Sexual Health in Menopause
Abstract
1. Introduction
2. Menopause and Sexual Function
2.1. Prevalence, Characteristics and Risk Factors for Sexual Symptoms
2.2. Hormonal Changes and Sexual Behavior: Beyond Estrogen Deficiency
2.3. Genitourinary Syndrome of Menopause (GSM)
2.4. Psychological and Relational Predictors for Sexual Dysfunction in Menopause
2.5. Associated Morbidities: Cardiovascular and Metabolic Diseases
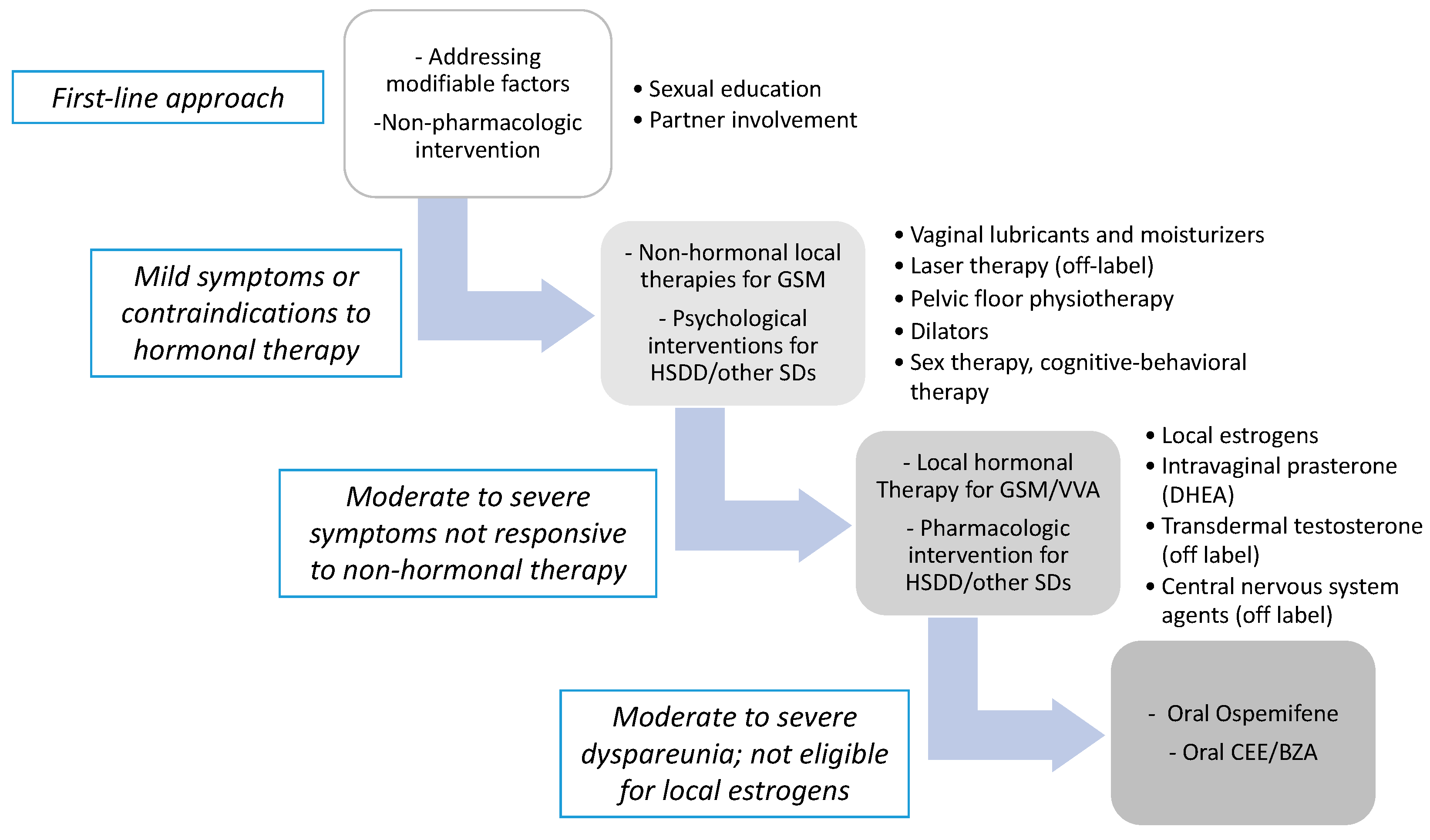
3. Treatment Options for Menopause—Related Sexual Dysfunctions
3.1. Hormonal Treatment
3.1.1. Hormonal Replacement Therapy (HRT)
3.1.2. Local Estrogen Therapy
3.1.3. Ospemifene and Tissue-Selective Estrogen Complex (T-SEC)
3.1.4. Androgens: Systemic and Local Treatment
3.2. Non-Hormonal Treatment
3.2.1. Central Nervous System Agents for HSDD
3.2.2. Vaginal Moisturizers and Lubricants
3.2.3. Laser Therapies
3.2.4. Future Options
4. Management of SD in Postmenopausal Breast Cancer Survivors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vignozzi, L.; Maseroli, E. Hormones and sex behaviour. In Endocrinology Female Reproductive Dysfunction; Petraglia, F., Fauser, B.C.J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; in press. [Google Scholar]
- WHO. Defining Sexual Health: Report of a Technical Consultation on Sexual Health; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Cain, V.S.; Johannes, C.B.; Avis, N.E.; Mohr, B.; Schocken, M.; Skurnick, J.; Ory, M. Sexual functioning and practices in a multi-ethnic study of midlife women: Baseline results from SWAN. J. Sex. Res. 2003, 40, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Damsted Petersen, C. Female Sexual Function in Midlife in Kirana. The EFS and ESSM Syllabus of Clinical Sexology, 1st ed.; Medix Publishers: Amsterdam, The Netherlands, 2013; pp. 1173–1197. [Google Scholar]
- Caruso, S.; Rapisarda, A.M.; Cianci, S. Sexuality in menopausal women. Curr. Opin. Psychiatry 2016, 29, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Graziottin, A.; Leiblum, S.R. Biological and psychosocial pathophysiology of female sexual dysfunction during the menopausal transition. J. Sex Med. 2005, 2, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Dennerstein, L.; Dudley, E.; Burger, H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001, 76, 456–460. [Google Scholar] [CrossRef]
- Avis, N.E.; Stellato, R.; Crawford, S.; Johannes, C.; Longcope, C. Is there an association between menopause status and sexual functioning? Menopause 2000, 7, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Nijland, E.A. Women’s perception of sexuality around the menopause: Outcomes of a European telephone survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.H.; Goldstein, I.; Kim, N.N.; Althof, S.E.; Faubion, S.S.; Fought, B.M.; Parish, S.J.; Simon, J.A.; Vignozzi, L.; Christiansen, K.; et al. The international society for the study of women’s sexual health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin. Proc. 2018, 93, 467–487. [Google Scholar] [CrossRef]
- Worsley, R.; Bell, R.J.; Gartoulla, P.; Davis, S.R. Prevalence and predictors of low sexual desire, sexually related personal distress, and hypoactive sexual desire dysfunction in a community-based sample of midlife women. J. Sex. Med. 2017, 14, 675–686. [Google Scholar] [CrossRef]
- Clayton, A.H.; Vignozzi, L. Pathophysiology and medical management of hypoactive sexual desire disorder. In Textbook of Female Sexual Function and Dysfunction—Diagnosis and Treatment; Goldstein, I., Clayton, A.H., Goldstein, A.T., Kim, N.N., Kingsber, S.A., Eds.; Wiley Blackwell: Oxford, UK, 2018; pp. 59–100. [Google Scholar]
- Kim, G.; Jeong, G.W. Menopause-related brain activation patterns during visual sexual arousal in menopausal women: An fMRI pilot study using time-course analysis. Neuroscience 2017, 343, 449–458. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Buster, J.; Lobo, R. Treatment of Postmenopausal Women; Lippocott: Boston, MA, USA, 1999. [Google Scholar]
- Kokcu, A.; Kurtoglu, E.; Bildircin, D.; Celik, H.; Kaya, A.; Alper, T. Does surgical menopause affect sexual performance differently from natural menopause? J. Sex. Med. 2015, 12, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Tobiansky, D.J.; Wallin Miller, K.G.; Floresco, S.B.; Wood, R.I.; Soma, K.K. Androgen regulation of the mesocorticolimbic system and executive function. Front. Endocrinol. 2018, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.P.; Packard, M.G. Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neurosci. Lett. 2000, 282, 17–20. [Google Scholar] [CrossRef]
- Portman, D.; Gass, M. Vulvovaginal atrophy terminology consensus conference panel genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the international society for the study of women’s sexual health and the North American menopause society. Climacteric 2014, 17, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sturdee, D.W.; Panay, N. Recommendations for the management of postmenopausal vaginal atrophy. International menopause society writing group. Climacteric 2010, 13, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.; Kokot-Kierepa, M. Vaginal health: Insights, views & attitudes (VIVA): Results from an international survey. Climacteric 2012, 15, 36–44. [Google Scholar] [PubMed]
- Traish, A.M.; Vignozzi, L.; Simon, J.A.; Goldstein, I.; Kim, N.N. Role of androgens in female genitourinary tissue structure and function: Implications in the genitourinary syndrome of menopause. Sex. Med. Rev. 2018, 6, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Kurita, T.; Cao, M.; Shen, J.; Robboy, S.; Baskin, L. Molecular mechanisms of development of the human fetal female reproductive tract. Differentiation 2017, 97, 54–72. [Google Scholar] [CrossRef] [PubMed]
- DiBonaventura, M.; Luo, X.; Moffatt, M.; Bushmakin, A.G.; Kumar, M.; Bobula, J. The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and western Europe. J. Womens Health 2015, 24, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.M. The royal Australian college of general practitioners. Reprinted from. AFP 2017, 46, 7. [Google Scholar]
- Mernone, L.; Fiacco, S.; Ehlert, U. Psychobiological factors of sexual functioning in aging women—Findings from the women 40+ healthy aging study. Front. Psychol. 2019, 13, 546. [Google Scholar] [CrossRef]
- Nazarpour, S.; Simbar, M.; Tehrani, F.R. Factors affecting sexual function in menopause: A review article. Taiwan J. Obstet. Gynecol. 2016, 55, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Bandini, E.; Fisher, A.; Elisa, M.; Boddi, V.; Balercia, G.; Maggi, M. Psychobiological correlates of women’s sexual interest as perceived by patients with erectile dysfunction. J. Sex. Med. 2010, 7, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Conaglen, H.M.; O’Connor, E.J.; McCabe, M.P.; Conaglen, J.V. An investigation of sexual dysfunction in female partners of men with erectile dysfunction: How interviews expand on questionnaire responses. Int. J. Impot. Res. 2010, 22, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Nappi, R.E. Couplepause: A new paradigm in treating sexual dysfunction during menopause and andropause. Sex. Med. Rev. 2018, 6, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Miner, M.; Esposito, K.; Guay, A.; Montorsi, P.; Goldstein, I. Cardiometabolic risk and female sexual health: The princeton III summary. J. Sex. Med. 2012, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Maseroli, E.; Scavello, I.; Vignozzi, L. Cardiometabolic risk and female sexuality-part, I. Risk factors and potential pathophysiological underpinnings for female vasculogenic sexual dysfunction syndromes. Sex. Med. Rev. 2018, 6, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.L. The epidemiology of cardiovascular disease in postmenopausal women. Ann. N. Y. Acad. Sci. 1990, 592, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Pontiroli, A.E.; Cortelazzi, D.; Morabito, A. Female sexual dysfunction and diabetes: A systematic review and metaanalysis. J. Sex. Med. 2013, 10, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Nackers, L.M.; Appelhans, B.M.; Segawa, E.; Janssen, I.; Dugan, S.A.; Kravitz, H.M. Associations between body mass index and sexual functioning in midlife women: The study of women’s health across the Nation. Menopause 2015, 22, 1175–1181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maseroli, E.; Fanni, E.; Cipriani, S.; Scavello, I.; Pampaloni, F.; Battaglia, C.; Fambrini, M.; Mannucci, E.; Jannini, E.A.; Maggi, M.; et al. Cardiometabolic risk and female sexuality: Focus on clitoral vascular resistance. J. Sex. Med. 2016, 13, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Rech, C.M.; Clapauch, R.; de Souza, M.D.; Bouskela, E. Low testosterone levels are associated with endothelial dysfunction in oophorectomized early postmenopausal women. Eur. J. Endocrinol. 2016, 174, 297–306. [Google Scholar] [CrossRef]
- Kingsberg, S.A.; Rezaee, R.L. Hypoactive sexual desire in women. Menopause 2013, 20, 1284–1300. [Google Scholar] [CrossRef] [PubMed]
- Pyke, R.E.; Clayton, A.H. Psychological treatment trials for hypoactive sexual desire disorder: A sexual medicine critique and perspective. J. Sex. Med. 2015, 12, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Nastri, C.O.; Lara, L.A.; Ferriani, R.A.; Rosa-e-Silva, A.C.J.; Figueiredo, J.B.; Martins, W.P. Hormone therapy for sexual function in perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2013, 5, CD009672. [Google Scholar] [CrossRef]
- Barnabei, V.M.; Cochrane, B.B.; Aragaki, A.K.; Nygaard, I.; Williams, R.S.; McGovern, P.G.; Young, R.L.; Wells, E.C.; O’Sullivan, M.J.; Chen, B.; et al. Women’s health initiative investigators. Menopausal symptoms and treatment-related effects of estrogen and progestin in the women’s health initiative. Obstet. Gynecol. 2005, 105, 1063–1073. [Google Scholar] [CrossRef]
- Rodriguez, M.; Shoupe, D. Surgical menopause. Endocrinol. Metab. Clin. North. Am. 2015, 44, 531–542. [Google Scholar] [CrossRef]
- Pinkerton, J.A.V.; Aguirre, F.S.; Blake, J.; Cosman, F.; Hodis, H.; Hoffstetter, S.; Marchbanks, P. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2018, 25, 1362–1387. [Google Scholar]
- Rahn, D.D.; Carberry, C.; Sanses, T.V.; Mamik, M.M.; Ward, R.M.; Meriwether, K.V.; Murphy, M. Society of gynecologic surgeons systematic review group. Vaginal estrogen for genitourinary syndrome of menopause: A systematic review. Obstet. Gynecol. 2014, 124, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J. Vaginal administration of estradiol: Effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015, 18, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Del Pup, L.; Di Francia, R.; Cavaliere, C.; Facchini, G.; Giorda, G.; De Paoli, P.; Berretta, M. Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: Is it safe even in cancer patients? Anticancer Drugs 2013, 24, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Ayeleke, R.O.; Roberts, H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst. Rev. 2016, 31, CD001500. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Nachtigall, L.; Ulrich, L.G.; Eugster-Hausmann, M.; Gut, R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet. Gynecol. 2010, 116, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, L.Å.; Ericsson, Å.; Bøgelund, M.; Maamari, R. Women’s preferences toward attributes of local estrogen therapy for the treatment of vaginal atrophy. Maturitas 2013, 74, 259–263. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Stanczyk, F.Z. Clinical effects of selective estrogen receptor modulators on vulvar and vaginal atrophy. Menopause 2014, 21, 309–319. [Google Scholar] [CrossRef]
- Mirkin, S.; Komm, B.S. Tissue-selective estrogen complexes for postmenopausal women. Maturitas 2013, 76, 213–220. [Google Scholar] [CrossRef]
- Burich, R.A.; McCall, J.L.; Mehta, N.R.; Greenberg, B.E.; Bell, K.E.; Griffey, S.M.; Wurz, G.T. Ospemifene and 4-hydroxyospemifene effectively prevent and treat breast cancer in the MTag. Tg transgenic mouse model. Menopause 2012, 19, 96–103. [Google Scholar] [CrossRef][Green Version]
- Archer, D.F.; Goldstein, S.R.; Simon, J.A.; Waldbaum, A.S.; Sussman, S.A.; Altomare, C.; Zhu, J.; Yoshida, Y.; Schaffer, S.; Soulban, G. Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: A phase 3, randomized, double-blind, placebo-controlled, multicenter trial. Menopause 2019, 26, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Bondi, C.; Ferrero, S.; Scala, C.; Tafi, E.; Racca, A.; Venturini, P.L.; Leone Roberti Maggiore, U. Pharmacokinetics, pharmacodynamics and clinical efficacy of ospemifene for the treatment of dyspareunia and genitourinary syndrome of menopause. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.J.; Gillespie, J.A. The evolving role of oral hormonal therapies and review of conjugated estrogens/bazedoxifene for the management of menopausal symptoms. Postgrad. Med. 2017, 129, 340–351. [Google Scholar] [CrossRef]
- Wierman, M.E.; Arlt, W.; Basson, R.; Davis, S.R.; Miller, K.K.; Murad, M.H.; Rosner, W.; Santoro, N. Androgen therapy in women: A reappraisal: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3489–3510. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Goldstein, I.; Kim, N.N.; Davis, S.R.; Kellogg-Spadt, S.; Lowenstein, L.; Pinkerton, J.V.; Stuenkel, C.A.; Traish, A.M.; Archer, D.F.; et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International society for the study of women’s sexual health (ISSWSH) expert consensus panel review. Menopause 2018, 25, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Somboonporn, W.; Davis, S.; Seif, M.W.; Bell, R. Testosterone for peri-and postmenopausal women. Cochrane Database Syst. Rev. 2005, 4, CD004509. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in women—The clinical significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Islam, R.M.; Bell, R.J.; Green, S.; Page, M.J.; Davis, S.R. Safety and efficacy of testosterone for women: A systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019. [Google Scholar] [CrossRef]
- Glaser, R.; Dimitrakakis, C. Testosterone therapy in women: Myths and misconceptions. Maturitas 2013, 74, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Elraiyah, T.; Sonbol, M.B.; Wang, Z.; Khairalseed, T.; Asi, N.; Undavalli, C.; Nabhan, M.; Altayar, O.; Prokop, L.; Montori, V.M.; et al. Clinical review: The benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Derogatis, L.; Archer, D.F.; Koltun, W.; Vachon, A.; Young, D.; Parent, J. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J. Sex. Med. 2015, 12, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Maseroli, E.; Cellai, I.; Corno, C.; Rastrelli, G.; Filippi, S.; Comeglio, P.; Amoriello, R.; Ballerini, C.; Sarchielli, E.; Morelli, A.; et al. Study of the anti-inflammatory effects of dihydrotestosterone in human vaginal smooth muscle cells. Endocrine Abstracts 2019, 63, 1123. [Google Scholar] [CrossRef]
- Simon, J.A.; Thorp, J.; Millheiser, L. Flibanserin for premenopausal hypoactive sexual desire disorder: Pooled analysis of clinical trials. J. Womens Health 2019, 28, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, L.; Feys, F.; Bramer, W.M.; Franco, O.H.; Leusink, P.; Laan, E.T. Efficacy and safety of flibanserin for the treatment of hypoactive sexual desire disorder in women: A systematic review and meta-analysis. JAMA Int. Med. 2016, 176, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Meixel, A.; Yanchar, E.; Fugh-Berman, A. Hypoactive sexual desire disorder: Inventing a disease to sell low libido. J. Med. Ethics 2015, 41, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Chańska, W.; Grunt-Mejer, K. The unethical use of ethical rhetoric: The case of flibanserin and pharmacologisation of female sexual desire. J. Med. Ethics 2016, 42, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Goldstein, I.; Kim, N.N.; Freedman, M.A.; Parish, S.J. Flibanserin approval: Facts or feelings? J. Sex. Med. 2016, 4, e69–e70. [Google Scholar] [CrossRef][Green Version]
- Portman, D.J.; Brown, L.; Yuan, J.; Kissling, R.; Kingsberg, S.A. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: Results of the plumeria study. J. Sex. Med. 2017, 14, 834–842. [Google Scholar] [CrossRef]
- Clayton, A.H.; Althof, S.E.; Kingsberg, S.; DeRogatis, L.R.; Kroll, R.; Goldstein, I.; Kaminetsky, J.; Spana, C.; Lucas, J.; Jordan, R.; et al. Bremelanotide for female sexual dysfunctions in premenopausal women: A randomized, placebo-controlled dose-finding trial. Womens Health 2016, 12, 325–337. [Google Scholar] [CrossRef]
- Sinha, A.; Ewies, A.A.A. Non-hormonal topical treatment of vulvovaginal atrophy: An up-to-date overview. Climacteric 2013, 16, 305–312. [Google Scholar] [CrossRef]
- Nachtigall, L.E. Comparative study: Replens versus local estrogen in menopausal women. Fertil. Steril. 1994, 61, 178–180. [Google Scholar] [CrossRef]
- Chen, J.; Geng, L.; Song, X.; Li, H.; Giordan, N.; Liao, Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: A multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J. Sex. Med. 2013, 10, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Losa, F.; Dexeus, D.; Cortés, J. Beneficial effects of a Coriolus versicolor-based vaginal gel on cervical epithelization, vaginal microbiota and vaginal health: A pilot study in asymptomatic women. BMC Womens Health 2017, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S. Managing urogenital atrophy. Maturitas 2009, 63, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Pitsouni, E.; Grigoriadis, T.; Douskos, A.; Kyriakidou, M.; Falagas, M.E.; Athanasiou, S. Efficacy of vaginal therapies alternative to vaginal estrogens on sexual function and orgasm of menopausal women: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Panay, N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition? Climacteric 2016, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.J.; Lee, L.A.; Torbenson, M.S.; Parsons, T.L.; Bakshi, R.P.; Guidos, A.M.; Hendrix, C.W. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission. J. Infect. Dis. 2007, 195, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Dayal, M.B.; Wheeler, J.; Williams, C.J.; Barnhart, K.T. Disruption of the upper female reproductive tract epithelium by nonoxynol-9. Contraception 2003, 68, 273–279. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists, American Congress of Obstetricians and Gynecologists. Position Statement Fractional Laser Treatment of Vulvovaginal Atrophy and U.S. Food and Drug Administration Clearance. Available online: www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance (accessed on 25 June 2019).
- Lee, M.S. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90° and 360° scanning scopes: A pilot study and short-term results. Laser Ther. 2014, 23, 129–138. [Google Scholar] [CrossRef]
- Gambacciani, M.; Torelli, M.G.; Martella, L.; Bracco, G.L.; Casagrande, A.G.; Albertin, E.; Cervigni, M. Rationale and design for the vaginal erbium laser academy study (VELAS): An international multicenter observational study on genitourinary syndrome of menopause and stress urinary incontinence. Climacteric 2015, 18 (Suppl. 1), 43–48. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Belkacemi, Y.; Darmon, F.; Bastuji-Garin, S.; Werkoff, G.; Bosc, R.; Niddam, J.; Hermeziu, O.; La Padula, S.; et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: A phase 2 pilot study. Menopause 2018, 25, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 4, 173. [Google Scholar]
- Taylor, C.E.; Meisel, J.L. Management of breast cancer therapy-related sexual dysfunction. Oncology 2017, 31, 726–729. [Google Scholar] [PubMed]
- Jing, L.; Zhang, C.; Li, W.; Jin, F.; Wang, A. Incidence and severity of sexual dysfunction among women with breast cancer: A meta-analysis based on female sexual function index. Support Care Cancer 2019, 27, 1171–1180. [Google Scholar] [CrossRef]
- Hummel, S.B.; Hahn, D.E.E.; van Lankveld, J.J.D.M.; Oldenburg, H.S.A.; Broomans, E.; Aaronson, N.K. Factors associated with specific diagnostic and statistical manual of mental disorders, fourth edition sexual dysfunctions in breast cancer survivors: A study of patients and their partners. J. Sex. Med. 2017, 14, 1248–1259. [Google Scholar] [CrossRef]
- Labrie, F.; Luu, T.V.; Labrie, C.; Bélanger, A.; Simard, J.; Lin, S.-X.; Pelletier, G. Endocrine and intracrine sources of androgens in women: Inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr. Rev. 2003, 24, 152–182. [Google Scholar] [CrossRef]
- Somboonporn, W.; Davis, S.R.; National Health and Medical Research Council. Testosterone effects on the breast: Implications for testosterone therapy for women. Endocr. Rev. 2004, 25, 374–388. [Google Scholar] [CrossRef]
- Gera, R.; Tayeh, S.; Chehade, H.E.-H.; Mokbel, K. Does transdermal testosterone increase the risk of developing breast cancer? A systematic review. Anticancer Res. 2018, 38, 6615–6620. [Google Scholar] [CrossRef]
- Witherby, S.; Johnson, J.; Demers, L.; Mount, S.; Littenberg, B.; Maclean, C.D.; Wood, M.; Muss, H. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: A phase I/II study. Oncologist 2011, 16, 424–431. [Google Scholar] [CrossRef]
- Dahir, M.; Travers-Gustafson, D. Breast cancer, aromatase inhibitor therapy, and sexual functioning: A pilot study of the effects of vaginal testosterone therapy. Sex. Med. 2014, 2, 8–15. [Google Scholar] [CrossRef]
- Davis, S.R.; Robinson, P.J.; Jane, F.; White, S.; White, M.; Bell, R.J. Intravaginal testosterone improves sexual satisfaction and vaginal symptoms associated with aromatase inhibitors. J. Clin. Endocrinol. Metab. 2018, 103, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Sulaica, E.; Han, T.; Wang, W.; Bhat, R.; Trivedi, M.V.; Niravath, P. Vaginal estrogen products in hormone receptor-positive breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res. Treat. 2016, 157, 203–210. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice; Farrell, R. ACOG Committee Opinion No. 659: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet. Gynecol. 2016, 127, e93–e96. [Google Scholar] [PubMed]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American menopause society and the international society for the study of women’s sexual health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef] [PubMed]
| Factors Possibly Affecting Sexual Function in Menopause | ||
|---|---|---|
| Predisposing factors | Biological | Gynecological or surgical interventions |
| Premature Ovarian Insufficiency (POI) | ||
| Endometriosis | ||
| Iatrogenic menopause (bilateral oophorectomy, chemotherapy, radiotherapy) | ||
| Endocrine factors | ||
| Psychosexual | Previous sex life | |
| Body image | ||
| Personality traits | ||
| History of sexual abuse/violence | ||
| Affective disorders | ||
| Coping strategies | ||
| Contextual | Ethnic/cultural/religious expectations and constraints | |
| Support and network | ||
| Precipitating factors | Biological | Age at menopause (POI) |
| Biological vs. iatrogenic menopause | ||
| Iatrogenic menopause | ||
| Extent and severity of menopausal symptoms | ||
| Current disorders | ||
| Substance abuse | ||
| Psychosexual | Relationship | |
| Sexual experience | ||
| Affective disorders | ||
| Loss of partner | ||
| Contextual | Life stressors (divorce, separation, partner infidelity) | |
| Loss or death of close kin | ||
| Lack of access to medical treatment | ||
| Economic difficulties | ||
| Maintaining factors | Biological | Changes secondary to menopause (hormonal, vascular, muscular, neurological, immunological) |
| Contraindications to hormone therapy | ||
| Inadequacy of hormone therapy | ||
| Pharmacological treatments | ||
| Substance abuse | ||
| Psychosexual | Perception of menopause changes | |
| Loss of sexual confidence | ||
| Affective disorder | ||
| Distress (personal, emotional, occupational, partner) | ||
| Partner’s general health or sexual problems | ||
| Contextual | Lack of access to care | |
| Interpersonal conflicts | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scavello, I.; Maseroli, E.; Di Stasi, V.; Vignozzi, L. Sexual Health in Menopause. Medicina 2019, 55, 559. https://doi.org/10.3390/medicina55090559
Scavello I, Maseroli E, Di Stasi V, Vignozzi L. Sexual Health in Menopause. Medicina. 2019; 55(9):559. https://doi.org/10.3390/medicina55090559
Chicago/Turabian StyleScavello, Irene, Elisa Maseroli, Vincenza Di Stasi, and Linda Vignozzi. 2019. "Sexual Health in Menopause" Medicina 55, no. 9: 559. https://doi.org/10.3390/medicina55090559
APA StyleScavello, I., Maseroli, E., Di Stasi, V., & Vignozzi, L. (2019). Sexual Health in Menopause. Medicina, 55(9), 559. https://doi.org/10.3390/medicina55090559




