The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bianchi, D.W.; Wilkins-Haug, L. Integration of noninvasive DNA testing for aneuploidy into prenatal care: What has happened since the rubber met the road? Clin. Chem. 2014, 60, 78–87. [Google Scholar] [CrossRef]
- Lo, Y.D.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Hahn, S.; Huppertz, B.; Holzgreve, W. Fetal cells and cell free fetal nucleic acids in maternal blood: New tools to study abnormal placentation? Placenta 2005, 26, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, A.; Samura, O.; Zhen, D.K.; Falco, V.; Farina, A.; Bianchi, D.W. Apoptosis in fetal nucleated erythrocytes circulating in maternal blood. Prenat. Diagn. 2000, 20, 886–889. [Google Scholar] [CrossRef]
- Quezada, M.S.; Gil, M.M.; Francisco, C.; Oròsz, G.; Nicolaides, K.H. Screening for trisomies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10-11 weeks’ gestation and the combined test at 11-13 weeks. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 36–41. [Google Scholar] [CrossRef]
- Gil, M.M.; Quezada, M.S.; Revello, R.; Akolekar, R.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 249–266. [Google Scholar] [CrossRef]
- Lo, Y.D.; Tein, M.S.; Lau, T.K.; Haines, C.J.; Leung, T.N.; Poon, P.M.; Wainscoat, J.S.; Johnson, P.J.; Chang, A.M.; Hjelm, N.M. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 1998, 62, 768–775. [Google Scholar] [CrossRef]
- Greeley, E.T.; Kessler, K.A.; Vohra, N. Clinical Applications of Noninvasive Prenatal Testing. J. Fetal. Med. 2015, 2, 11–17. [Google Scholar] [CrossRef][Green Version]
- Skrzypek, H.; Hui, L. Noninvasive prenatal testing for fetal aneuploidy and single gene disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 42, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Noninvasive Prenatal Testing for Trisomies 21, 18, and 13, Sex Chromosome Aneuploidies, and Microdeletions: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2019, 19, 1–166. [Google Scholar]
- Serapinas, D.; Bekasene, D.; Bandzeviciene, R.; Narbekovas, A.; Valantinas, A. Current position of legislative approaches to the grant of patent law on isolated human genes. Med Stud./Studia Med. 2018, 34, 264–266. [Google Scholar] [CrossRef]
- Mackie, F.L.; Hemming, K.; Allen, S.; Morris, R.K.; Kilby, M.D. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: A systematic review and bivariate meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Iwarsson, E.; Jacobsson, B.; Dagerhamn, J.; Davidson, T.; Bernabé, E.; Heibert Arnlind, M. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population—A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2017, 96, 7–18. [Google Scholar] [CrossRef]
- Serapinas, D.; Sukys, M.; Bardziliauskas, P. Associations of prenatally detected choroid plexus cysts with biochemical risk for congenital disorders. Med Stud./Studia Med. 2014, 30, 71–74. [Google Scholar] [CrossRef]
- Serapinas, D.; Bartkeviciene, D. Child’s respiratory and sleep health following mid-trimester amniocentesis Danielius Serapinas and Daiva Bartkeviciene Special articles. Arch. Argent Pediatr. 2019, 117, 401–404. [Google Scholar]
- Hooks, J.; Wolfberg, A.J.; Wang, E.T.; Struble, C.A.; Zahn, J.; Juneau, K.; Mohseni, M.; Huang, S.; Bogard, P.; Song, K.; et al. Non-invasive risk assessment of fetal sex chromosome aneuploidy through directed analysis and incorporation of fetal fraction. Prenat. Diagn. 2014, 34, 496–499. [Google Scholar] [CrossRef]
- Mujezinovic, F.; Alfirevic, Z. Procedure-related complications of amniocentesis and chorionic villous sampling: A systematic review. Obstet. Gynecol. 2007, 110, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Audibert, F.; Kagan, K.O.; Paladini, D.; Yeo, G.; Raine-Fenning, N. ISUOG updated consensus statement on the impact of cfDNA aneuploidy testing on screening policies and prenatal ultrasound practice. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 815–816. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Gil, M.; Atanasova, V.; Markova, D. Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X., and Y. Prenat. Diagn. 2013, 33, 575–579. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; del Mar Gil, M.; Quezada, M.S.; Zinevich, Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood. Fetal. Diagn. Ther. 2014, 35, 212–217. [Google Scholar] [CrossRef]
- Eiben, B.; Krapp, M.; Borth, H.; Kutur, N.; Kreiselmaier, P.; Glaubitz, R.; Deutinger, J.; Merz, E. Single Nucleotide Polymorphism-Based Analysis of Cell-Free Fetal DNA in 3000 Cases from Germany and Austria. Ultrasound Int. Open 2015, 1, E8–E11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, I.C.; Puri, R.; Venkataswamy, E.; Tayal, T.; Nampoorthiri, S.; Andrew, C.; Kabra, M.; Bagga, R.; Gowda, M.; Batra, M.; et al. Single Nucleotide Polymorphism-Based Noninvasive Prenatal Testing: Experience in India. J. Obstet. Gynaecol. India 2018, 68, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Curnow, K.J.; Gross, S.J.; Hall, M.P.; Stosic, M.; Demko, Z.; Zimmermann, B.; Hill, M.; Sigurjonsson, S.; Ryan, A.; Banjevic, M.; et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am. J. Obstet. Gynecol. 2014, 211, 527-e1–527-e17. [Google Scholar]
- Ashoor, G.; Syngelaki, A.; Poon, L.C.Y.; Rezende, J.C.; Nicolaides, K.H. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: Relation to maternal and fetal characteristics. Ultrasound Obstet. Gynecol. 2013, 41, 26–32. [Google Scholar] [CrossRef]
- White, K.; Wang, Y.; Kunz, L.H.; Schmid, M. Factors associated with obtaining results on repeat cell-free DNA testing in samples redrawn due to insufficient fetal fraction. J. Matern. Fetal Neonatal Med. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Haghiac, M.; Vora, N.L.; Basu, S.; Johnson, K.L.; Presley, L.; Bianchi, D.W.; De Mouzon, S.H. Increased death of adipose cells, a path to release cell free DNA into systemic circulation of obese women. Obes. Silver Spring Md. 2012, 20, 2213–2219. [Google Scholar] [CrossRef]
- Canick, J.A.; Palomaki, G.E.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat. Diagn. 2013, 33, 667–674. [Google Scholar] [CrossRef]
- Kinnings, S.L.; Geis, J.A.; Almasri, E.; Wang, H.; Guan, X.; McCullough, R.M.; Bombard, A.T.; Saldivar, J.S.; Oeth, P.; Deciu, C. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat. Diagn. 2015, 35, 816–822. [Google Scholar] [CrossRef]
- Wang, E.; Batey, A.; Struble, C.; Musci, T.; Song, K.; Oliphant, A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat. Diagn. 2013, 33, 662–666. [Google Scholar] [CrossRef]
- Hestand, M.S.; Bessem, M.; van Rijn, P.; de Menezes, R.X.; Sie, D.; Bakker, I.; Boon, E.M.; Sistermans, E.A.; Weiss, M.M. Fetal fraction evaluation in non-invasive prenatal screening (NIPS). Eur. J. Hum. Genet. 2019, 27, 198–202. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Messerlian, G.M.; Halliday, J.V. Prenatal Screening for Common Aneuploidies Using Cell-free DNA.; Wilkins-Haug, L., Ed.; UpToDate: Waltham, MA, USA, 2017. [Google Scholar]
- Lutgendorf, M.A.; Stoll, K.A.; Knutzen, D.M.; Foglia, L.M. Noninvasive prenatal testing: Limitations and unanswered questions. Genet. Med. Off. J. Am. Coll. Med. Genet. 2014, 16, 281–285. [Google Scholar] [CrossRef]
- Gray, K.J.; Wilkins-Haug, L.E. Have we done our last amniocentesis? Updates on cell-free DNA for Down syndrome screening. Pediatr. Radiol. 2018, 48, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pergament, E.; Cuckle, H.; Zimmermann, B.; Banjevic, M.; Sigurjonsson, S.; Ryan, A.; Hall, M.P.; Dodd, M.; Lacroute, P.; Stosic, M.; et al. Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet. Gynecol. 2014, 124 Pt 1, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.P.; Srinivasan, A.; Sehnert, A.J.; Bianchi, D.W. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin. Chem. 2014, 60, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Suzumori, N.; Ebara, T.; Yamada, T.; Samura, O.; Yotsumoto, J.; Nishiyama, M.; Miura, K.; Sawai, H.; Murotsuki, J.; Kitagawa, M.; et al. Fetal cell-free DNA fraction in maternal plasma is affected by fetal trisomy. J. Hum. Genet. 2016, 61, 647–652. [Google Scholar] [CrossRef]
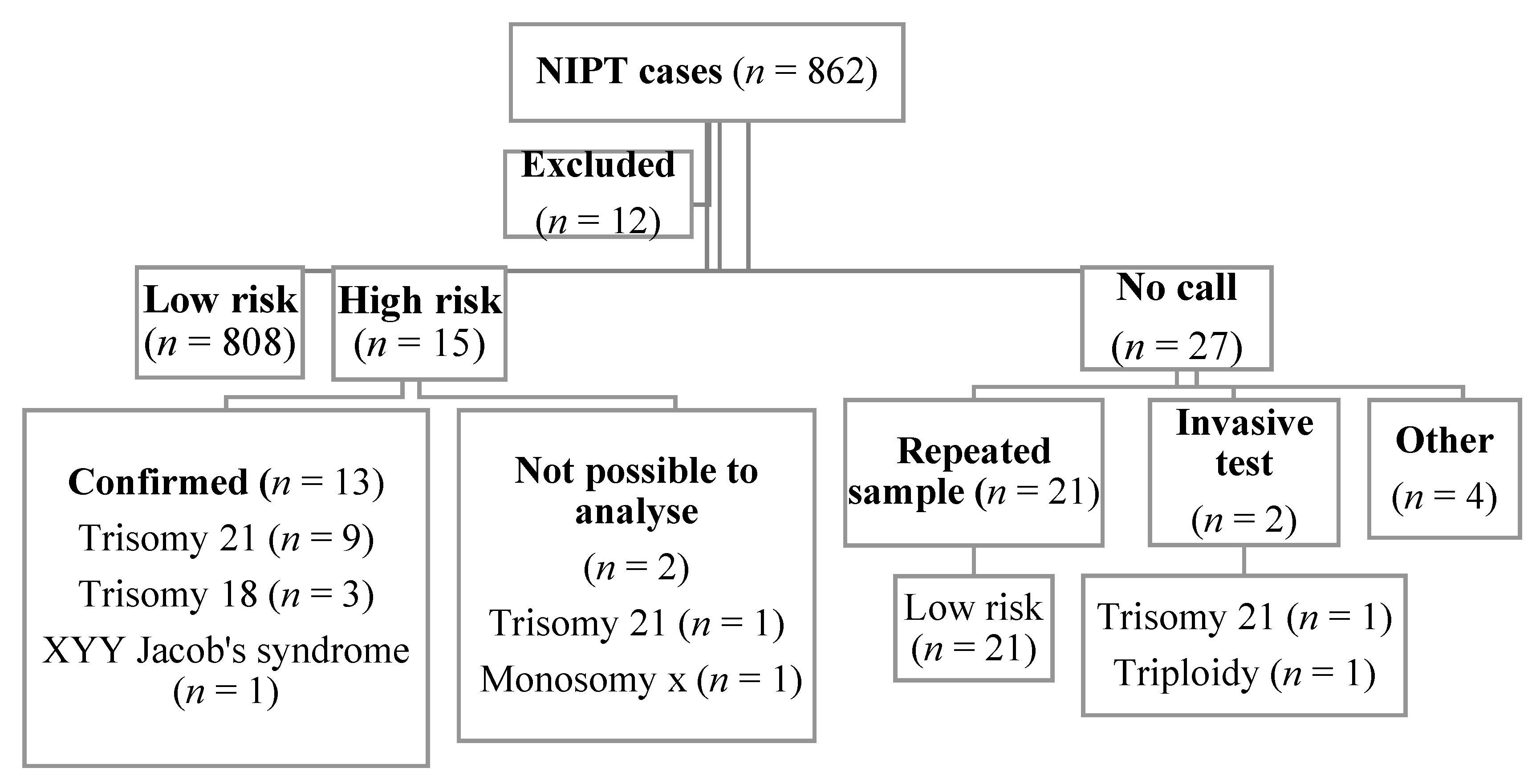
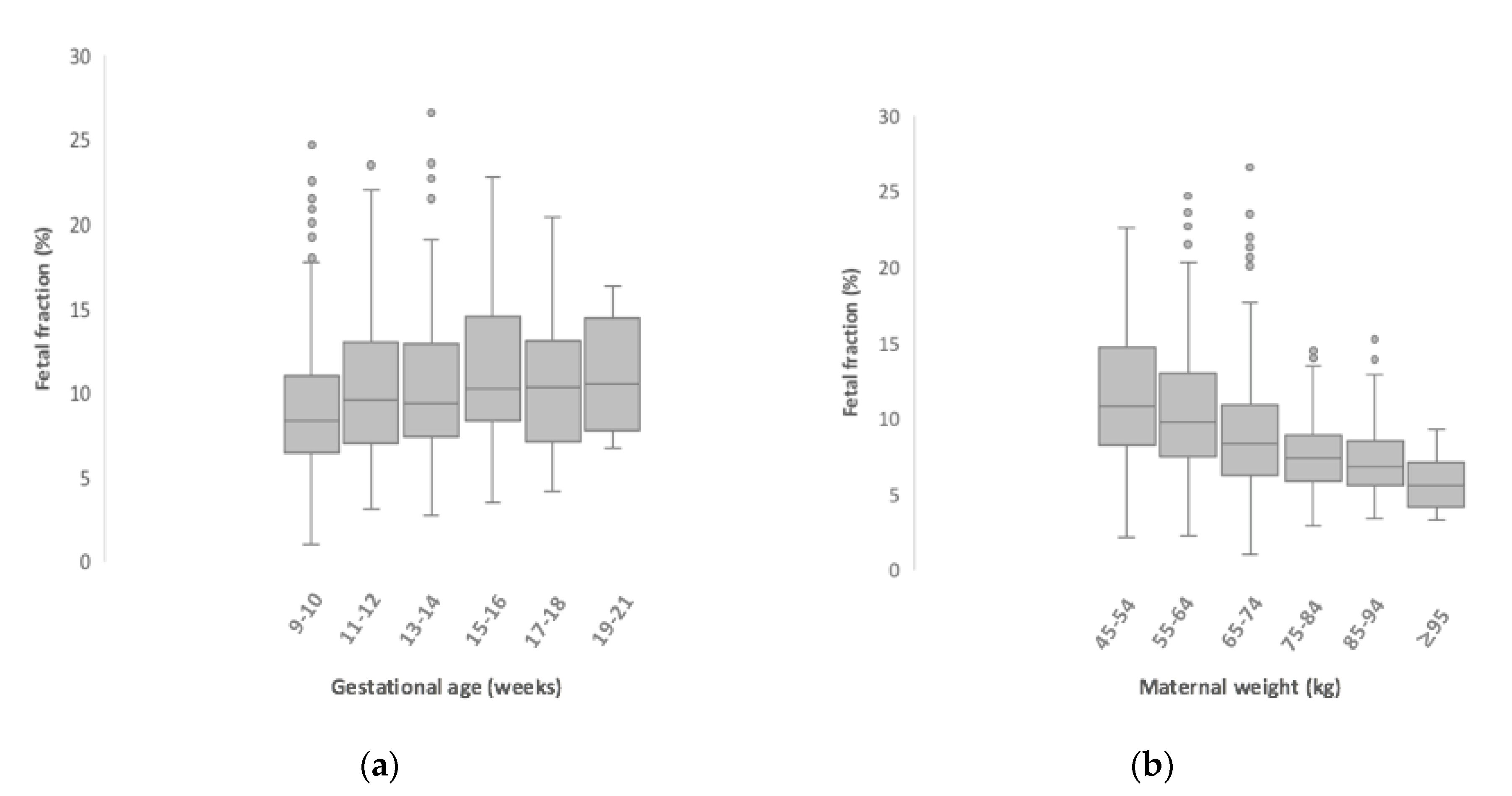
| n | Median (Min–Max) | |
|---|---|---|
| Maternal age (in years) | ||
| All | 850 | 35 (19–49) |
| Low risk | 808 | 34 (19–49) |
| High risk | 15 | 38 (29–47) |
| No call | 27 | 35 (27–42) |
| Gestational age (in weeks) | ||
| All | 850 | 11 (9–21) |
| Low risk | 808 | 11 (9–21) |
| High risk | 15 | 11 (9–18) |
| No call | 27 | 10 (9–16) |
| Maternal weight (kg) | ||
| All | 850 | 62.9 (44.6–174.8) |
| Low risk | 808 | 63.0 (44.6–174.8) |
| High risk | 15 | 63.1 (51.0–90.1) |
| No call | 27 | 67.0 (51.8–94.3) |
| Fetal fraction (%) | ||
| All | 840 | 9.0 (1–26.6) |
| Low risk | 808 | 9.2 (2.9–26.6) |
| High risk | 15 | 6.4 (4.8–20.0) |
| No call | 17 | 3.1 (1–5.9) |
| n | Confirmatory Test | FP | FF (%) | PPV | |
|---|---|---|---|---|---|
| High risk | 15 | 13 | 0 | 6.4 | 100% |
| • Trisomy 21 | 10 | 9 | 0 | 9.8 | 100% |
| • Trisomy 18 | 3 | 3 | 0 | 7.4 | 100% |
| • Monosomy x | 1 | 0 | 8.4 | ||
| • XYY (Jacob’s syndrome) | 1 | 1 | 0 | 6.4 | 100% |
| n (%) | Risk | |
|---|---|---|
| Repeated samples | 21 (77.8%) | Low risk |
| Invasive tests | 2 (7.4%) | Trisomy 21 |
| Triploidy | ||
| Miscarriage | 2 (7.4%) | - |
| Refused to repeat a sample | 2 (7.4%) | - |
| Total | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serapinas, D.; Boreikaitė, E.; Bartkevičiūtė, A.; Norvilaitė, K.; Narbekovas, A.; Bartkevičienė, D. The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region. Medicina 2020, 56, 579. https://doi.org/10.3390/medicina56110579
Serapinas D, Boreikaitė E, Bartkevičiūtė A, Norvilaitė K, Narbekovas A, Bartkevičienė D. The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region. Medicina. 2020; 56(11):579. https://doi.org/10.3390/medicina56110579
Chicago/Turabian StyleSerapinas, Danielius, Evelina Boreikaitė, Agnė Bartkevičiūtė, Kristina Norvilaitė, Andrius Narbekovas, and Daiva Bartkevičienė. 2020. "The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region" Medicina 56, no. 11: 579. https://doi.org/10.3390/medicina56110579
APA StyleSerapinas, D., Boreikaitė, E., Bartkevičiūtė, A., Norvilaitė, K., Narbekovas, A., & Bartkevičienė, D. (2020). The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region. Medicina, 56(11), 579. https://doi.org/10.3390/medicina56110579





