Local Vibrational Therapy for Essential Tremor Reduction: A Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Clinical Study
2.2. Data and Statistical Analysis
2.3. Ethical Statement
2.4. Investigated Medical Device (IMD)
2.5. Efficacy and Safety Evaluations
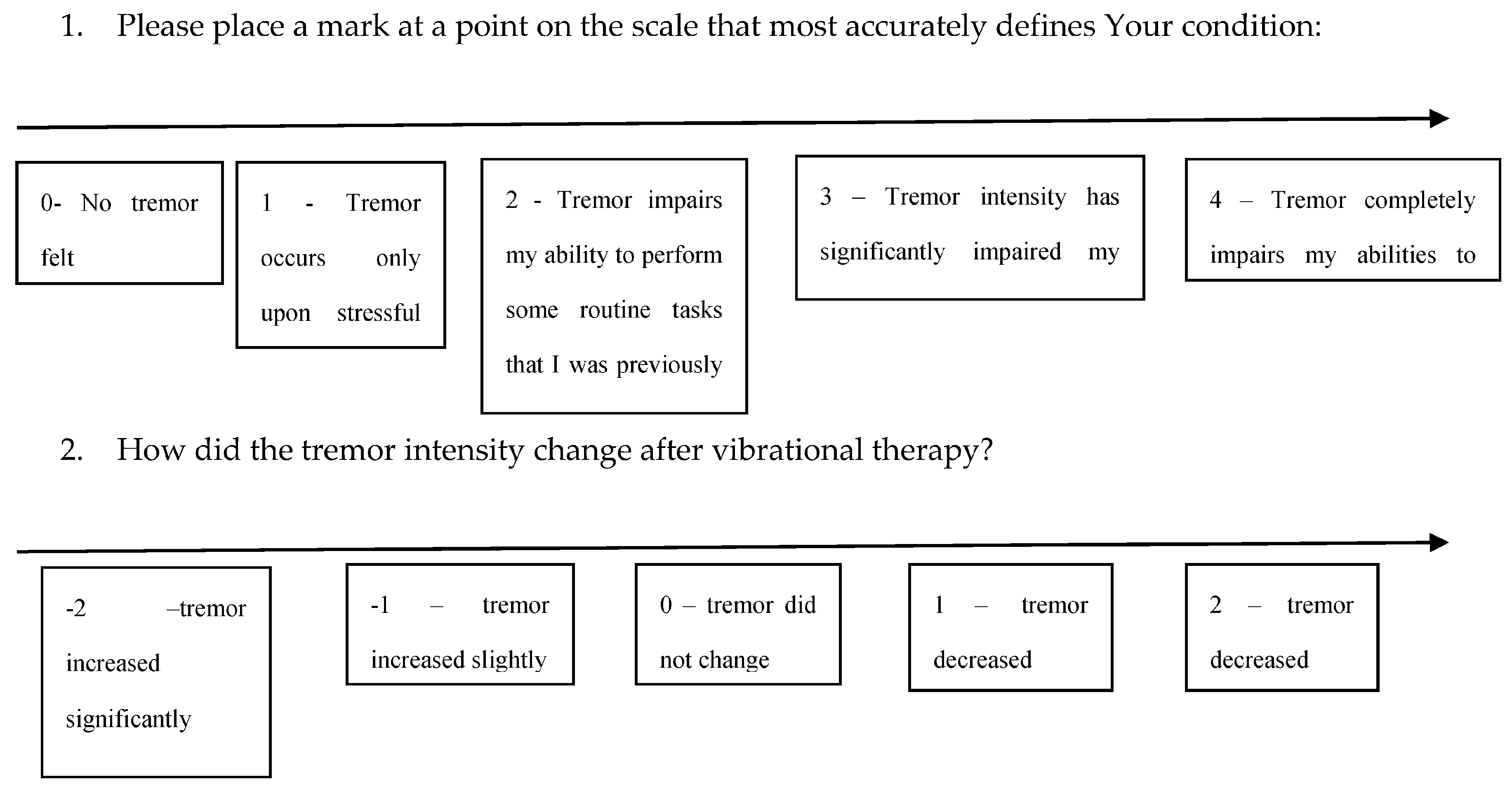
2.5.1. Efficacy Evaluation
2.5.2. The Secondary Efficacy Endpoint
2.5.3. Exploratory Endpoint
2.5.4. Sample Size
2.6. Selection of Study Population
2.7. Additional Extended Safety Study
3. Results
3.1. Clinical Efficacy Assessment
3.2. Exploratory Analysis in the Clinical Efficacy Assessment
3.3. Additional Extended Safety Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bötzel, K.; Tronnier, V.; Gasser, T. Differenzialdiagnose und therapie des tremors. Dtsch. Arztebl. 2014, 111, 225–235. [Google Scholar]
- Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G.; Jiménez-Jiménez, F.J. Current and Future Neuropharmacological Options for the Treatment of Essential Tremor. Curr. Neuropharmacol. 2020, 18, 518–537. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.S.; Domingo, R.A.; Wharen, R.E. Left Radiofrequency Thalamotomy for Drug-Refractory Essential Tremor. World Neurosurg. 2020, 134, 438. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Marder, K.; Cote, L.; Pullman, S.; Ford, B.; Wilder, D.; Tang, M.X.; Lantigua, R.; Gurland, B.; Mayeux, R. Differences in the Prevalence of Essential Tremor Among Elderly African Americans, Whites, and Hispanics in Northern Manhattan, NY. Arch. Neurol. 1995, 52, 1201–1205. [Google Scholar] [CrossRef]
- Louis, E.D.; Ferreira, J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef]
- Louis, E.D.; Dogu, O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology 2008, 29, 208–212. [Google Scholar] [CrossRef]
- Hopfner, F.; Ahlf, A.; Lorenz, D.; Klebe, S.; Zeuner, K.E.; Kuhlenbäumer, G.; Deuschl, G. Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov. Disord. 2016, 31, 1560–1566. [Google Scholar] [CrossRef]
- Haubenberger, D.; McCrossin, G.; Lungu, C.; Considine, E.; Toro, C.; Nahab, F.B.; Auh, S.; Buchwald, P.; Grimes, G.J.; Starling, J.; et al. Octanoic acid in alcohol-responsive essential tremor: A randomized controlled study. Neurology 2013, 80, 933–940. [Google Scholar] [CrossRef]
- Voller, B.; Lines, E.; McCrossin, G.; Tinaz, S.; Lungu, C.; Grimes, G.; Starling, J.; Potti, G.; Buchwald, P.; Haubenberger, D.; et al. Dose-escalation study of octanoic acid in patients with essential tremor. J. Clin. Investig. 2016, 126, 1451–1457. [Google Scholar] [CrossRef]
- Gironell, A.; Pascual-Sedano, B.; Marín-Lahoz, J. Perampanel, a new hope for Essential tremor: An open label trial. Parkinsonism Relat. Disord. 2019, 60, 171–172. [Google Scholar] [CrossRef]
- Deep Brain Stimulation: Treating Neurological and Psychiatric Disorders by Modulating Brain Activity-PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18356594 (accessed on 26 April 2020).
- Lin, P.T.; Ross, E.K.; Chidester, P.; Rosenbluth, K.H.; Hamner, S.R.; Wong, S.H.; Sanger, T.D.; Hallett, M.; Delp, S.L. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham-controlled pilot trial. Mov. Disord. 2018, 33, 1182–1183. [Google Scholar] [CrossRef]
- Shih, L.C.; Pascual-Leone, A. Non-invasive brain stimulation for essential tremor. Tremor Other Hyperkinetic Mov. 2017, 7, 458. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic trials. N. Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Morant, A.V.; Jagalski, V.; Vestergaard, H.T. Characteristics of Single Pivotal Trials Supporting Regulatory Approvals of Novel Non-orphan, Non-oncology Drugs in the European Union and United States from 2012−2016. Clin. Transl. Sci. 2019, 12, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Manto, M. Neurological tremor: Sensors, signal processing and emerging applications. Sensors 2010, 10, 1399–1422. [Google Scholar] [CrossRef]
- Daneault, J.F.; Carignan, B.; Codère, C.É.; Sadikot, A.F.; Duval, C. Using a smart phone as a standalone platform for detection and monitoring of pathological tremors. Front. Hum. Neurosci. 2012, 6, 1–31. [Google Scholar] [CrossRef]
- Pan, D.; Dhall, R.; Lieberman, A.; Petitti, D.B. A Mobile Cloud-Based Parkinson’s Disease Assessment System for Home-Based Monitoring. JMIR mHealth uHealth 2015, 3, e29. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.; Comella, C.; Fahn, S.; Hallett, M.; Jankovic, J.; Juncos, J.L.; LeWitt, P.; Lyons, K.; Ondo, W.; Pahwa, R.; et al. Reliability of a new scale for essential tremor. Mov. Disord. 2012, 27, 1567–1569. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bahgat, D.; Raslan, A.M.; McCartney, S.; Burchiel, K.J. Lesioning and Stimulation in Tremor-Predominant Movement Disorder Patients: An Institutional Case Series and Patient-Reported Outcome. Stereotact. Funct. Neurosurg. 2012, 90, 181–187. [Google Scholar] [CrossRef]
- Chuanasa, J.; Songschon, S. Essential tremor suppression by a novel self-balancing device. Prosthet. Orthot. Int. 2015, 39, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, K.; Hayashi, Y. Upper-limb tremor suppression with a 7DOF exoskeleton power-assist robot. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6679–6682. [Google Scholar]
- Pahwa, R.; Dhall, R.; Ostrem, J.; Gwinn, R.; Lyons, K.; Ro, S.; Dietiker, C.; Luthra, N.; Chidester, P.; Hamner, S.; et al. An Acute Randomized Controlled Trial of Noninvasive Peripheral Nerve Stimulation in Essential Tremor. Neuromodul. Technol. Neural Interface 2019, 22, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Trenado, C.; Amtage, F.; Huethe, F.; Schulte-Mönting, J.; Mendez-Balbuena, I.; Baker, S.N.; Baker, M.; Hepp-Reymond, M.-C.; Manjarrez, E.; Kristeva, R. Suppression of Enhanced Physiological Tremor via Stochastic Noise: Initial Observations. PLoS ONE 2014, 9, e112782. [Google Scholar] [CrossRef] [PubMed]
- Trenado, C.; Mikulić, A.; Manjarrez, E.; Mendez-Balbuena, I.; Schulte-Mönting, J.; Huethe, F.; Hepp-Reymond, M.-C.; Kristeva, R. Broad-band Gaussian noise is most effective in improving motor performance and is most pleasant. Front. Hum. Neurosci. 2014, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.O.; Scheidt, R.A.; Schmit, B.D. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehabilit. Neural Repair 2011, 25, 61–70. [Google Scholar] [CrossRef]
- Nilsson, T.; Wahlström, J.; Burström, L. Hand-arm vibration and the risk of vascular and neurological diseases-A systematic review and meta-analysis. PLoS ONE 2017, 12, e0180795. [Google Scholar] [CrossRef]
- Sauni, R.; Toivio, P.; Pääkkönen, R.; Malmström, J.; Uitti, J. Work disability after diagnosis of hand-arm vibration syndrome. Int. Arch. Occup. Environ. Health 2015, 88, 1061–1068. [Google Scholar] [CrossRef]
- Kluger, N. National survey of health in the tattoo industry: Observational study of 448 French tattooists. Int. J. Occup. Med. Environ. Health 2017, 30, 111–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.-R.; Lee, K.-S.; Bae, H.-G. Chronic subdural hematoma after eccentric exercise using a vibrating belt machine. J. Korean Neurosurg. Soc. 2013, 54, 265. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Mauro, M.; Bovenzi, M.; Griffin, M.J. Reduction in finger blood flow induced by hand-transmitted vibration: Effect of hand elevation. Int. Arch. Occup. Environ. Health 2015, 88, 981–992. [Google Scholar] [CrossRef][Green Version]
- Obelenis, V.; Malinauskienė, V. The influence of occupational environment and professional factors on the risk of cardiovascular disease. Medicina 2006, 43, 96. [Google Scholar] [CrossRef]
- Bovenzi, M.; Prodi, A.; Mauro, M. A longitudinal study of neck and upper limb musculoskeletal disorders and alternative measures of vibration exposure. Int. Arch. Occup. Environ. Health 2016, 89, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.T.; Bovenzi, M. Rheumatic effects of vibration at work. Best Pract. Res. Clin. Rheumatol. 2015, 29, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Gudas, R.; Šiupšinskas, L.; Gudaitė, A.; Vansevičius, V.; Stankevičius, E.; Smailys, A.; Vilkytė, A.; Simonaitytė, R. The Patello-Femoral Joint Degeneration and the Shape of the Patella in the Population Needing an Arthroscopic Procedure. Medicina 2018, 54, 21. [Google Scholar] [CrossRef] [PubMed]
- Popević, M.B.; Janković, S.M.; Borjanović, S.S.; Jovičić, S.R.; Tenjović, L.R.; Milovanović, A.P.S.; Bulat, P. Assessment of coarse and fine hand motor performance in asymptomatic subjects exposed to hand-arm vibration. Arch. Ind. Hyg. Toxicol. 2014, 65, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abramavičius, S.; Volkevičiūtė, A.; Tunaitytė, A.; Venslauskas, M.; Bubulis, A.; Bajoriūnas, V.; Stankevičius, E. Low-Frequency (20 kHz) Ultrasonic Modulation of Drug Action. Ultrasound Med. Biol. 2020, 46, 3017–3031. [Google Scholar] [CrossRef]
- Matoba, T. Human response to vibration stress in Japanese workers: Lessons from our 35-year studies: A narrative review. Ind. Health 2015, 53, 522–532. [Google Scholar] [CrossRef][Green Version]
- Krajnak, K. Health effects associated with occupational exposure to hand-arm or whole body vibration. J. Toxicol. Environ. Health Part B Crit. Rev. 2018, 21, 320–334. [Google Scholar] [CrossRef]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Azoto oksido ir kitu endotelio isskiriamu medziagu svarba [Role of nitric oxide and other endothelium-derived factors]. Medicina 2003, 39, 333–341. [Google Scholar]
- Wang, Y.J.; Huang, X.L.; Yan, J.W.; Wan, Y.N.; Wang, B.X.; Tao, J.H.; Chen, B.; Li, B.Z.; Yang, G.J.; Wang, J. The association between vibration and vascular injury in rheumatic diseases: A review of the literature. Autoimmunity 2015, 48, 61–68. [Google Scholar] [CrossRef]

| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
| Tremor Increased Slightly | Tremor Did Not Change | Tremor Decreased Slightly | |
|---|---|---|---|
| Tremor occurs only upon stressful situation | 0 | 0 | 1 |
| Tremor impairs my ability to perform some routine tasks that I was previously capable of performing | 0 | 4 | 3 |
| Tremor intensity has significantly impaired my ability to perform daily tasks | 2 | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramavičius, S.; Venslauskas, M.; Vaitkus, A.; Gudžiūnas, V.; Laucius, O.; Stankevičius, E. Local Vibrational Therapy for Essential Tremor Reduction: A Clinical Study. Medicina 2020, 56, 552. https://doi.org/10.3390/medicina56100552
Abramavičius S, Venslauskas M, Vaitkus A, Gudžiūnas V, Laucius O, Stankevičius E. Local Vibrational Therapy for Essential Tremor Reduction: A Clinical Study. Medicina. 2020; 56(10):552. https://doi.org/10.3390/medicina56100552
Chicago/Turabian StyleAbramavičius, Silvijus, Mantas Venslauskas, Antanas Vaitkus, Vaidotas Gudžiūnas, Ovidijus Laucius, and Edgaras Stankevičius. 2020. "Local Vibrational Therapy for Essential Tremor Reduction: A Clinical Study" Medicina 56, no. 10: 552. https://doi.org/10.3390/medicina56100552
APA StyleAbramavičius, S., Venslauskas, M., Vaitkus, A., Gudžiūnas, V., Laucius, O., & Stankevičius, E. (2020). Local Vibrational Therapy for Essential Tremor Reduction: A Clinical Study. Medicina, 56(10), 552. https://doi.org/10.3390/medicina56100552







