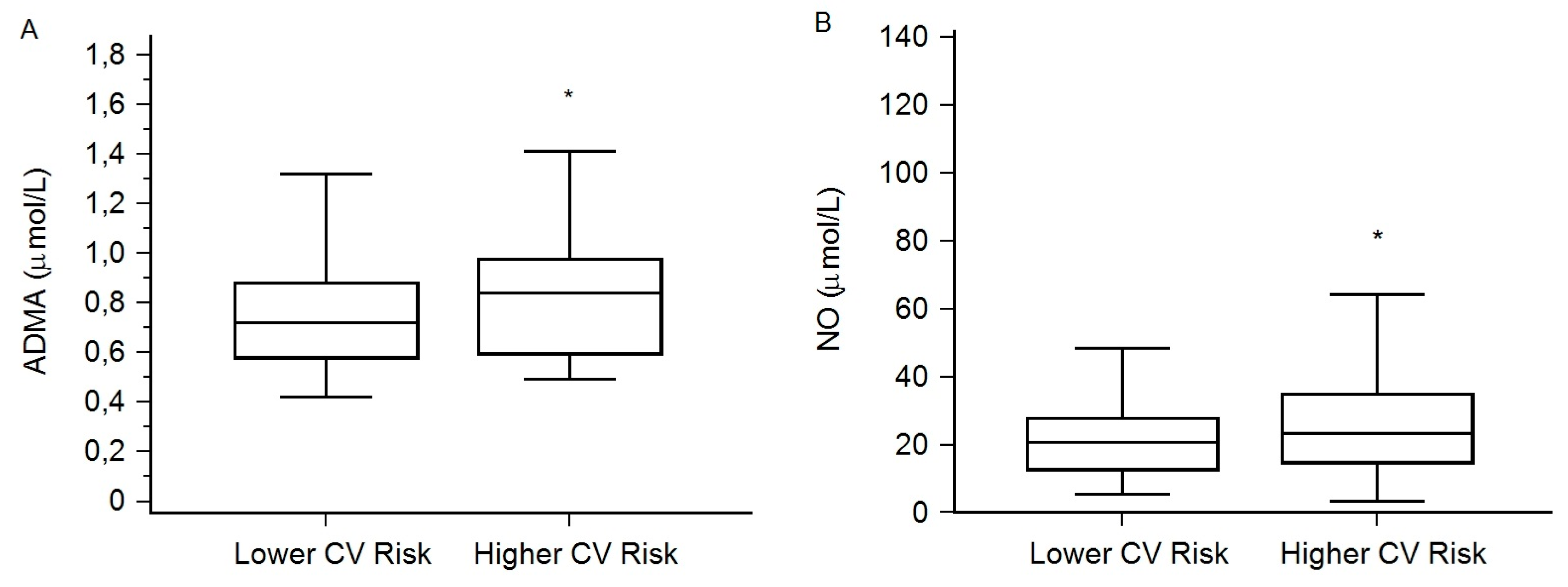
| Characteristics | Lower CV Risk (N = 87) | Higher CV Risk (N = 73) | P (Lower vs. Higher CV Risk) |
|---|---|---|---|
| Male (%) | 64 | 78 | 0.058 |
| HCC (%) | 23 | 34 | 0.114 |
| MELD | 16 (13–19) | 17 (11–21) | 0.515 |
| BMI (kg/m2) | 26.0 (23.1–28.8) | 25.59 (23.6–29.4) | 0.546 |
| CRP (mg/L) | 6.0 (2.6–13.0) | 10.8 (3.6–28.9) | 0.012 |
| HDL cholesterol (mmol/L) | 0.97 (0.63–1.28) | 0.75 (0.52–1.04) | 0.022 |
| LDL cholesterol (mmol/L) | 2.30 (1.60–3.10) | 1.80 (1.06–2.70) | 0.041 |
| Triglycerides (mmol/L) | 0.80 (0.63–1.22) | 0.77 (0.58–1.16) | 0.844 |
| FPG (mmol/L) | 5.2 (4.8–5.8) | 5.7 (5.0–6.6) | 0.001 |
| Insulin (pmol/L) | 114 (81–191) | 123 (82–187) | 0.770 |
| HOMA2-B (%) | 179 (141–240) | 166 (110–219) | 0.134 |
| HOMA2-IS (%) | 41 (25–61) | 38 (24–55) | 0.528 |
| IRI | 2.5 (1.6–4.0) | 2.6 (1.8–3.9) | 0.596 |
| Variable | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| ADMA (µmol/L) per 1 µmol/L increase | 3.6629 | 1.0442–12.8376 | 0.042 |
| NO (µmol/L) per 1 µmol/L increase | 1.0180 | 1.0002–1.0361 | 0.048 |
| FPG (mmol/L) per 1 mmol/L increase | 1.6514 | 1.0923–2.4966 | 0.017 |
| HDL-C (mmol/L) per 1 mmol/L increase | 0.4064 | 0.1672–0.9877 | 0.047 |
| HCC absence vs. presence | 0.6221 | 0.3921–0.9866 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragičević, M.; Košuta, I.; Kruezi, E.; Lovrenčić, M.V.; Mrzljak, A. Association of Asymmetric Dimethylarginine and Nitric Oxide with Cardiovascular Risk in Patients with End-Stage Liver Disease. Medicina 2020, 56, 622. https://doi.org/10.3390/medicina56110622
Dragičević M, Košuta I, Kruezi E, Lovrenčić MV, Mrzljak A. Association of Asymmetric Dimethylarginine and Nitric Oxide with Cardiovascular Risk in Patients with End-Stage Liver Disease. Medicina. 2020; 56(11):622. https://doi.org/10.3390/medicina56110622
Chicago/Turabian StyleDragičević, Maro, Iva Košuta, Egon Kruezi, Marijana Vučić Lovrenčić, and Anna Mrzljak. 2020. "Association of Asymmetric Dimethylarginine and Nitric Oxide with Cardiovascular Risk in Patients with End-Stage Liver Disease" Medicina 56, no. 11: 622. https://doi.org/10.3390/medicina56110622
APA StyleDragičević, M., Košuta, I., Kruezi, E., Lovrenčić, M. V., & Mrzljak, A. (2020). Association of Asymmetric Dimethylarginine and Nitric Oxide with Cardiovascular Risk in Patients with End-Stage Liver Disease. Medicina, 56(11), 622. https://doi.org/10.3390/medicina56110622





