Vitamin A Enhances Macrophages Activity Against B16-F10 Malignant Melanocytes: A New Player for Cancer Immunotherapy?
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Dose–Response Assay to H2O2-Induced Oxidative Stress
2.3. TBARS Assay
2.4. Dose–Response Assay to Vitamin A
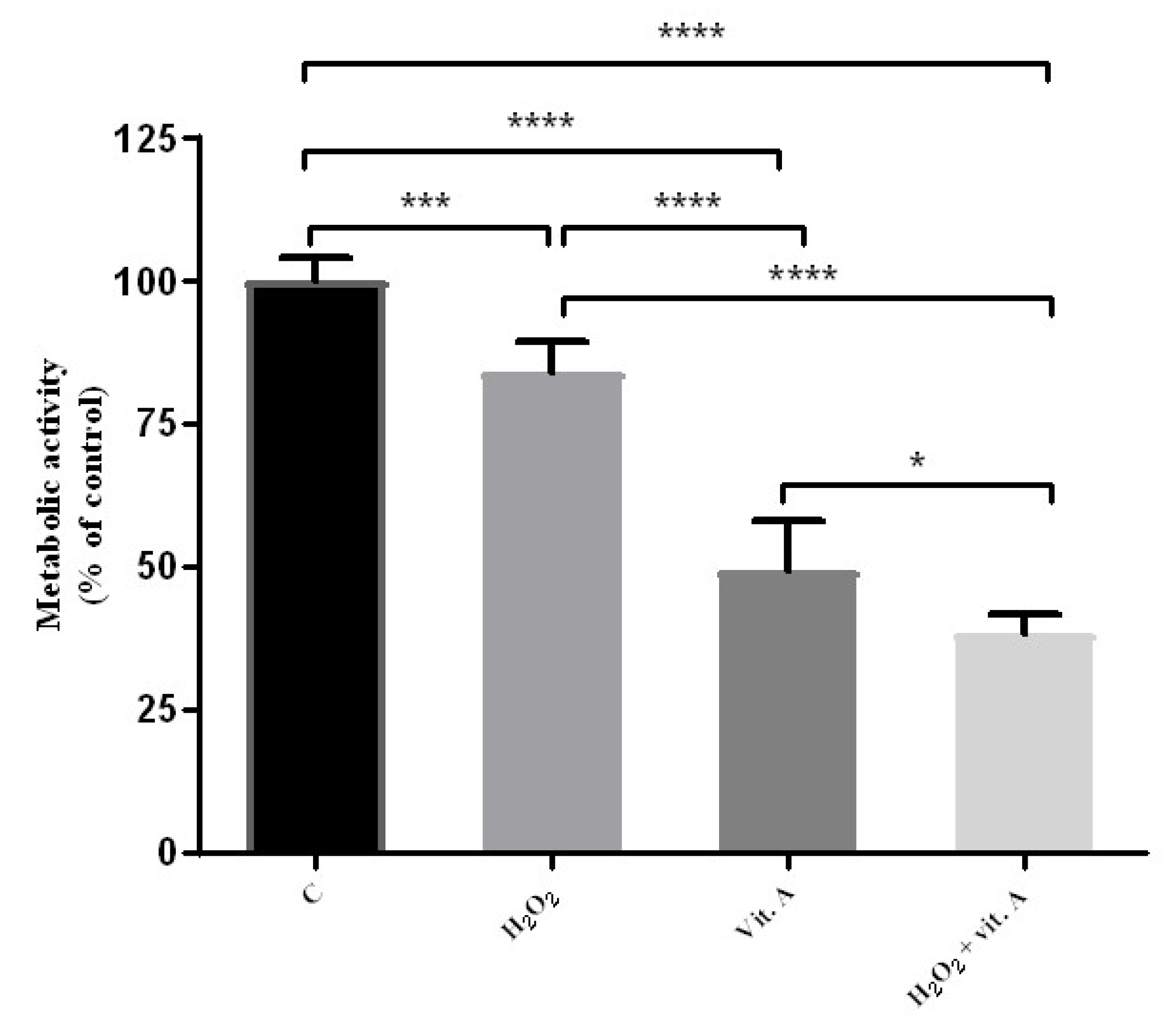
2.5. Combined Effects of Vitamin A and H2O2
2.6. Metabolic Activity Determination: MTT Assay
2.7. Macrophage-Mediated Cytotoxicity
2.8. Statistical Analysis
3. Results
3.1. Lower Concentrations of Vitamin A May Be Related to Its Possible Antioxidant Properties
3.2. Higher Concentrations of Vitamin A May Be Related to Its Possible Pro-Oxidant Properties
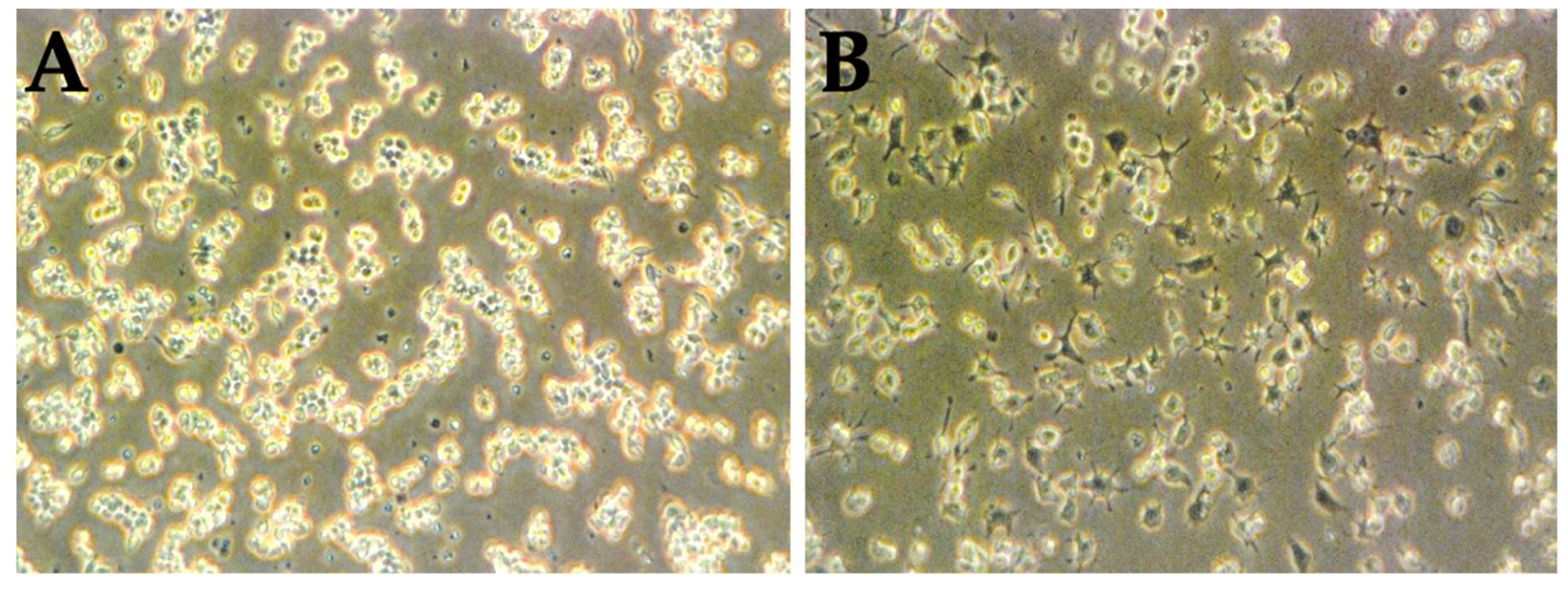
3.3. LPS Is Capable of the Activation of RAW 264.7 Naïve Cells
3.4. The Percentage of Macrophage-Mediated Cells Cytotoxicity Is Inversely Correlated to Cell Culture Results Alone
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, S.; Coelho, P.; Prudêncio, C.; Vieira, M.; Soares, R.; Guerreiro, S.G.; Fernandes, R. Melanoma and obesity: Should antioxidant vitamins be addressed? Life Sci. 2016, 165, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.B.; Bonamigo, R.R.; Segatto, M.M.; Costa, M.M.; Mastroeni, S.; Fortes, C. Could a specific dietary intake be a risk factor for cutaneous melanoma? Cutis 2016, 97, 421–425. [Google Scholar] [PubMed]
- Wick, M.R. Cutaneous melanoma: A current overview. Semin. Diagn. Pathol. 2016, 33, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Vidwans, S.J.; Flaherty, K.T.; Fisher, D.E.; Tenenbaum, J.M.; Travers, M.D.; Shrager, J. A Melanoma Molecular Disease Model. PLoS ONE 2011, 6, e18257. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.C.; Delman, K.A. Comparative Effectiveness in Melanoma. In Comparative Effectiveness in Surgical Oncology; Springer: Cham, Switzerland, 2015; pp. 31–49. [Google Scholar]
- Abildgaard, C.; Guldberg, P. Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol. Med. 2015, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chartrain, M.; Riond, J.; Stennevin, A.; Vandenberghe, I.; Gomes, B.; Lamant, L.; Meyer, N.; Gairin, J.E.; Guilbaud, N.; Annereau, J.P. Melanoma Chemotherapy Leads to the Selection of ABCB5-Expressing Cells. PLoS ONE 2012, 7, e36762. [Google Scholar] [CrossRef] [PubMed]
- Bandarchi, B.; Ma, L.; Navab, R.; Seth, A.; Rasty, G. From Melanocyte to Metastatic Malignant Melanoma. Dermatol. Res. Pract. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Liu-Smith, F.; Meyskens, F.L. Continuing to illuminate the mechanisms underlying UV-mediated melanomagenesis. J. Photochem. Photobiol. B Biol. 2014, 138, 317–323. [Google Scholar] [CrossRef]
- Tong, L.X.; Young, L.C. Nutrition: The future of melanoma prevention? J. Am. Acad. Dermatol. 2014, 71, 151–160. [Google Scholar] [CrossRef]
- Millen, A.E.; Tucker, M.A.; Hartge, P.; Halpern, A.; Elder, D.E.; Guerry, D.; Holly, E.A.; Sagebiel, R.W.; Potischman, N. Diet and melanoma in a case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1042–1051. [Google Scholar]
- Murzaku, E.C.; Bronsnick, T.; Rao, B.K. Diet in dermatology. J. Am. Acad. Dermatol. 2014, 71, 1053.e1–1053.e16. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Caroppo, F.; Alaibac, M. Vitamins and Melanoma. Cancers 2015, 7, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Costin, G.E.; Shellman, Y.; Kechris, K.; Olteanu, E.D.; Filip, A.; Cosgarea, M.R.; Norris, D.A.; Birlea, S.A. Biphasic pro-melanogenic and pro-apoptotic effects of all-trans-retinoic acid (ATRA) on human melanocytes: Time-course study. J. Dermatol. Sci. 2013, 72, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.D.; Dreher, F. Comparison of a skin-lightening cream targeting melanogenesis on multiple levels to triple combination cream for melasma. J. Drugs Dermatol. 2013, 12, 270–274. [Google Scholar]
- Jimbow, K.; Salopek, T.G.; Dixon, W.T.; Searles, G.E.; Yamada, K. The epidermal melanin unit in the pathophysiology of malignant melanoma. Am. J. Dermatopathol. 1991, 13, 179–188. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Slominski, A.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.P.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef]
- Oh, T.I.; Lee, Y.M.; Lim, B.O.; Lim, J.H. Inhibition of NAT10 Suppresses Melanogenesis and Melanoma Growth by Attenuating Microphthalmia-Associated Transcription Factor (MITF) Expression. Int. J. Mol. Sci. 2017, 18, 1924. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Melanoma: Genetic Abnormalities, Tumor Progression, Clonal Evolution and Tumor Initiating Cells. Med. Sci. 2017, 5, 28. [Google Scholar]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of fatty acids on melanogenesis and tumor cell growth in melanoma cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Brożyna, A.A.; VanMiddlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Almeida, J.; Prudêncio, C.; Fernandes, R.; Soares, R. Effect of Adipocyte Secretome in Melanoma Progression and Vasculogenic Mimicry. J. Cell. Biochem. 2016, 117, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Gülden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef]
- Kiyoshima, T.; Enoki, N.; Kobayashi, I.; Sakai, T.; Nagata, K.; Wada, H.; Fujiwara, H.; Ookuma, Y.; Sakai, H. Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. Int. J. Mol. Med. 2012, 30, 1007–1012. [Google Scholar] [CrossRef]
- Fang, I.J.; Trewyn, B.G. Application of Mesoporous Silica Nanoparticles in Intracellular Delivery of Molecules and Proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2012; Volume 508, pp. 41–59. [Google Scholar]
- Coelho, P.; Silva, L.; Faria, I.; Vieria, M.; Monteiro, A.; Pinto, G.; Prudêncio, C.; Fernandes, R.; Soares, R. Adipocyte Secretome Increases Radioresistance of Malignant Melanocytes by Improving Cell Survival and Decreasing Oxidative Status. Radiat. Res. 2017, 187, 581–588. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, C.W.; Choi, D.J.; Lee, J.; Lee, J.Y.; Park, Y. Il Ginseng marc-derived low-molecular weight oligosaccharide inhibits the growth of skin melanoma cells via activation of RAW264.7 cells. Int. Immunopharmacol. 2015, 29, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Dellinger, R.; Meyskens, F.L. Updates of reactive oxygen species in melanoma etiology and progression. Arch. Biochem. Biophys. 2014, 563, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fukunaga-Kalabis, M.; Li, L.; Herlyn, M. Developmental pathways activated in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: a double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.D.; Wing, G.J.; Dellavalle, R.P. Nutrition and melanoma prevention. Clin. Dermatol. 2010, 28, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Frömberg, A.; Gutsch, D.; Schulze, D.; Vollbracht, C.; Weiss, G.; Czubayko, F.; Aigner, A. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemother. Pharmacol. 2010, 67, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J. The Prospects of Vitamin C in Cancer Therapy. Immune Netw. 2009, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, H.; Kong, J.M.; Bae, S.; Kim, Y.S.; Lee, N.; Cho, B.J.; Lee, S.K.; Kim, H.R.; Hwang, Y.; et al. Vitamin C down-regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J. Cell. Biochem. 2011, 112, 894–901. [Google Scholar] [CrossRef]

| Cells and Treatments | Cytotoxicity (%) |
|---|---|
| Control (without vitamin A) | 73.8% |
| Vitamin A treatment [0.1 µg/mL] | 86.8% |
| Vitamin A treatment [100 µg/mL] | 75.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.; Costa, J.; Faria, I.; Guerreiro, S.G.; Fernandes, R. Vitamin A Enhances Macrophages Activity Against B16-F10 Malignant Melanocytes: A New Player for Cancer Immunotherapy? Medicina 2019, 55, 604. https://doi.org/10.3390/medicina55090604
Oliveira S, Costa J, Faria I, Guerreiro SG, Fernandes R. Vitamin A Enhances Macrophages Activity Against B16-F10 Malignant Melanocytes: A New Player for Cancer Immunotherapy? Medicina. 2019; 55(9):604. https://doi.org/10.3390/medicina55090604
Chicago/Turabian StyleOliveira, Sofia, José Costa, Isabel Faria, Susana G. Guerreiro, and Rúben Fernandes. 2019. "Vitamin A Enhances Macrophages Activity Against B16-F10 Malignant Melanocytes: A New Player for Cancer Immunotherapy?" Medicina 55, no. 9: 604. https://doi.org/10.3390/medicina55090604
APA StyleOliveira, S., Costa, J., Faria, I., Guerreiro, S. G., & Fernandes, R. (2019). Vitamin A Enhances Macrophages Activity Against B16-F10 Malignant Melanocytes: A New Player for Cancer Immunotherapy? Medicina, 55(9), 604. https://doi.org/10.3390/medicina55090604






