Long-Term Efficacy of Variable-Thread Tapered Implants—A Retrospective, Clinical and Radiological Evaluation
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Baseline Characteristics
3.2. Implant Survival
3.3. Implant Success
3.4. Peri-Implant Health
3.5. Correlation with Risk Factors
4. Discussion
4.1. Implant Survival
4.2. Implant Success
4.3. Peri-Implantitis
4.4. Risk Factors
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Nardi, D.; Piattelli, A. 10-Year Follow-Up of Immediately Loaded Implants with TiUnite Porous Anodized Surface. Clin. Implant Dent. Relat. Res. 2012, 14, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDP s) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Håkansson, J.; Wennström, J.L.; Klinge, B.; Berglundh, T. Patient-reported outcomes of dental implant therapy in a large randomly selected sample. Clin. Oral Implant. Res. 2015, 26, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, S.K.; Wagner, W.; Noelken, R. Retrospective cohort study of a tapered implant with high primary stability in patients with local and systemic risk factors—7-year data. Int. J. Implant Dent. 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Kolinski, M.L.; Cherry, J.E.; McAllister, B.S.; Parrish, K.D.; Pumphrey, D.W.; Schroering, R.L. Evaluation of a variable-thread tapered implant in extraction sites with immediate temporization: A 3-year multicenter clinical study. J. Periodontol. 2014, 85, 386–394. [Google Scholar] [CrossRef]
- Ho, D.S.W.; Yeung, S.C.H.; Zee, K.Y.; Curtis, B.; Hell, P.; Tumuluri, V. Clinical and radiographic evaluation of NobelActive(TM) dental implants. Clin. Oral Implant. Res. 2013, 24, 297–304. [Google Scholar] [CrossRef]
- Babbush, C.A.; Kanawati, A.; Brokloff, J. A new approach to the All-on-Four treatment concept using narrow platform NobelActive implants. J. Oral Implantol. 2013, 39, 314–325. [Google Scholar] [CrossRef]
- Orentlicher, G.; Horowitz, A.; Goldsmith, D.; Delgado-Ruiz, R.; Abboud, M. Cumulative survival rate of implants placed “fully guided” using CT-guided surgery: A 7-year retrospective study. Compend. Contin. Educ. Dent. 2014, 35, 590–598, 600. [Google Scholar]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy analyzed in a Swedish Population: Prevalence of Peri-Implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Doornewaard, R.; Jacquet, W.; Cosyn, J.; De Bruyn, H. How do peri-implant biologic parameters correspond with implant survival and peri-implantitis? A critical review. Clin. Oral Implant. Res. 2018, 29, 100–123. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient Selection and Preparation. In Tissue-Integrated Prosthesis: Osseointegration in Clinical Dentistry; Branemark, P.-I., Ed.; Quintessence Publishing Co.: Chicago, IL, USA; London, UK; Berlin, Germany; Sao Paulo, Brazil; Tokyo, Japan, 1985; pp. 199–209. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef]
- Derks, J.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Early and Late Implant Loss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef]
- Friberg, B.; Ekestubbe, A.; Mellström, D.; Sennerby, L. Brånemark implants and osteoporosis: A clinical exploratory study. Clin. Implant Dent. Relat. Res. 2001, 3, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G.O. Success Criteria in Implant Dentistry. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Francetti, L.; Rodolfi, A.; Barbaro, B.; Taschieri, S.; Cavalli, N.; Corbella, S. Implant success rates in full-arch rehabilitations supported by upright and tilted implants: A retrospective investigation with up to five years of follow-up. J. Periodontal Implant Sci. 2015, 45, 210–215. [Google Scholar] [CrossRef]
- Albrektsson, T.; Isidor, F. Consensus Report of Session IV. In Proceedings of the 1st European Workshop on Periodontology; Lang, N.P., Karring, T., Eds.; Quintessence: Chicago, IL, USA; London, UK; Berlin, Germany; Sao Paulo, Brazil; Tokyo, Japan; Moscow, Russia; Prague, Czech Republic; Warsaw, Poland, 1994; pp. 365–369. [Google Scholar]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Wilson Jr, T.G. The positive relationship between excess cement and peri-implant disease: A prospective clinical endoscopic study. J. Periodontol. 2009, 80, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Puisys, A.; Vindasiute, E.; Linkeviciene, L.; Apse, P. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin. Oral Implant. Res. 2013, 24, 1179–1184. [Google Scholar] [CrossRef]
- Branemark, P.-I.; Zarb, G.A.; Albrektsson, T.; Rosen, H.M. Tissue-Integrated Prostheses. Osseointegration in Clinical Dentistry; Quintessence Publishing Co.: Chicago, IL, USA; London, UK; Berlin, Germany; Sao Paulo, Brazil; Tokyo, Japan, 1985. [Google Scholar]
- Schnitman, P.A.; Wöhrle, P.S.; Rubenstein, J.E.; DaSilva, J.D.; Wang, N.-H. Ten-year results for Brånemark implants immediately loaded with fixed prostheses at implant placement. Int. J. Oral Maxillofac. Implant. 1997, 12, 495–503. [Google Scholar]
- Baelum, V.; Ellegaard, B. Implant survival in periodontally compromised patients. J. Periodontol. 2004, 75, 1404–1412. [Google Scholar] [CrossRef]
- Rasouli Ghahroudi, A.; Talaeepour, A.; Mesgarzadeh, A.; Rokn, A.; Khorsand, A.; Mesgarzadeh, N.; Kharazi Fard, M. Radiographic Vertical Bone Loss Evaluation around Dental Implants Following One Year of Functional Loading. J. Dent. (Tehran) 2010, 7, 89–97. [Google Scholar]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef] [PubMed]
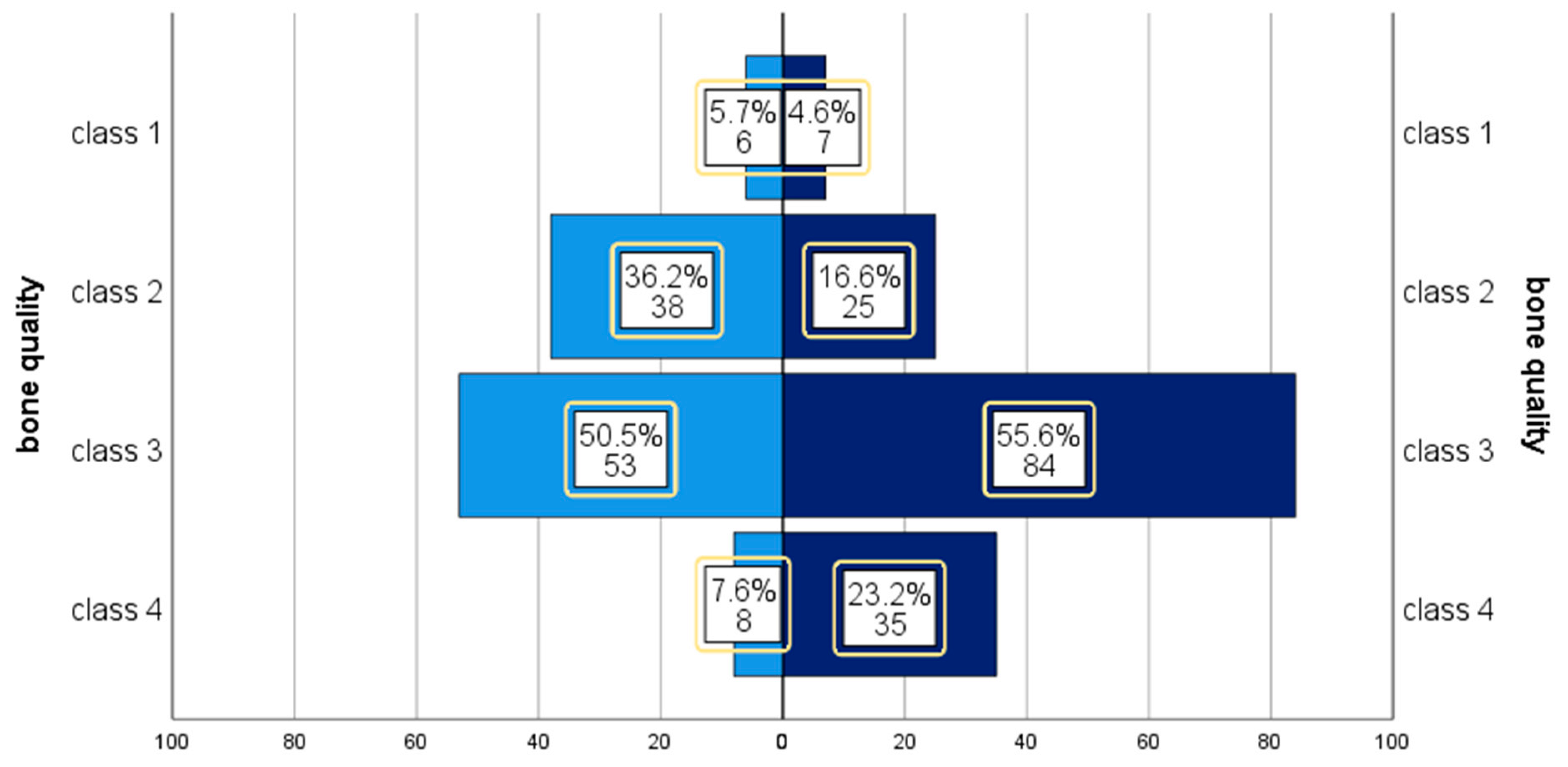
| Risk Factor | Events | % | |
|---|---|---|---|
| current smoker | 6 | 7.4 | |
| smoking history in pack years | 1–9 10–19 20–39 >40 | 15 6 7 7 | 18.5 7.4 8.6 8.6 |
| consume of alcohol | occasionally daily | 29 21 | 35.8 25.9 |
| diabetes mellitus | 7 | 8.6 | |
| HbA1C in percent (n = 5) | <6.5 6.5–7.5 >7.5 | 2 1 2 | 40.0 20.0 40.0 |
| history of immunosuppressant | 4 | 4.9 | |
| Carcinosis | 9 | 11.1 | |
| Bisphosphonate-intake at follow-up | oral intravenous | 3 3 | 3.7 3.7 |
| periodontitis (n = 80) according to PSR (Periodontal Screening and Recording) | 35 | 43.8 | |
| oral hygiene status | insufficient | 8 | 9.9 |
| Bruxism | 38 | 46.9 | |
| frequency of professional dental cleaning | never 1/year >1/year >2/year >3/year | 3 14 41 16 7 | 3.7 17.3 50.6 19.8 8.6 |
| VTTI (n = 149) | Control Group (n = 108) | |
|---|---|---|
| plaque 1,2 | 35.6% (53) | 42.5% (45) |
| spontaneous bleeding 2 | 2.0% (3) | 0.9% (1) |
| exudation 2 | 0.7% (1) | 0.0% (0) |
| BOP | 66.4% (99) | 63.0% (68) |
| probing depth 2 in mm * | 3.6 ± 1.5 | 3.6 ± 1.4 |
| recession in mm ± SD | 0.33 ± 0.88 | 1.17 ± 1.24 |
| keratinized mucosa 2 in mm * | 2.5 ± 2.1 | 1.2 ± 1.5 |
| prosthodontics | ||
| crown | 55.0% (82) | 50.9% (55) |
| implant-supported fixed prosthodontics | 40.9% (61) | 44.4% (48) |
| anchorage for removable prosthodontics | 4.0% (6) | 4.6% (5) |
| fixation | ||
| cemented | 74.5% (111) | 66.7% (72) |
| screw-retained | 25.5% (38) | 33.3% (36) |
| patient satisfaction * | 9.54 ± 0.941 | 9.30 ± 1.665 |
| foreign body sensation | 3.18% (5) | 0.00% (0) |
| radiological bone loss in mm 3,* | 1.12 ± 1.50 | 1.62 ± 1.68 |
| Outcome Variable | n | Events | 95.0% CI | Time In Situ * |
|---|---|---|---|---|
| Implant survival | ||||
| VTTI | 157 | 149 (94.9%) | 90.2–97.8% | 8.51 ± 0.70 |
| control group | 113 | 108 (95.6%) | 90.0–98.5% | 12.46 ± 3.91 |
| Implant success | ||||
| VTTI | 87 | 66 (75.9%) | 65.5–84.4% | 8.55 ± 0.73 |
| control group | 71 | 43 (60.6%) | 48.3–72.0% | 12.84 ± 3.38 |
| Peri-implantitis | ||||
| VTTI | 79 | 29 (36.7%) | 27.3–48.0% | 8.47 ± 0.67 |
| control group | 66 | 31 (47.0%) | 35.6–58.8% | 12.75 ± 3.41 |
| Implant Quality Scale Group and Criteria | VTTI (n = 87) | Control Group (n = 71) |
|---|---|---|
| I. Success (optimum health) | 66 (75.9%) | 43 (60.6%) |
| (a) No pain or tenderness upon function | ||
| (b) 0 mobility | ||
| (c) <2 mm radiographic bone loss from initial surgery | ||
| (d) No exudates history | ||
| II. Satisfactory survival | 2 (2.3%) | 12 (16.9%) |
| (a) No pain on function | ||
| (b) 0 mobility | ||
| (c) 2–4 mm radiographic bone loss | ||
| (d) No exudates history | ||
| III. Compromised survival | 9 (10.3%) | 9 (12.7%) |
| (a) May have sensitivity on function | ||
| (b) No mobility | ||
| (c) Radiographic bone loss > 4 mm (less than 1/2 of implant body) | ||
| (d) Probing depth > 7 mm | ||
| (e) May have exudates history | ||
| IV. Failure (absolute or clinical failure) | 10 (11.5%) | 7 (9.9%) |
| Any of following: | ||
| (a) Pain on function | ||
| (b) Mobility | ||
| (c) Radiographic bone loss > 1/2 length of implant | 1 (1.1%) | |
| (d) Uncontrolled exudate | 1 (1.1%) | |
| (e) No longer in mouth | 8 (9.2%) | 5 (7.0%) |
| or scheduled for explantation | 2 (2.8%) | |
| time in situ ** (years) | 8.55 ± 0.73 | 12.84 ± 3.38 |
| minimum–maximum | 7.42–11.29 | 8.22–22.02 |
| Step | Regression-Coefficient B | Standard Deviation | Wald | df | p | Exp (B) = OR 95.0% CI | |
|---|---|---|---|---|---|---|---|
| (1) | presence of plaque | 1.586 | 0.502 | 9.992 | 1 | 0.002 | 4.886 (1.827–13.064) |
| constant | 0.488 | 0.251 | 3.779 | 1 | 0.052 | 1.629 | |
| (2) | presence of plaque | 1.568 | 0.527 | 8.863 | 1 | 0.003 | 4.798 (1.709–13.473) |
| submerged healing mode | 1.402 | 0.572 | 5.998 | 1 | 0.014 | 4.063 (1.323–12.478) | |
| constant | 0.695 | 0.285 | 5.942 | 1 | 0.015 | 2.004 | |
| (3) | absence of keratinized mucosa | 1.502 | 0.637 | 5.553 | 1 | 0.018 | 4.489 (1.288–15.649) |
| presence of plaque | 1.697 | 0.560 | 9.170 | 1 | 0.002 | 5.457 (1.820–16.367) | |
| submerged healing mode | 1.442 | 0.605 | 5.679 | 1 | 0.017 | 4.230 (1.292–13.851) | |
| constant | 0.339 | 0.331 | 1.051 | 1 | 0.305 | 1.404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blume, O.; Schnödt, E.M.; Back, M.; Wildenhof, J.I.; Probst, F.A.; Otto, S. Long-Term Efficacy of Variable-Thread Tapered Implants—A Retrospective, Clinical and Radiological Evaluation. Medicina 2020, 56, 564. https://doi.org/10.3390/medicina56110564
Blume O, Schnödt EM, Back M, Wildenhof JI, Probst FA, Otto S. Long-Term Efficacy of Variable-Thread Tapered Implants—A Retrospective, Clinical and Radiological Evaluation. Medicina. 2020; 56(11):564. https://doi.org/10.3390/medicina56110564
Chicago/Turabian StyleBlume, Oliver, Eva Maria Schnödt, Michael Back, Jan IR Wildenhof, Florian A. Probst, and Sven Otto. 2020. "Long-Term Efficacy of Variable-Thread Tapered Implants—A Retrospective, Clinical and Radiological Evaluation" Medicina 56, no. 11: 564. https://doi.org/10.3390/medicina56110564
APA StyleBlume, O., Schnödt, E. M., Back, M., Wildenhof, J. I., Probst, F. A., & Otto, S. (2020). Long-Term Efficacy of Variable-Thread Tapered Implants—A Retrospective, Clinical and Radiological Evaluation. Medicina, 56(11), 564. https://doi.org/10.3390/medicina56110564





