Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review
Abstract
1. Introduction
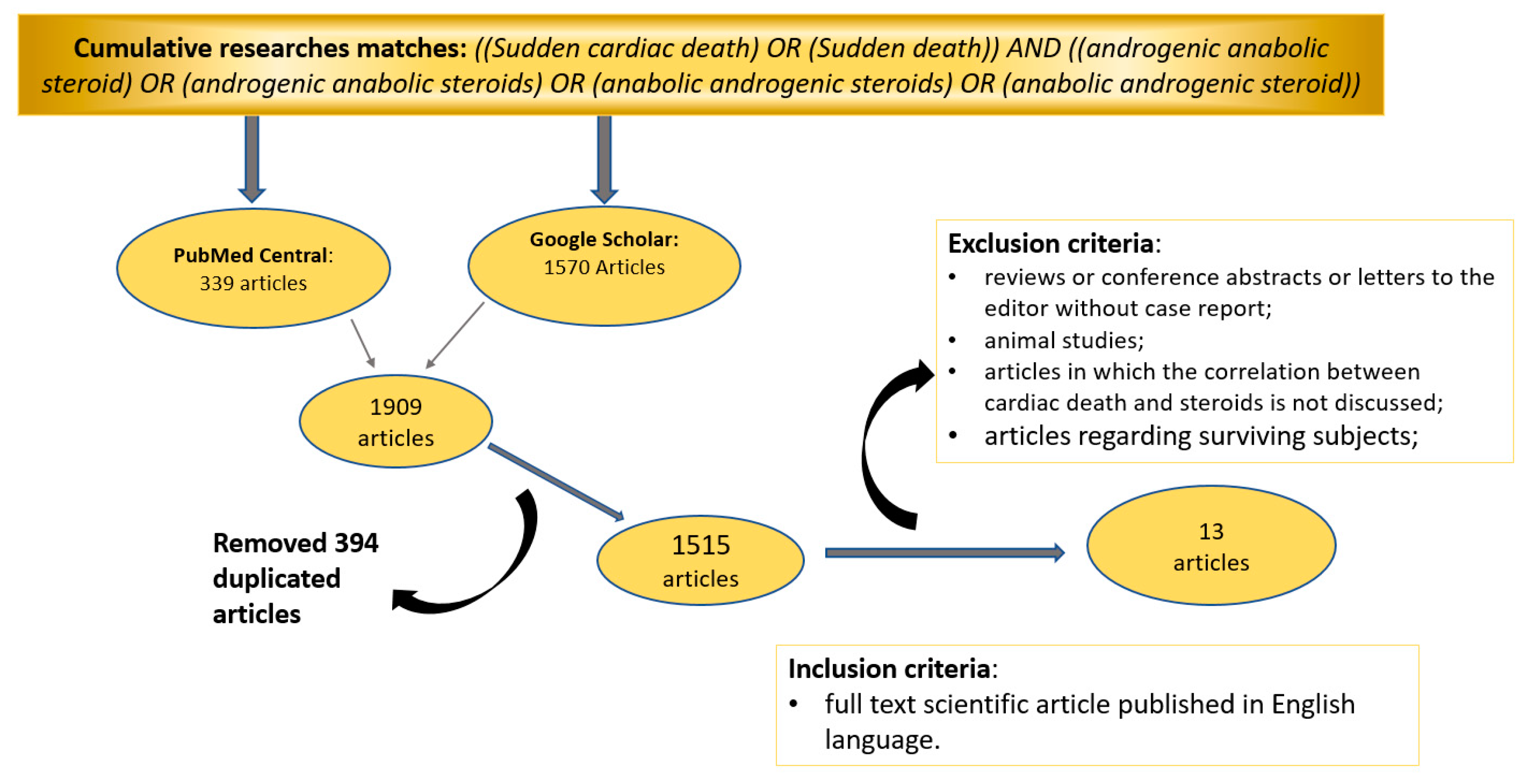
2. Materials and Methods
2.1. Database Search Terms and Timeline
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Author (Year) | Age (Years); Sex; Height; Weight | BMI | Personal and Family Medical History | Kind of Sport Activity | ASS Reported Use—Time of Assumption | Use of Other Drugs | Circumstance of Death | Macroscopic Heart Findings | Histological Heart Findings | Toxicological Analysis | Cause of Death |
| Campbell, S. E. et al. (1993) | 21; M | NR | Absence of significant diseases | Bodybuilder | Testosterone; nandrolone—several months | NR | Collapse during a weight-lifting workout at the gym | 530 g—Marked left and right ventricular hypertrophy | Extensive perivascular fibrosis of intramural coronary arteries—interstitial fibrosis | NR | Unspecified SCD |
| Dickerman, R. D. et al. (1995) | 20; M; 180 cm; 100.7 kg | 31.08 | No past or family history of cardiac disease | Bodybuilder | Methenolone depot; veterinarian testosterone enanthate—just complete a 3-month cycle | NR | Sudden witnessed death | 515 g (0.51% of body weight)—Signs of concentric left ventricular hypertrophy | Mild atherosclerosis | NR | Unspecified SCD |
| Hausmann, R. et al. (1998) | 23; M; 192 cm; 94 kg | 25.50 | NR | Bodybuilder | Testosterone cyclopentilpropionate; methenolone enanthate; mesterolone—9 months | Other performance-enhancing drugs (liothyronine hydrochloride, clenbuterol hydrochloride) | Found unconscious at home in his bed | 500 g (0.53% of body weight)—Cardiac hypertrophy, right ventricle dilatation, focal induration of endocardium | Enlargement and nuclear polymorphism of the left ventricular muscle fibers. Disseminated focal necrosis and interstitial fibrosis | Urine: Mesterolone, methandienone, testosterone, nandrolone, clenbuterol | Unspecified SCD |
| Fineschi, V. et al. (2001) | 32; M; 189 cm; 90 kg | 25.20 | No history of disease | Bodybuilder | Testosterone propionate; nandrolone—several months | NR | Sudden loss of consciousness during a weight lifting workout | 450 g (0.50% of body weight)—Normal heart measures (14 × 14 × 4 cm)—Normal valves, endocardium, and coronary arteries—one grayish zone in the left ventricle myocardium | Infarct necrosis corresponding to the grayish zone—some foci of contraction band necrosis and fibrosis | Urine: Metabolites of nandrolone, metabolites of stanozolol | SCD most likely related to adrenergic stress |
| 29; M; 166 cm; 72 kg | 26.13 | His medical history was unremarkable | Bodybuilder | Nandrolone; stanozolol—several months | NR | Found unconscious at home in his bed | 390 g (0.54% of body weight)—Normal heart measures (11 × 10 × 5 cm)—Normal valves, endocardium, and coronary arteries | Occasional isolated myocardial cells with contraction band and segmentation | Urine: Metabolites of nandrolone, metabolites of stanozolol | Unspecified SCD | |
| Fineschi, V. et al. (2005) | 30; M; 178 cm; 90 kg | 28.41 | NR | Bodybuilder | Nandrolone decanoate—6 months | Unspecified other drugs | Sudden collapse at home | 400g (0.44% of body weight) —Scattered fatty streaks in coronary arteries | Focal myocardial fibrosis | Urine: Norandrosterone. Blood: nandrolone | Unspecified SCD |
| Di Paolo, M. et al. (2007) | 29; M; 190 cm; 127 kg | 35.2 | No prior history of disease. No family history of cardiac disease under the age of 50 | Bodybuilder | History of use of unspecified AAS—unspecified | NR | Sudden loss of consciousness during the first minutes of a spin bike lesson | 490 g (0.39% of body weight)—Normal hearth wall thickness, normal valve, normal coronary arteries | Severe epicardial interstitial fibrosis, small vessel disease | Negative | Unspecified SCD |
| 27; M; 190 cm; 100 kg | 25.8 | No prior history of disease. No family history of cardiac disease under the age of 50 | Bodybuilder | History of use of unspecified AAS—unspecified | NR | Sudden illness while he was at a night club | 360 g (0.36% of body weight)—Normal hearth wall thickness, normal valve, normal coronary arteries | Mild focal epicardial interstitial fibrosis, small vessel disease | Urine: Stanozolol, testosterone | Unspecified SCD | |
| 37; F; 161 cm; 71 kg | 27.4 | No prior history of disease. No family history of cardiac disease under the age of 50 | Bodybuilder and weight lifter | History of use of unspecified AAS—unspecified | NR | Found dead in her car | 310 g (0.44% of body weight) —Normal hearth wall thickness, normal valve, normal coronary arteries | Moderate focal epicardial interstitial fibrosis, small vessel disease | Negative | Unspecified SCD | |
| 31; M; 175 cm; 79 kg | 25.8 | No prior history of disease. No family history of cardiac disease under the age of 50 | Bodybuilder | History of use of unspecified AAS—unspecified | NR | Found dead in his bedroom: alive 7 h before | 400 g (0.51% of body weight)—Normal hearth wall thickness, normal valve, normal coronary arteries | Moderate epicardial interstitial fibrosis, small vessel disease | Urine: Stanozolol | Unspecified SCD | |
| Fanton, L. et al. (2009) | 19; M | NR | No history of cardiac disease | Weight lifter | History of use of unspecified AAS—unspecified | NR | SD during training | 360 g—Left ventricle apoplexy | Multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD |
| 22; M | NR | No history of cardiac disease | PE teacher | History of use of unspecified AAS—unspecified | NR | SD during training | 520 g—Left ventricle apoplexy | Multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD | |
| 25; M | NR | No history of cardiac disease | Bodybuilder | History of use of unspecified AAS—unspecified | NR | SD during training | 460 g—Disseminated myocarditis | Multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD | |
| 28; M | NR | No history of cardiac disease | Soccer player | History of use of unspecified AAS—unspecified | NR | SD during training | 380 g—Disseminated myocarditis | Multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD | |
| 54; M | NR | No history of cardiac disease | Marathon runner | History of use of unspecified AAS—unspecified | NR | SD during training | 410 g—Coronary thrombosis and dilated cardiomyopathy | multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD | |
| 48; M | NR | No history of cardiac disease | Marathon runner | History of use of unspecified AAS—unspecified | NR | SD during training | 430 g—Left ventricle hypertrophy | Multiple focal areas of necrosis, myolysis, scarring fibrosis | NR | Unspecified SCD | |
| Thiblin, I. et al. (2009) | 29; F; 172 cm; 76 kg | 25.7 | No history of disease | Fitness athlete | History of use of unspecified AAS—unspecified | Unspecified other drugs | Found naked in a prone position on the floor beside her bed, with a pillow partly under her body | 331 g (0.44% of body weight)—Normal heart measures—Normal coronary arteries, with an isolated flat area of fatty thickening in the proximal part of the left anterior descending (LAD) coronary artery. | Lymphocytic infiltration around several middle-sized and small intramural vessels—minimal myocardial necrosis | Blood: ephedrine, norephedrine. Urine: testosterone, metabolites of stanozolol, boldenone | Sudden cardiac arrhythmia, possibly related to the combination of an otherwise unspecified inflammatory process in the heart and the acute influence of ASS and ephedrine |
| Montisci, M. et al. (2012) | 32; M; 180 cm; 110 kg | 33.95 | NR | Bodybuilder | History of use of unspecified AAS—7 years (recently withdraw) | NR | Found dead at home in his bed | 450 g (0.41% of body weight)—11 × 9.5 cm—cardiomegaly, concentric left ventricular hypertrophy, normal valve, normal coronary arteries | Hypertrophic myocytes, focal disarray, interstitial and replacement fibrosis, foci of lymphoplasma cellular infiltrates (CD3+), with edema and patchy necrosis | Negative | Concentric left ventricular hypertrophy, focal acute myocarditis. |
| 32; M; 178 cm; 94 kg | 29.67 | At last screening, nonspecific repolarization changes were found at ECG | Cycler | History of use of unspecified AAS—several years | NR | SD after a dentistry visit | 580 g (0.62% of body weight)—12.5 × 11 cm—Cardiomegaly, hypertrophy, biventricular dilatation, normal valve, non-obstructive LAD stenosis | Hypertrophic myocytes, foci of necrosis, replacement fibrosis, LAD 50% stenosis, fibrofatty replacement | Negative | Inflammatory dilated cardiomyopathy with subacute-chronic stages, hemorrhagic pulmonary infarction | |
| 25; M, 185 cm; 125 kg | 36.52 | An ECG performed 5y before death was normal | Bodybuilder | Circumstantial finding of unspecified use of AAS—unspecified | Unspecified other performance-enhancing drugs | SD while sleeping | 390 g (0.31% of body weight)—10.5 × 9.5 cm—normal hearth wall thickness, normal valve, normal coronary arteries | Inflammatory infiltrate, myocyte necrosis | Urine: Testosterone, epitestosterone, nortestosterone | Eosinophilic myocarditis | |
| Lusetti, M. et al. (2015) | 39 (mean age); M (All 6 cases) | NR | NR | NR | History of use of unspecified AAS—unspecified | NR | Sudden unwitnessed death | Normal hearth wall thickness, normal valve, normal coronary arteries. In one case: 490 g (0.54% of body weight) | Interstitial fibrosis (6 cases); perivascular fibrosis (4 cases); perineural fibrosis within the left ventricle (2 cases); fibroadipous metaplasia (2 cases); contraction band necrosis (2 cases); Myocyte segmentation (2 cases); Intercalated disc widening (2 cases); myocyte hypertrophy (3 cases); coronary intimal and media thickening (4 cases) | Blood: Ethanol (1 case). Urine and hair: nandrolone (3 cases), Testosterone (3 cases) | Sudden cardiac arrhythmia |
| Lichtenfeld, J. et al. (2016) | 13; M | NR | No prior history of disease. An episode of syncope with exertion 1 week before cardiac arrest. No family history of sudden death, hypertrophic cardiomyopathy, or heart rhythm abnormalities | Sprinter | Physical Phenotype suggesting AAS use | NR | Sudden cardiac arrest while performing timed wind sprints at a competitive sports camp | 465 g—Cardiomegaly, marked LV Hypertrophy | Foci of myofibrillar disarray, the proliferation of fibroblasts consistent with early fibrosis, and enlarged myofibers with the heterogeneity of nuclear size including “box-car” nuclei | NR | Sudden cardiac arrest followed by brain death |
| Lusetti, M. et al. (2018) | 32; M | NR | No “officially” medically prescribed drug treatment at the time of death. | NR | History of use of unspecified AAS—unspecified | NR | unspecified SD | 390 g—Left ventricular hypertrophy | Myocardial fibrosis | Urine: Nandrolone, Testosterone. Blood: Methadone, Citalopram, Clozapine, Venlafaxine, Lorazepam, Phenobarbital, THC | Unspecified SCD |
| 32; M | NR | No “officially” medically prescribed drug treatment at the time of death. | NR | History of use of unspecified AAS—unspecified | NR | unspecified SD | 360 g | Fatty streaks, intima, and media thickening within the coronary arteries | Urine: Boldenone, Clomiphene, Methenolone, Oxandrolone, Stanozolol. Blood: Lorazepam, THC | Unspecified SCD | |
| 33; M | NR | No “officially” medically prescribed drug treatment at the time of death. | NR | History of use of unspecified AAS—unspecified | NR | unspecified SD | 425 g—Left ventricular hypertrophy | Myocyte necrosis | Urine: Testosterone. Blood: Methadone, Cocaine | Unspecified SCD | |
| 39; M | NR | No “officially” medically prescribed drug treatment at the time of death. | NR | History of use of unspecified AAS—unspecified | NR | unspecified SD | 480 g—Left and right ventricular hypertrophy | Myocyte necrosis, Myocardial fibrosis | Urine: Nandrolone. Blood: Morphine, THC | Unspecified SCD | |
| 29; M | NR | No “officially” medically prescribed drug treatment at the time of death. | NR | History of use of unspecified AAS—unspecified | NR | unspecified SD | 340 g | NR | Urine: Nandrolone, Testosterone. Blood: morphine, THC, Ethanol | Unspecified SCD | |
| Hernandez-Guerra, A. I. et al. (2019) | 24; M; 178 cm; 85 Kg | 26.8 | No past or family history of cardiac disease. One episode of precordial pain some months before. | NR | stanozolol, testosterone, mesterolone, nandrolone—6 months | tamoxifen | Sudden death at home | 420 g (0.49% of body weight)—Cardiomegaly, Normal ventricular thickness, >75% Stenosis of the left main trunk and the LAD, areas of scarring located at the intersection between the posterior wall and the posterior third of the septum | Acute myocardial infarction, myocytes hypertrophy, small intramyocardial vessel disease | Blood: Ethanol, Stanozolol, Nandrolone | Acute myocardial infarction |
References
- Hernández-Guerra, A.I.; Tapia, J.; Menéndez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic steroids abuse: Case report and literature review. For. Sci. Res. 2019, 4, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, D.; D Kotzalidis, G.; Del Casale, A.; Rosaria Aromatario, M.; Pomara, C.; Girardi, P.; Sani, G. Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr. Neuropharmacol. 2015, 13, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Al-harbi, F.F.; Gamaleddin, I.; Alsubaie, E.G.; Al-Surimi, K.M. Prevalence and Risk Factors Associated with Anabolic-androgenic Steroid Use: A Cross-sectional Study among Gym Users in Riyadh, Saudi Arabia. Oman Med. J. 2020, 35, e110. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E. Current topics in testosterone replacement of hypogonadal men. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, R.; Chu, K.; Ramasamy, R. Novel therapy for male hypogonadism. Curr. Urol. Rep. 2018, 19, 63. [Google Scholar] [CrossRef]
- Orr, R.; Singh, M.F. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders. Drugs 2004, 64, 725–750. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kovac, J.R. Novel uses for the anabolic androgenic steroids nandrolone and oxandrolone in the management of male health. Curr. Urol. Rep. 2016, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Frati, P.; P Busardo, F.; Cipolloni, L.; De Dominicis, E.; Fineschi, V. Anabolic androgenic steroid (AAS) related deaths: Autoptic, histopathological and toxicological findings. Curr. Neuropharmacol. 2015, 13, 146–159. [Google Scholar] [CrossRef]
- Pereira, E.; Moyses, S.J.; Ignácio, S.A.; Mendes, D.K.; Da Silva, D.S.; Carneiro, E.; Johann, A.C.B.R. Anabolic steroids among resistance training practitioners. PLoS ONE 2019, 14, e0223384. [Google Scholar]
- Reyes-Vallejo, L. Current use and abuse of anabolic steroids. Actas Urol. Esp. 2020, 44, 309–313. [Google Scholar] [CrossRef]
- Fineschi, V.; Neri, M.; Di Donato, S.; Pomara, C.; Riezzo, I.; Turillazzi, E. An immunohistochemical study in a fatality due to ovarian hyperstimulation syndrome. Int. J. Legal Med. 2006, 120, 293–299. [Google Scholar] [CrossRef]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef]
- Kanayama, G.; Pope Jr, H.G. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol. Cell Endocrinol. 2018, 464, 4–13. [Google Scholar] [CrossRef]
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef]
- Bertozzi, G.; Salerno, M.; Pomara, C.; Sessa, F. Neuropsychiatric and behavioral involvement in AAS abusers. A literature review. Medicina 2019, 55, 396. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Cipolloni, L.; Bertozzi, G.; Messina, G.; Di Mizio, G.; Pomara, C. Anabolic-androgenic steroids and brain injury: miRNA evaluation in users compared to cocaine abusers and elderly people. Aging 2020, 12, 15314. [Google Scholar] [CrossRef] [PubMed]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Sani, G. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Sessa, F.; Maglietta, F.; Cipolloni, L.; Salerno, M.; Fiore, C.; Pomara, C. Immunodeficiency as a side effect of anabolic androgenic steroid abuse: A case of necrotizing myofasciitis. For. Sci. Med. Pathol. 2019, 15, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Sessa, F.; Albano, G.D.; Sani, G.; Maglietta, F.; Roshan, M.H.; Salerno, M. The role of anabolic androgenic steroids in disruption of the physiological function in discrete areas of the central nervous system. Mol. Neurobiol. 2018, 55, 5548–5556. [Google Scholar] [CrossRef]
- Pomara, C.; Neri, M.; Bello, S.; Fiore, C.; Riezzo, I.; Turillazzi, E. Neurotoxicity by synthetic androgen steroids: Oxidative stress, apoptosis, and neuropathology: A review. Curr. Neuropharmacol. 2015, 13, 132–145. [Google Scholar] [CrossRef]
- Pomara, C.; Barone, R.; Marino Gammazza, A.; Sangiorgi, C.; Barone, F.; Pitruzzella, A.; Locorotondo, N.; Di Gaudio, F.; Salerno, M.; Maglietta, F.; et al. Effects of nandrolone stimulation on testosterone biosynthesis in leydig cells. J. Cell Physiol. 2016, 231, 1385–1391. [Google Scholar] [CrossRef]
- Albano, G.D.; Sessa, F.; Messina, A.; Monda, V.; Bertozzi, G.; Maglietta, F.; Giugliano, P.; Vacchiano, G.; Gabriella, M.; Salerno, M. AAS and organs damage: A focus on Nandrolone effects. Acta Med. Mediter. 2017, 6, 939–946. [Google Scholar]
- Joukar, S.; Yoosefnia, M.; Naderi-Boldaji, V.; Nasri, H.; Rafie, F. Heart reaction to nandrolone decanoate plus two different intensities of endurance exercise: Electrocardiography and stereological approach. Addict. Health 2018, 10, 180. [Google Scholar]
- Wadthaisong, M.; Witayavanitkul, N.; Bupha-Intr, T.; Wattanapermpool, J.; De Tombe, P.P. Chronic high-dose testosterone treatment: Impact on rat cardiac contractile biology. Physiol. Rep. 2019, 7, e14192. [Google Scholar] [CrossRef]
- Climstein, M.; O’Shea, P.; Adams, K.J.; DeBeliso, M. The effects of anabolic-androgenic steroids upon resting and peak exercise left ventricular heart wall motion kinetics in male strength and power athletes. J. Sci. Med. Sport 2003, 6, 387–397. [Google Scholar] [CrossRef]
- Pomara, C.; D’Errico, S.; Riezzo, I.; De Cillis, G.P.; Fineschi, V. Sudden cardiac death in a child affected by Prader-Willi syndrome. Int. J. Legal Med. 2005, 119, 153–157. [Google Scholar] [CrossRef]
- Chatwin, C.; Measham, F.; O’Brien, K.; Sumnall, H. New drugs, new directions? Research priorities for new psychoactive substances and human enhancement drugs. Int. J. Drug Policy 2017, 40, 1–5. [Google Scholar] [CrossRef]
- Nieschlag, E.; Vorona, E. Mechanisms in endocrinology: Medical consequences of doping with anabolic androgenic steroids: Effects on reproductive functions. Eur. J. Endocrinol. 2015, 173, 47–58. [Google Scholar] [CrossRef]
- Ahlgrim, C.; Guglin, M. Anabolics and cardiomyopathy in a bodybuilder: Case report and literature review. J. Card Fail. 2009, 15, 496–500. [Google Scholar] [CrossRef]
- Angoorani, H.; Narenjiha, H.; Tayyebi, B.; Ghassabian, A.; Ahmadi, G.; Assari, S. Amphetamine use and its associated factors in body builders: A study from Tehran, Iran. Arch. Med. Sci. 2012, 8, 362. [Google Scholar] [CrossRef]
- Bilard, J.; Ninot, G.; Hauw, D. Motives for illicit use of doping drugs among athletes calling a national antidoping phone-help service: An exploratory study. Subst. Use Misuse 2011, 46, 359–367. [Google Scholar] [CrossRef]
- Sagoe, D.; McVeigh, J.; Bjørnebekk, A.; Essilfie, M.S.; Andreassen, C.S.; Pallesen, S. Polypharmacy among anabolic-androgenic steroid users: A descriptive metasynthesis. Subst. Abuse Treat. Prev. Policy 2015, 10, 12. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of sudden cardiac death: Global and regional perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Doolan, A.; Semsarian, C.; Langlois, N. Causes of sudden cardiac death in young Australians. Med. J. Aust. 2004, 180, 110–112. [Google Scholar] [CrossRef]
- Muller, D.; Agrawal, R.; Arntz, H.R. How sudden is sudden cardiac death. Circulation 2006, 114, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Farb, A.; Weber, K.T. Pathologic remodeling of the myocardium in a weightlifter taking anabolic steroids case report. Blood Press 1993, 2, 213–216. [Google Scholar] [CrossRef]
- Dickerman, R.D.; Schaller, F.; Prather, I.; McConathy, W.J. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology 1995, 86, 172–173. [Google Scholar] [CrossRef]
- Hausmann, R.; Hammer, S.; Betz, P. Performance enhancing drugs (doping agents) and sudden death–a case report and review of the literature. Int. J. Legal Med. 1998, 111, 261–264. [Google Scholar] [CrossRef]
- Fineschi, V.; Baroldi, G.; Monciotti, F.; Reattelli, L.P.; Turillazzi, E. Anabolic steroid abuse and cardiac sudden death: A pathologic study. Arch. Pathol. Lab. Med. 2001, 125, 253–255. [Google Scholar]
- Fineschi, V.; Riezzo, I.; Centini, F.; Silingardi, E.; Licata, M.; Beduschi, G.; Karch, S.B. Sudden cardiac death during anabolic steroid abuse: Morphologic and toxicologic findings in two fatal cases of bodybuilders. Int. J. Legal Med. 2007, 121, 48–53. [Google Scholar] [CrossRef]
- Di Paolo, M.; Agozzino, M.; Toni, C.; Luciani, A.B.; Molendini, L.; Scaglione, M.; Arbustini, E. Sudden anabolic steroid abuse-related death in athletes. Int. J. Cardiol. 2007, 114, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Fanton, L.; Belhani, D.; Vaillant, F.; Tabib, A.; Gomez, L.; Descotes, J.; Timour, Q. Heart lesions associated with anabolic steroid abuse: Comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp. Toxicol. Pathol. 2009, 61, 317–323. [Google Scholar] [CrossRef]
- Thiblin, I.; Mobini-Far, H.; Frisk, M. Sudden unexpected death in a female fitness athlete, with a possible connection to the use of anabolic androgenic steroids (AAS) and ephedrine. For. Sci. Int. 2009, 184, 7–11. [Google Scholar] [CrossRef]
- Montisci, M.; El Mazloum, R.; Cecchetto, G.; Terranova, C.; Ferrara, S.D.; Thiene, G.; Basso, C. Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012, 217, 13–18. [Google Scholar] [CrossRef]
- Lusetti, M.; Licata, M.; Silingardi, E.; Bonetti, L.R.; Palmiere, C. Pathological changes in anabolic androgenic steroid users. J. For. Leg. Med. 2015, 33, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Lichtenfeld, J.; Deal, B.J.; Crawford, S. Sudden cardiac arrest following ventricular fibrillation attributed to anabolic steroid use in an adolescent. Cardiol. Young 2016, 26, 996–998. [Google Scholar] [CrossRef]
- Lusetti, M.; Licata, M.; Silingardi, E.; Bonsignore, A.; Palmiere, C. Appearance/image- and performance-enhancing drug users: A forensic approach. Am. J. For. Med. Pathol. 2018, 39, 325–329. [Google Scholar] [CrossRef]
- Narayan, S.M.; Wang, P.J.; Daubert, J.P. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 70–88. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Hutter, A.M.; Weiner, R.B. Sudden cardiac death in athletes. Methodist DeBakey Cardiovasc. J. 2016, 12, 76. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Klossner, D.; Drezner, J.A. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation 2011, 123, 1594–1600. [Google Scholar] [CrossRef]
- Ackerman, M.; Atkins, D.L.; Triedman, J.K. Sudden cardiac death in the young. Circulation 2016, 133, 1006–1026. [Google Scholar] [CrossRef]
- Sheppard, M.N. Aetiology of sudden cardiac death in sport: A histopathologist’s perspective. Br. J. Sports Med. 2012, 46, i15–i21. [Google Scholar] [CrossRef]
- Wolk, R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace 2000, 2, 216–223. [Google Scholar] [CrossRef]
- Aro, A.L.; Reinier, K.; Phan, D.; Teodorescu, C.; Uy-Evanado, A.; Nichols, G.A.; Chugh, S.S. Left-ventricular geometry and risk of sudden cardiac arrest in patients with preserved or moderately reduced left-ventricular ejection fraction. Europace 2017, 19, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Lippi, G.; Franchini, M.; Banfi, G.; Guidi, G.C. Sudden cardiac death in young athletes. Intern. Med. 2008, 47, 1373–1378. [Google Scholar] [CrossRef][Green Version]
- Smit, D.L.; De Hon, O.; Venhuis, B.J.; Den Heijer, M.; De Ronde, W. Baseline characteristics of the HAARLEM study: 100 male amateur athletes using anabolic androgenic steroids. Scand. J. Med. Sci. Sports 2020, 30, 531–539. [Google Scholar] [CrossRef]
- De Ronde, W.; Smit, D.L. Anabolic androgenic steroid abuse in young males. Endocronol. Connect. 2020, 9, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Cascio, O.; Bertozzi, G.; Sessa, F.; Messina, A.; Monda, V.; Pomara, C. Anabolic androgenic steroids and carcinogenicity focusing on Leydig cell: A literature review. Oncotarget 2018, 9, 19415. [Google Scholar] [CrossRef]
- Monda, V.; Salerno, M.; Sessa, F.; Bernardini, R.; Valenzano, A.; Marsala, G.; Zammit, C.; Avola, R.; Carotenuto, M.; Messina, G.; et al. Functional changes of orexinergic reaction to psychoactive substances. Mol. Neurobiol. 2018, 55, 6362–6368. [Google Scholar] [CrossRef]
- Rothman, R.D.; Weiner, R.B.; Pope, H.; Kanayama, G.; Hutter, A.M.; Fifer, M.A.; Baggish, A.L. Anabolic androgenic steroid induced myocardial toxicity: An evolving problem in an ageing population. BMJ Case Rep. 2011. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.Y.; Alqallaf, A.; Abdella, N. Anabolic androgenic steroid-induced cardiomyopathy, stroke and peripheral vascular disease. BMJ Case Rep. 2011. [Google Scholar] [CrossRef]
- Baggish, A.L.; Weiner, R.B.; Kanayama, G.; Hudson, J.I.; Lu, M.T.; Hoffmann, U.; Pope, H.G., Jr. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 2017, 135, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Melchert, R.B.; Welder, A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1995, 27, 1252–1262. [Google Scholar] [CrossRef]
- Monda, V.; Salerno, M.; Fiorenzo, M.; Villano, I.; Viggiano, A.; Sessa, F.; Triggiani, A.I.; Cibelli, G.; Valenzano, A.; Marsala, G.; et al. Role of sex hormones in the control of vegetative and metabolic functions of middle-aged women. Front. Physiol. 2017, 8, 773. [Google Scholar] [CrossRef]
- Shirpoor, A.; Heshmatian, B.; Tofighi, A.; Eliasabad, S.N.; Kheradmand, F.; Zerehpoosh, M. Nandrolone administration with or without strenuous exercise increases cardiac fatal genes overexpression, calcium/calmodulin-dependent protein kinaseiiδ, and monoamine oxidase activities and enhances blood pressure in adult wistar rats. Gene 2019, 697, 131–137. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Mullens, W.; Delrue, L.; Goethals, M.; De Bruyne, B.; Wijns, W.; Geelen, P.; Verstreken, S.; Wellens, F.; Bartunek, J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J. Am. Coll. Cardiol. 2008, 51, 129–136. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Scholz, D.G.; Hagen, P.T.; Ilstrup, D.M.; Edwards, W.D. Age-related changes in normal human hearts during the first 10 decades of life. Part II (maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin. Proc. 1988, 63, 137–146. [Google Scholar] [CrossRef]
- Mandal, R.; Loeffler, A.G.; Salamat, S.; Fritsch, M.K. Organ weight changes associated with body mass index determined from a medical autopsy population. Am. J. For. Med. Pathol. 2012, 33, 382–389. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pomara, C.; Schiavone, S.; Turillazzi, E. Oxidative-nitrosative stress and myocardial dysfunctions in sepsis: Evidence from the literature and postmortem observations. Mediat. Inflamm. 2016, 2016, 3423450. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.C.; Lawrence, C. Anabolic steroid abuse and cardiac death. Med. J. Aust. 1993, 158, 346–348. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Martinez, C.M.; Gennis, P.; Gallagher, E.J. The cardiac toxicity of anabolic steroids. Prog. Cardiovasc. Dis. 1998, 41, 1–15. [Google Scholar] [CrossRef]
- Sculthorpe, N.; Grace, F.; Jones, P.; Davies, B. Evidence of altered cardiac electrophysiology following prolonged androgenic anabolic steroid use. Cardiovasc. Toxicol. 2010, 10, 239–243. [Google Scholar] [CrossRef][Green Version]
- Carbone, A.; D’Andrea, A.; Riegler, L.; Scarafile, R.; Pezzullo, E.; Martone, F.; America, R.; Liccardo, B.; Galderisi, M.; Bossone, E.; et al. Cardiac damage in athlete’s heart: When the “supernormal” heart fails! World J. Cardiol. 2017, 9, 470. [Google Scholar] [CrossRef]
- Papamitsou, T.; Barlagiannis, D.; Papaliagkas, V.; Kotanidou, E.; Dermentzopoulou-Theodoridou, M. Testosterone-induced hypertrophy, fibrosis and apoptosis of cardiac cells–an ultrastructural and immunohistochemical study. Med. Sci. Monit. 2011, 17, 266. [Google Scholar] [CrossRef]
- Riezzo, I.; Di Paolo, M.; Neri, M.; Bello, S.; Cantatore, S.; D’Errico, S.; Dinucci, D.; Parente, R.; Pomara, C.; Rabozzi, R.; et al. Anabolic steroid-and exercise-induced cardio-depressant cytokines and myocardial β1 receptor expression in CD1 mice. Curr. Pharm. Biotechnol. 2011, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Hudson, J.I.; Pope, H.G., Jr. Anabolic-androgenic steroid use and body image in men: A growing concern for clinicians. Psychother. Psychosom. 2020, 89, 65–73. [Google Scholar] [CrossRef]
- Pope, H.; Brower, K.J. Treatment of anabolic-androgenic steroid related disorders. In The American Psychiatric Publishing Textbook of Substance Abuse Treatment; American Psychiatric Publishing: Washington, DC, USA, 2008; pp. 237–246. [Google Scholar]
- Sessa, F.; Salerno, M.; Bertozzi, G.; Cipolloni, L.; Messina, G.; Aromatario, M.; Polo, L.; Turillazzi, E.; Pomara, C. miRNAs as novel biomarkers of chronic kidney injury in anabolic-androgenic steroid users: An experimental study. Front. Pharmacol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Arsenos, P.; Trachanas, K.; Dilaveris, P.; Antoniou, C.; Tsiachris, D.; Tousoulis, D. Signal-averaged electrocardiography: Past, present, and future. J. Arrhythm. 2018, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Maior, A.S.; Menezes, P.; Pedrosa, R.C.; Carvalho, D.P.; Soares, P.P.; Nascimento, J.H.M. Abnormal cardiac repolarization in anabolic androgenic steroid users carrying out submaximal exercise testing. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.; Bozkurt, I.; Ozdemir, M.; Yavuz, O. Side effect of metenolone enanthate on rats heart in puberty: Morphometrical study. Exp. Toxicol. Pathol. 2013, 65, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Strano-Rossi, S.; Fiore, C.; Chiarotti, M.; Centini, F. Analytical techniques in androgen anabolic steroids (AASs) analysis for antidoping and forensic purposes. Mini Rev. Med. Chem. 2011, 11, 451–458. [Google Scholar] [CrossRef]
- Sessa, F.; Franco, S.; Picciocchi, E.; Geraci, D.; Chisari, M.G.; Marsala, G.; Polito, A.N.; Sorrentino, M.; Tripi, G.; Salerno, M.; et al. Addictions substance free during lifespan. Acta Med. Mediter. 2018, 34, 2081–2087. [Google Scholar]
- Moacir, M.; Silva-Neto, J.A.; Neto, O.B. Acute interruption of treatment with nandrolone decanoate is not sufficient to reverse cardiac autonomic dysfunction and ventricular repolarization disturbances in rats. Steroids 2018, 132, 12–17. [Google Scholar]
- Olivares, E.L.; Silveira, A.L.; Fonseca, F.V.; Silva-Almeida, C.; Côrtes, R.S.; Pereira-Junior, P.P.; Nascimento, J.H.M.; Reis, L.C. Administration of an anabolic steroid during the adolescent phase changes the behavior, cardiac autonomic balance and fluid intake in male adult rats. Physiol. Behav. 2014, 126, 15–24. [Google Scholar] [CrossRef]
- Marocolo, M.; Maior, A.S.; Katayama, P.L.; Mota, G.R.D.; Neto, O.B.; Lauria, A.D.A.; Santos, E.L. Anabolic steroid treatment induces cardiac autonomic dysfunction in rats: Time-course of heart rate variability. Am. J. Biomed. Eng. 2013, 3, 54–62. [Google Scholar]
- Tanno, A.P.; Cunha, T.S.; Fernandes, T.; Guzzoni, V.; da Silva, C.A.; de Oliveira, E.M.; Costa Sampaio Moura, M.J.; Marcondes, F.K. Effects of nandrolone and resistance training on the blood pressure, cardiac electrophysiology, and expression of atrial β-adrenergic receptors. Life Sci. 2013, 92, 1029–1035. [Google Scholar]
- Medei, E.; Marocolo, M.; de Carvalho Rodrigues, D.; Arantes, P.C.; Takiya, C.M.; Silva, J.; Rondinelli, E.; dos Santos Goldenberg, R.C.; Campos de Carvalho, A.C.; Nascimento, J.H.M. Chronic treatment with anabolic steroids induces ventricular repolarization disturbances: Cellular, ionic and molecular mechanism. J. Mol. Cell Cardiol. 2010, 49, 165–175. [Google Scholar] [CrossRef]
- Phillis, B.D.; Abeywardena, M.Y.; Adams, M.J.; Kennedy, J.A.; Irvine, R.J. Nandrolone potentiates arrhythmogenic effects of cardiac ischemia in the rat. Toxicol. Sci. 2007, 99, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Binayi, F.; Joukar, S.; Najafipour, H.; Karimi, A.; Abdollahi, F.; Masumi, Y. The effects of nandrolone decanoate along with prolonged low-intensity exercise on susceptibility to ventricular arrhythmias. Cardiovasc. Toxicol. 2016, 16, 23–33. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Di Mizio, G.; Bertozzi, G.; Messina, G.; Tomaiuolo, B.; Pisanelli, D.; Maglietta, F.; Ricci, P.; Pomara, C. Anabolic androgenic steroids: Searching new molecular biomarkers. Front. Pharmacol. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Sessa, F.; Maglietta, F.; Bertozzi, G.; Salerno, M.; Di Mizio, G.; Messina, G.; Montana, A.; Ricci, P.; Pomara, C. Human brain injury and mirnas: An experimental study. Int. J. Mol. Sci. 2019, 20, 1546. [Google Scholar] [CrossRef]
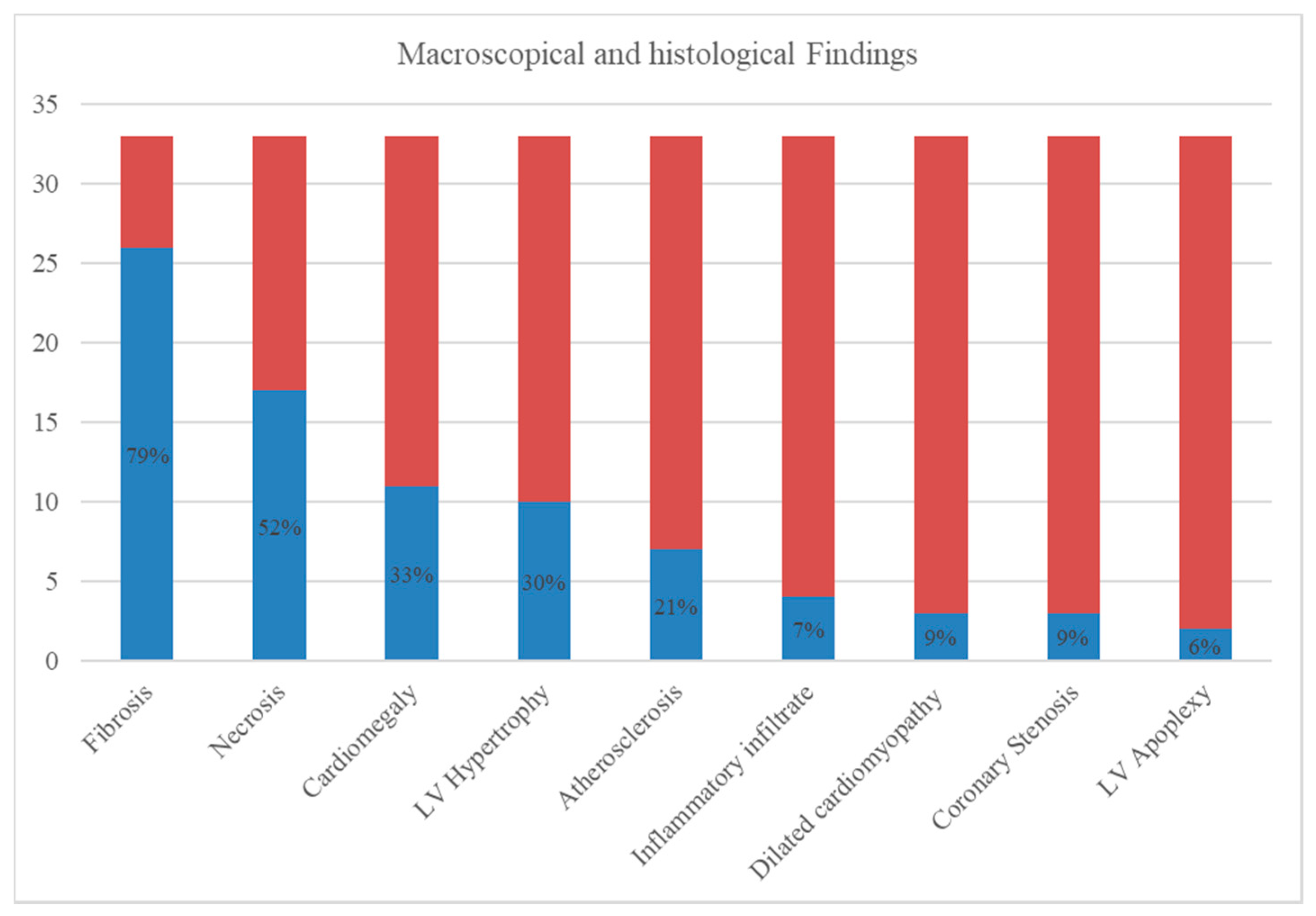
| Author | Year | Number of Cases | Study Type |
|---|---|---|---|
| Campbell, S.E. et al. [37] | 1993 | 1 | Case report |
| Dickerman, R.D. et al. [38] | 1995 | 1 | Case report |
| Hausmann, R. et al. [39] | 1998 | 1 | Case report |
| Fineschi, V. et al. [40] | 2001 | 2 | Case series |
| Fineschi, V. et al. [41] | 2007 | 1 1 | Case series |
| Di Paolo, M. et al. [42] | 2007 | 4 | Letter to the editor |
| Fanton, L. et al. [43] | 2009 | 6 2 | Retrospective study |
| Thiblin, I. et al. [44] | 2009 | 1 | Case report |
| Montisci, M. et al. [45] | 2012 | 3 3 | Case series |
| Lusetti, M. et al. [46] | 2015 | 6 | Retrospective study |
| Lichtenfeld, J. et al. [47] | 2016 | 1 | Case report |
| Lusetti, M. et al. [48] | 2018 | 5 | Retrospective study |
| Hernandez-Guerra, A.I. et al. [1] | 2019 | 1 | Case report |
| Toxicological Findings | Number of Cases | % of Total Cases |
|---|---|---|
| Nandrolone | 10 | 30% |
| Testosterone | 9 | 27% |
| Stanozolol | 7 | 21% |
| Boldenon | 2 | 6% |
| Norandrosterone | 1 | 3% |
| Mesterolone | 1 | 3% |
| Methandienone | 1 | 3% |
| Epitestosterone | 1 | 3% |
| Nortestosterone | 1 | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Li Rosi, G.; Di Nunno, N.; et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina 2020, 56, 587. https://doi.org/10.3390/medicina56110587
Torrisi M, Pennisi G, Russo I, Amico F, Esposito M, Liberto A, Cocimano G, Salerno M, Li Rosi G, Di Nunno N, et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina. 2020; 56(11):587. https://doi.org/10.3390/medicina56110587
Chicago/Turabian StyleTorrisi, Marco, Giuliana Pennisi, Ilenia Russo, Francesco Amico, Massimiliano Esposito, Aldo Liberto, Giuseppe Cocimano, Monica Salerno, Giuseppe Li Rosi, Nunzio Di Nunno, and et al. 2020. "Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review" Medicina 56, no. 11: 587. https://doi.org/10.3390/medicina56110587
APA StyleTorrisi, M., Pennisi, G., Russo, I., Amico, F., Esposito, M., Liberto, A., Cocimano, G., Salerno, M., Li Rosi, G., Di Nunno, N., & Montana, A. (2020). Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina, 56(11), 587. https://doi.org/10.3390/medicina56110587









