Ganglion Cell Layer Thinning in Alzheimer’s Disease
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puliafito, C.A. OCT angiography: The next era of OCT technology emerges. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 360. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog. Brain Res. 2015, 220, 37–57. [Google Scholar] [PubMed]
- Casado, A.; Cerveró, A.; López-De-Eguileta, A.; Fernández, R.; Fonseca, S.; González, J.C.; Pacheco, G.; Gándara, E.; Gordo-Vega, M.Á. Topographic correlation and asymmetry analysis of ganglion cell layer thinning and the retinal nerve fiber layer with localized visual field defects. PLoS ONE 2019, 14, e0222347. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lapiscina, E.H.; Arnow, S.; Wilson, J.A.; Saidha, S.; Preiningerova, J.L.; Oberwahrenbrock, T.; Brandt, A.U.; Pablo, L.E.; Guerrieri, S.; Gonzalez, I.; et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016, 15, 574–584. [Google Scholar] [CrossRef]
- DeBuc, D.C.; Somfai, G.M.; Koller, A. Retinal microvascular network alterations: Potential biomarkers of cerebrovascular and neural diseases. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 201–212. [Google Scholar] [CrossRef]
- Zivkovic, M.; Dayanir, V.; Stamenović, J.; Ljubisavljević, S.; Pražić, A.; Zlatanović, M.; Jakšić, V.; Radenković, M.; Jovanovic, S. Retinal ganglion cell/inner plexiform layer thickness in patients with Parkinson’s disease. Folia Neuropathol. 2017, 55, 168–173. [Google Scholar] [CrossRef]
- Cerveró, A.; Casado, A.; Riancho, J. Retinal changes in amyotrophic lateral sclerosis: Looking at the disease through a new window. J. Neurol. 2019. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.K.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttilä, T. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.K.; Alkon, D.L. Alzheimer’s disease cerebrospinal fluid and neuroimaging biomarkers: Diagnostic accuracy and relationship to drug efficacy. J. Alzheimer’s Dis. 2015, 46, 817–836. [Google Scholar] [CrossRef]
- Tu, P.; Fu, H.; Cui, M. Compounds for imaging amyloid-β deposits in an Alzheimer’s brain: A patent review. Expert Opin. Ther. Pat. 2015, 25, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Thal, L.J.; Kantarci, K.; Reiman, E.M.; Klunk, W.E.; Weiner, M.W.; Zetterberg, H.; Galasko, D.; Praticò, D.; Griffin, S.; Schenk, D.; et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, R.F.; Larson, E.B.; Koepsell, T.D.; Rees, T.S.; Duckert, L.G. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J. Gen. Intern. Med. 1991, 6, 126–132. [Google Scholar] [CrossRef]
- Cronin-Golomb, A.; Corkin, S.; Rizzo, J.F.; Cohen, J.; Growdon, J.H.; Banks, K.S. Visual dysfunction in Alzheimer’s disease: Relation to normal aging. Ann. Neurol. 1991, 29, 41–52. [Google Scholar] [CrossRef]
- Pache, M.; Smeets, C.H.W.; Gasio, P.F.; Savaskan, E.; Flammer, J.; Wirz-Justice, A.; Kaiser, H.J. Colour vision deficiencies in Alzheimer’s disease. Age Ageing 2003, 32, 422–426. [Google Scholar] [CrossRef]
- Trick, G.L.; Trick, L.R.; Morris, P.; Wolf, M. Visual field loss in senile dementia of the Alzheimer’s type. Neurology 1995, 45, 68–74. [Google Scholar] [CrossRef]
- Gilmore, G.C.; Wenk, H.E.; Naylor, L.A.; Koss, E. Motion perception and Alzheimer’s disease. J. Gerontol. 1994, 49, 52–57. [Google Scholar] [CrossRef]
- Hinton, D.R.; Sadun, A.A.; Blanks, J.C.; Miller, C.A. Optic-nerve degeneration in Alzheimer’s disease. N. Engl. J. Med. 1986, 315, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Blanks, J.C.; Schmidt, S.Y.; Torigoe, Y.; Porrello, K.V.; Hinton, D.R.; Blanks, R.H. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol. Aging 1996, 17, 385–395. [Google Scholar] [CrossRef]
- Blanks, J.C.; Torigoe, Y.; Hinton, D.R.; Blanks, R.H.I. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol. Aging 1996, 17, 377–384. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage 2011, 4, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Parnell, M.; Guo, L.; Abdi, M.; Cordeiro, M.F. Ocular manifestations of Alzheimer’s disease in animal models. Int. J. Alzheimer’s Dis. 2012, 2012, 786494. [Google Scholar] [CrossRef] [PubMed]
- Ning, A.; Cui, J.; To, E.; Ashe, K.H.; Matsubara, J. Amyloid-β deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5136–5143. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Williams, P.A.; Thirgood, R.A.; Oliphant, H.; Frizzati, A.; Littlewood, E.; Votruba, M.; Sengpiel, F.; Williams, P.A.; Morgan, J.E. Retinal ganglion cell dendritic degeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1799–1806. [Google Scholar] [CrossRef]
- Ascaso, F.J.; Cruz, N.; Modrego, P.J.; Lopez-Anton, R.; Santabárbara, J.; Pascual, L.F.; Lobo, A.; Cristóbal, J.A. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: An optical coherence tomography study. J. Neurol. 2014, 261, 1522–1530. [Google Scholar] [CrossRef]
- Leuba, G.; Saini, K. Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 1995, 21, 410–422. [Google Scholar] [CrossRef]
- Moreno-Ramos, T.; Benito-León, J.; Villarejo, A.; Bermejo-Pareja, F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 34, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Tagaris, G.; Markopoulos, L.; Margetis, L.; Tsapakis, S.; Kanakis, M.; Koutsandrea, C.; Markopoulos, I.; Margetis, I. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur. J. Ophthalmol. 2011, 21, 24–29. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Satue, M.; Fuertes, I.; Otin, S.; Alarcia, R.; Herrero, R.; Bambo, M.P.; Fernandez, J.; Pablo, L.E. Ability and reproducibility of Fourier-domain optical coherence tomog-raphy to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology 2012, 119, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Wang, C.; Xu, L.; You, Q.S.; Wang, Y.X.; Zhao, L.; Bin Wei, W.; Zhao, X.; Jonas, J.B. Localized retinal nerve fiber layer defects and stroke. Stroke 2014, 45, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Kalesnykas, G.; Tuulos, T.; Uusitalo, H.; Jolkkonen, J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience 2008, 155, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Fujimoto, J.G.; Frohman, T.C.; Calabresi, P.A.; Cutter, G.; Balcer, L.J. Optical coherence tomography: A window into the mechanisms of multiple sclerosis. Nat. Clin. Pract. Neurol. 2008, 4, 664–675. [Google Scholar] [CrossRef]
- Monteiro, M.L.R.; Fernandes, D.B.; Apóstolos-Pereira, S.L.; Callegaro, D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3959–3966. [Google Scholar] [CrossRef]
- Fisher, J.B.; Jacobs, D.A.; Markowitz, C.E.; Galetta, S.L.; Volpe, N.J.; Nano-Schiavi, M.L.; Baier, M.L.; Frohman, E.M.; Winslow, H.; Frohman, T.C. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006, 113, 324–332. [Google Scholar] [CrossRef]
- Ong, Y.T.; Hilal, S.; Cheung, C.Y.; Venketasubramanian, N.; Niessen, W.J.; Vrooman, H.; Anuar, A.R.; Chew, M.; Chen, C.; Wong, T.Y.; et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci. Lett. 2015, 584, 12–16. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Muffat, J.A.; Cherny, R.A.; Moir, R.D.; Ericsson, M.H.; Huang, X.; Mavros, C.; Coccia, J.A.; Faget, K.Y.; Fitch, K.A.; et al. Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 2003, 361, 1258–1265. [Google Scholar] [CrossRef]
- Grimaldi, A.; Brighi, C.; Peruzzi, G.; Ragozzino, D.; Bonanni, V.; Limatola, C.; Ruocco, G.; Di Angelantonio, S. Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer’s disease in the 3xTg-AD mouse model. Cell Death Dis. 2018, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric ab, and frank neuronal loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef] [PubMed]
- Schön, C.; Hoffmann, N.A.; Ochs, S.M.; Burgold, S.; Filser, S.; Steinbach, S.; Seeliger, W.M.; Arzberger, T.; Goedert, M.; Kretzschmar, H.A.; et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS ONE 2012, 7, e53547. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Rasool, S.; Yang, Z.; Glabe, C.G.; Schreiber, S.S.; Ge, J.; Tan, Z. Amyloid-peptide vaccinations reduce β-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am. J. Pathol. 2009, 175, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E.; Lumayag, S.; Kovacs, B.; Mufson, E.J.; Xu, S. β-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 793–800. [Google Scholar] [CrossRef]
- Zabel, P.; Kałużny, J.J.; Wiłkość-Dębczyńska, M.; Gębska-Tołoczko, M.; Suwała, K.; Kucharski, R.; Araszkiewicz, A. Peripapillary retinal nerve fiber layer thickness in Patients with Alzheimer’s disease: A comparison of eyes of patients with Alzheimer’s disease, primary open-angle glaucoma, and preperimetric glaucoma and healthy controls. Med. Sci. Monit. 2019, 25, 1001–1008. [Google Scholar] [CrossRef]
- Paquet, C.; Boissonnot, M.; Roger, F.; Dighiero, P.; Gil, R.; Hugon, J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2007, 420, 97–99. [Google Scholar] [CrossRef]
- Parisi, V.; Restuccia, R.; Fattaposta, F.; Mina, C.; Bucci, M.G.; Pierelli, F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin. Neurophysiol. 2001, 112, 1860–1867. [Google Scholar] [CrossRef]
- Rebolleda, G.; Diez-Alvarez, L.; Casado, A.; Sánchez-Sánchez, C.; De Dompablo, E.; González-López, J.J.; Muñoz-Negrete, F.J. OCT: New perspectives in neuro-ophthalmology. Saudi J. Ophthalmol. 2015, 29, 9–25. [Google Scholar] [CrossRef]
- Kromer, R.; Serbecic, N.; Hausner, L.; Froelich, L.; Beutelspacher, S.C. Comparison of visual evoked potentials and retinal nerve fiber layer thickness in Alzheimer’s disease. Front. Neurol. 2013, 4, 203. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, X.; Ming, B.; Jia, J.; Wang, R.; Ma, D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: Evidence in optic coherence tomography. Neurosci. Lett. 2010, 480, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Lupidi, M.; Mishra, S.B.; Paez-Escamilla, M.; Querques, G.; Chhablani, J. Unique optical coherence tomographic features in age-related macular degeneration. Surv. Ophthalmol. 2020, 21, 451–457. [Google Scholar] [CrossRef]
- Iseri, P.K.; Altinas, O.; Tokay, T.; Yuksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol. 2006, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Markopoulos, I.; Chatziralli, I.; Rouvas, A.; Papageorgiou, S.G.; Ladas, I.; Vassilopoulos, D. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 782–788. [Google Scholar] [CrossRef]
- Cunha, J.P.; Proença, R.; Dias-Santos, A.; Almeida, R.; Águas, H.; Alves, M.; Papoila, A.L.; Louro, C.; Castanheira-Dinis, A. OCT in Alzheimer’s disease: Thinning of the RNFL and superior hemiretina. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1827–1835. [Google Scholar] [CrossRef]
- Gupta, V.B.; Chitranshi, N.; Haan, J.D.; Mirzaei, M.; You, Y.; Basavarajappa, D.; Godinez, A.; di Angelantonio, S.; Sachdev, P.; Salekdeh, G.H.; et al. Retinal changes in Alzheimer’s disease-integrated prospects of imaging, functional and molecular advances. Prog. Retin. Eye Res. 2020, 2, 100899. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-domain OCT measurements in Alzheimer’s disease: A systematic review and meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimer’s Dement. (Amst.) 2017, 6, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Masurkar, A.V.; Balcer, L.J. Afferent and efferent visual markers of Alzheimer’s disease: A review and update in early stage disease. Front. Aging Neurosci. 2020, 12, 293. [Google Scholar]
- Marziani, E.; Pomati, S.; Ramolfo, P.; Cigada, M.; Giani, A.; Mariani, C.; Staurenghi, G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5953–5958. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.-L.; Ong, Y.T.; Hilal, S.; Ikram, M.K.; Low, S.; Venketasubramanian, N.; Yap, P.; Seow, D.; Chen, C.L.H.; Wong, T.Y. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 5, 45–56. [Google Scholar] [CrossRef]
- Cheung, C.Y.-L.; Ong, Y.T.; Ikram, M.K.; Ong, S.Y.; Li, X.; Hilal, S.; Catindig, J.-A.S.; Venketasubramanian, N.; Yap, P.; Seow, D.; et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Ong, Y.T.; Ikram, M.K.; Chen, C.; Wong, T.Y. Retinal microvasculature in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 339–352. [Google Scholar] [CrossRef]
- López-De-Eguileta, A.; Lage, C.; López-García, S.; Pozueta, A.; García-Martínez, M.; Kazimierczak, M.; Bravo, M.; De Arcocha-Torres, M.; Banzo, I.; Jimenez-Bonilla, J.; et al. Ganglion cell layer thinning in prodromal Alzheimer’s disease defined by amyloid PET. Alzheimer’s Dement. (N. Y.) 2019, 5, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Ehlers, J.P.; Leverenz, J.B. Retinal nerve fiber layer thinning in Alzheimer’s disease: A case-control study in comparison to normal aging, Parkinson’s disease, and non-Alzheimer’s dementia. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 430–436. [Google Scholar] [CrossRef]
- Lad, E.M.; Mukherjee, D.; Stinnett, S.S.; Cousins, S.W.; Potter, G.G.; Burke, J.R. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLoS ONE 2018, 13, e0192646. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Bambo, M.P.; Marques, M.L.; Satue, M.; Otin, S.; Larrosa, J.M.; Polo, V.; Pablo, L.E. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016, 94, e454–e459. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, H.A.; Bayhan, S.A.; Celikbilek, A.; Tanik, N.; Gurdal, C. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2015, 43, 145–151. [Google Scholar] [CrossRef]
- Ferrari, C.; Lombardi, G.; Polito, C.; Lucidi, G.; Bagnoli, S.; Piaceri, I.; Nacmias, B.; Berti, V.; Rizzuto, D.; Fratiglioni, L.; et al. Alzheimer’s disease progression: Factors influencing cognitive decline. J. Alzheimer’s Dis. 2018, 61, 785–791. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar]
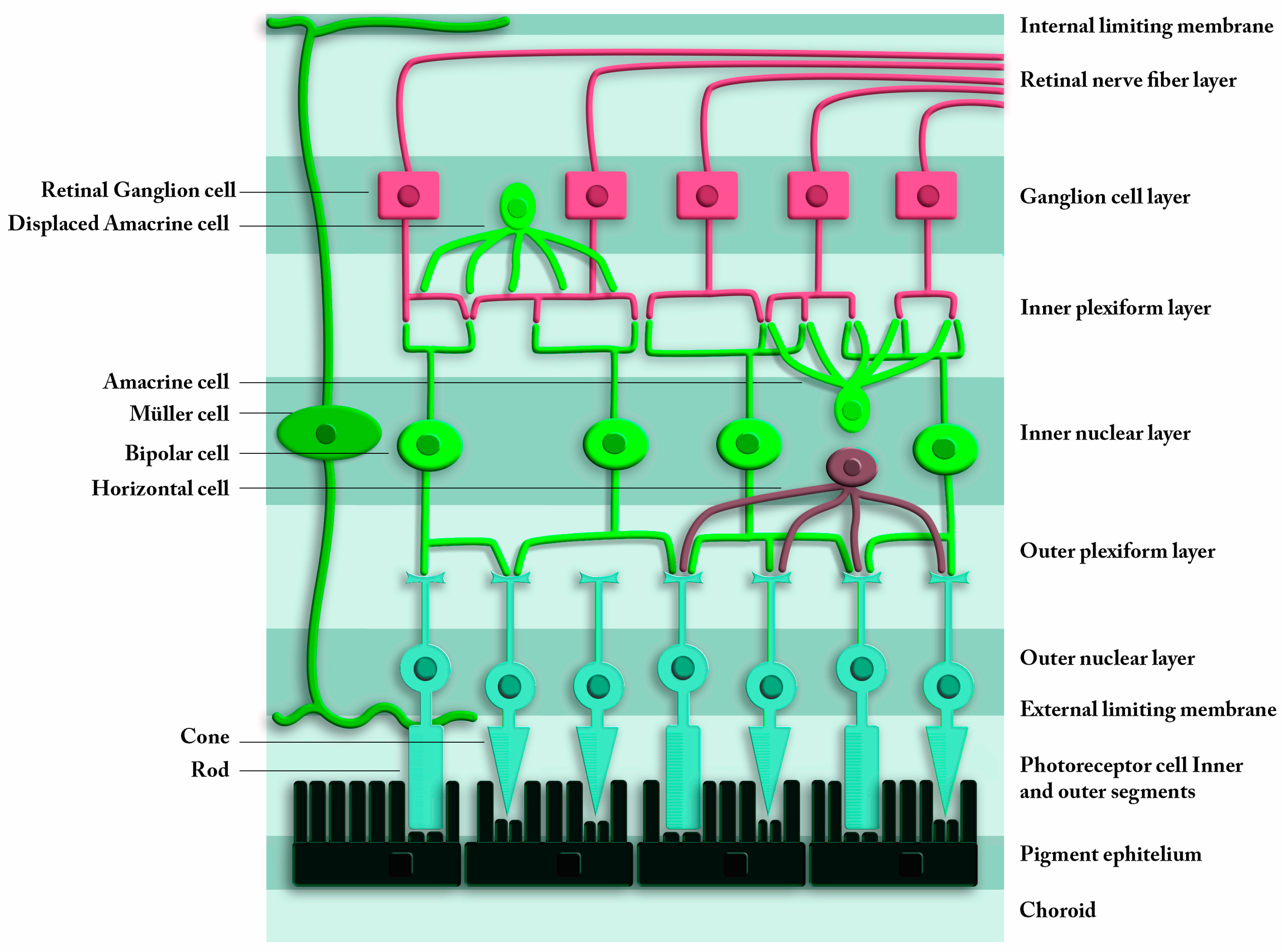
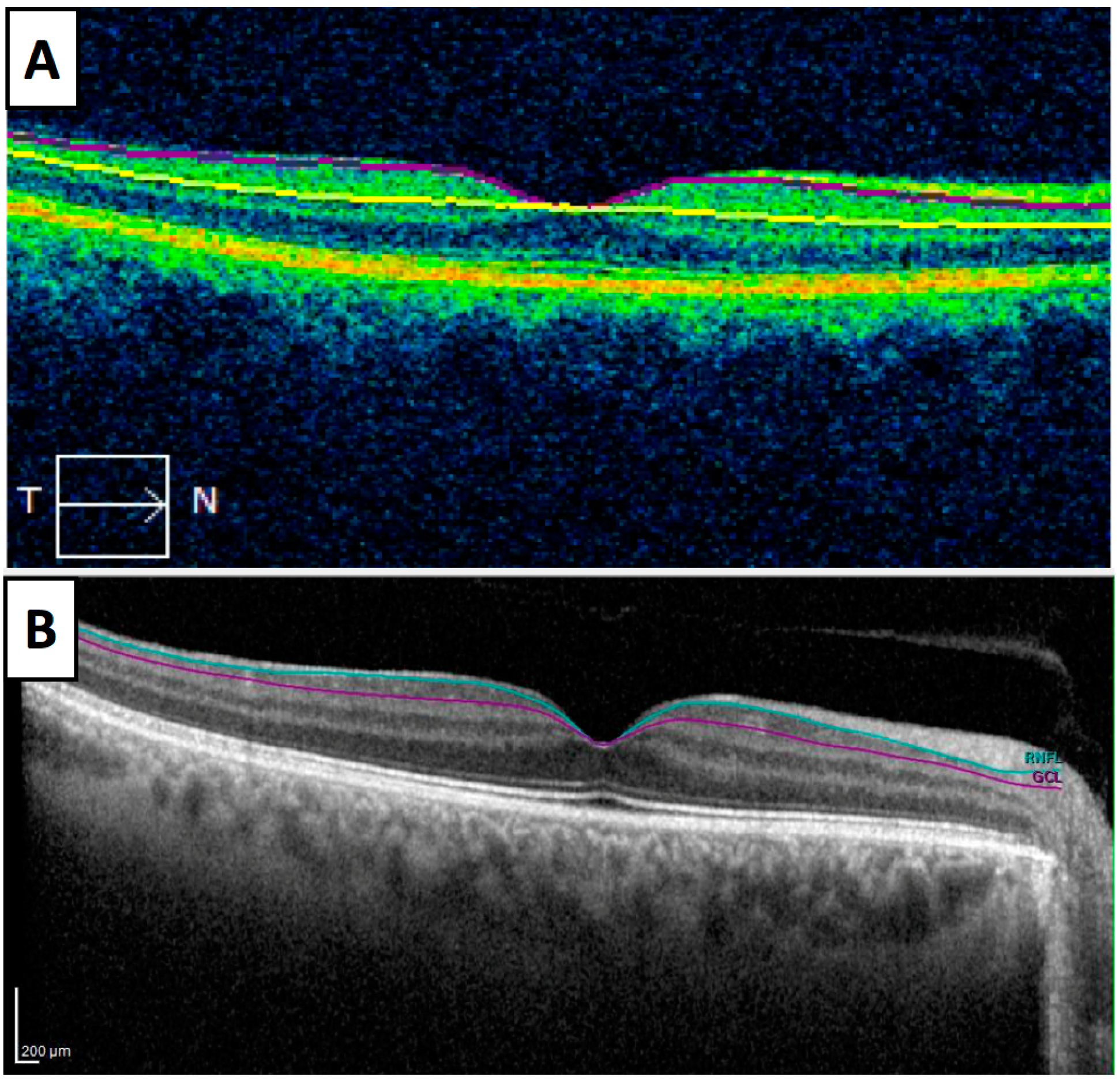
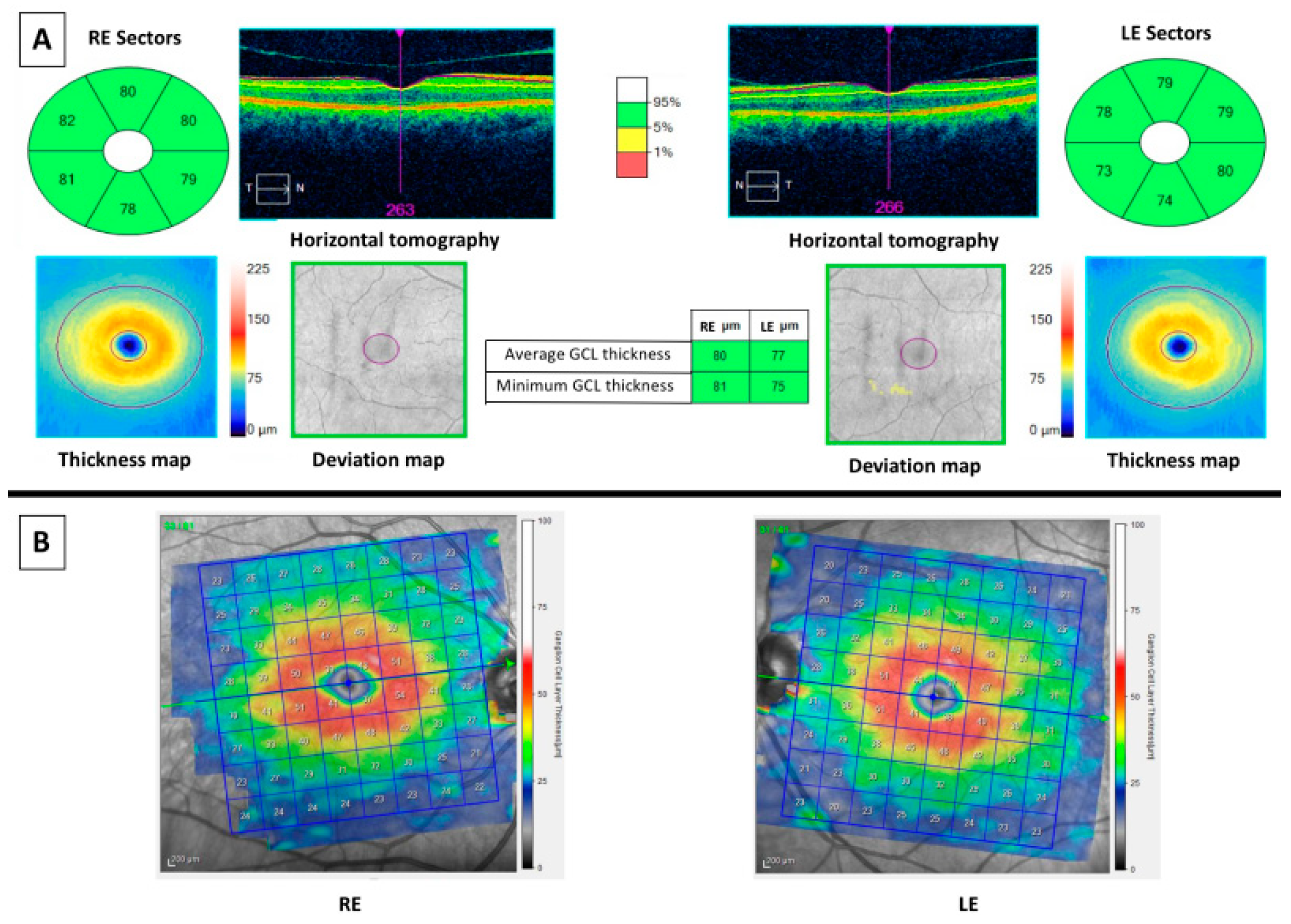

| Source | OCT Exam: Layers | Macular or GCL Results | AD Biomarkers | OCT Platform | Cross-Sectional | Subjects | Sample Size (Eyes) |
|---|---|---|---|---|---|---|---|
| Iseri et al. 2006 | RNFL and macula | Thinner (23%, p < 0.001) | No | Zeiss Stratus | Yes | AD HC | AD 28 eyes (n = 14) HC 30 eyes (n = 15) Age-matched |
| Moschos et al. 2012 | RNFL and macula | Thinner (7%, p = 0.034) | No | Zeiss Stratus | Yes | AD HC | AD (n = 30) HCs (n = 30) Age and sex matched |
| Marziani et al. 2013 | RNFL + GCL combined | Thinner (12.8%, p = 0.008) | No | RTVue-100 and Heidelberg Spectralis | Yes | AD HC | AD (n = 21) HC (n = 21) Age-matched |
| Garcia-Martin et al. 2014 | RNFL and macula | Mild AD had a significant decrease in RNFL (9.24%, p = 0.015), of some macular regions and in the total macular volume (9.34%, p = 0.024). | No | Topcon 3D OCT-100 | Yes | Mild AD HC | Mild AD (n = 20) HC (n = 28) Age-matched |
| Ascaso et al. 2014 | RNFL and macula | RNFL was thinner in -MCI vs. HC (8.5%, p = 0.001)-AD vs. MCI (24.8%, p = 0.001) -AD vs. HC (37.5%, p = 0.001) Macular volume in mm3: -HC had greater macular volume vs. AD (12.4%, p = 0.001) | No | Zeiss Stratus | Yes | AD MCI HC | AD (n = 18) MCI (n = 21) HC (n = 41) |
| Eraslan et al. 2015 | RNFL and GCL | -RNFL Thinner in AD and NTG vs. HC (8%, p = 0.004). -GCL (8.8%, p = 0.001) -No difference between AD and NTG. | No | RTVue-100 | Yes | NTG AD HC | NTG (n = 18) AD (n = 20) HC (n = 20) |
| Bayhan et al. 2015 | GCL and choroid | Reduced choroidal (12.1%, p = 0.01) and macular GCL (5.9%, p = 0.001) thicknesses in AD | CT or MRI | Zeiss Stratus | Yes | AD HC | AD (n = 31) HC (n = 30) Age matched |
| Cheung et al. 2015 | RNFL and GCIPL | - AD had GCIPL thinning in all sectors (AVG 5.4%, p = 0.039) and RNFL in Superior quadrant vs. HC (6.5%, p = 0.001) -GCIPL reduction in MCI (5.1%, p = 0.009) | CT or MRI | Zeiss Cirrus | Yes | MCI AD HC | AD (n = 100) MCI (n = 41) HC (n = 123) |
| Pillai et al. 2016 | RNFL, macula GCL | No differences (p = 0.35 and p = 0.17) | MRI | Zeiss Cirrus | Yes | AD MCI No AD Dementia Parkinson HC | AD (n = 21) MCI (n = 21) no AD dementia (n = 20) PD (n = 20) HC (n = 34) Age-/sex-matched |
| Garcia Martin et al. 2016 | RNFL, GCL, INL, IPL, ONL, OPL | Thinner RNFL (5.6%, p = 0.004), GCL (2.8%, p = 0.04) and IPL (2.3%, p = 0.018) | No | Heidelberg Spectralis | Yes | AD HC | AD (n = 150) HC (n = 75) Age-matched |
| Liu et al. 2016 | GCIPL | Thinner (2.1%, p = 0.003) | Yes. MRI | Zeiss Cirrus | Yes | MCI AD HC | MCI (n = 68) AD (n = 47) HC (n = 65) |
| Choi et al. 2016 | RNFL andGCIPL | -RNFL thinner in temporal sector (14.9%, p = 0.04). -GCIPL thinner in inferior sector (14.5%, p = 0.004). | Yes | Zeiss cirrus | Yes | MCI AD HC | AD (n = 42) MCI (n = 26) HC (n = 66) Age-matched, age as a covariate |
| Gimenéz Castejon et al. 2016 | Macula | Macular thickness reduction in MCI (5.7%, p = 0.05) vs. HC and in SMC vs. HC (4.9%, p = 0.05) | No | Zeiss cirrus | Yes | SMC MCI HC | SMC n = 24 MCI n = 33 HC n = 25 |
| Snyder et al. 2016 | IPL | Thicker (5.8%, p = 0.029) | Yes (florbetapir PET imaging) | Heidelberg Spectralis | Yes | SMC | SMC (n = 63) Age-matched, age as a covariate |
| Kwon et al. 2017 | RNFL and macula | RNFL average thinner in AD vs. MCI (7.8%, p = 0.011). Macular thickness was thinner from HC to MCI and to AD, but no significant. | Yes (MRI) | Zeiss Cirrus | Yes | Gender and race unknown | AD (n = 15) MCI (n = 15) HC (n = 15) |
| Ferrari et al. 2017 | RNFL and GCIPL | Thinning (6.4%, p = 0.023) (15.9%, p = 0.009) | No | Heidelberg Spectralis | Yes | MCI AD HC | AD (n = 39) MCI (n = 27) HC (n = 49) Age-matched, age as a covariate |
| Golzan et al. 2017 | RNFL and GCL | GCL thinner (5.2%, p = 0.02) No RNFL differences | Yes (MRI, florbetapir PET imaging) | Heidelberg Spectralis | Yes | AD HC | AD n = 73 HC n = 28 Age-matched, age as a covariate |
| Poroy et al. 2018 | RNFL and macula | Foveal thickness and volume were higher in AD (5.5%, p = 0.023). RNFL and other macular region not different. | No | Zeiss Stratus | Yes | AD HC | AD (n = 21) HC (n = 25) Age-matched |
| den Haan et al. 2018 | RNFL and macula | No differences | Yes (MRI, PET, CSF) | Heidelberg Spectralis | Yes | AD HC | Early onset AD (n = 15) HC (n = 15) |
| Lad et al. 2018 | RNFL, GCIP | No differences | No | Heidelberg Spectralis | Yes | MCI AD HC | MCI (n = 15) AD (n = 15) HC (n = 18) |
| Uchida et al. 2018 | ONL | No differences | Yes (MRI) | Zeiss Cirrus | Yes | AD MCI non-AD Dementia HC | AD (n = 24) MCI (n = 22) non-AD dementia (n = 20) HC (n = 36) |
| Santos et al. 2018 | RNFL, GCL, OPL, ONL, IPL, INL | RNFL volume (p = 0.05), OPL temporal (p = 0.04), ONL (p = 0.026) and IPL volume (p = 0.020) and inferior thinner over a 27-month follow-up | Yes (florbetapir PET imaging, head CT) | Heidelberg Spectralis | No, 27 months | Preclinical AD HC | Preclinical AD (n = 56) Age-matched |
| López de Eguileta et al. 2019 | RNFL, GCL, BMO-MRW, IPL, ONL, LC | RNFL (2.8%, p = 0.004), GCL (8.7%, p = 0.006), IPL (5.2%, p = 0.011) & ONL (7.9%, p = 0.010) showed significant thinning in eyes of patients with positive 11C-PiB PET/CT | Yes (11C-labeled Pittsburgh Compound-B PET imaging, head CT) | Heidelberg Spectralis | Yes | MCI AD HC | MCI (n =51) AD (n =12) HC (n = 63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-de-Eguileta, A.; Cerveró, A.; Ruiz de Sabando, A.; Sánchez-Juan, P.; Casado, A. Ganglion Cell Layer Thinning in Alzheimer’s Disease. Medicina 2020, 56, 553. https://doi.org/10.3390/medicina56100553
López-de-Eguileta A, Cerveró A, Ruiz de Sabando A, Sánchez-Juan P, Casado A. Ganglion Cell Layer Thinning in Alzheimer’s Disease. Medicina. 2020; 56(10):553. https://doi.org/10.3390/medicina56100553
Chicago/Turabian StyleLópez-de-Eguileta, Alicia, Andrea Cerveró, Ainara Ruiz de Sabando, Pascual Sánchez-Juan, and Alfonso Casado. 2020. "Ganglion Cell Layer Thinning in Alzheimer’s Disease" Medicina 56, no. 10: 553. https://doi.org/10.3390/medicina56100553
APA StyleLópez-de-Eguileta, A., Cerveró, A., Ruiz de Sabando, A., Sánchez-Juan, P., & Casado, A. (2020). Ganglion Cell Layer Thinning in Alzheimer’s Disease. Medicina, 56(10), 553. https://doi.org/10.3390/medicina56100553






