Polymorphism of Interleukin 1B May Modulate the Risk of Ischemic Stroke in Polish Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. Genotyping
2.3. Statistical Analysis
3. Results
3.1. Association between Overall IS and Genetic Variation in IL1B and IL1RN
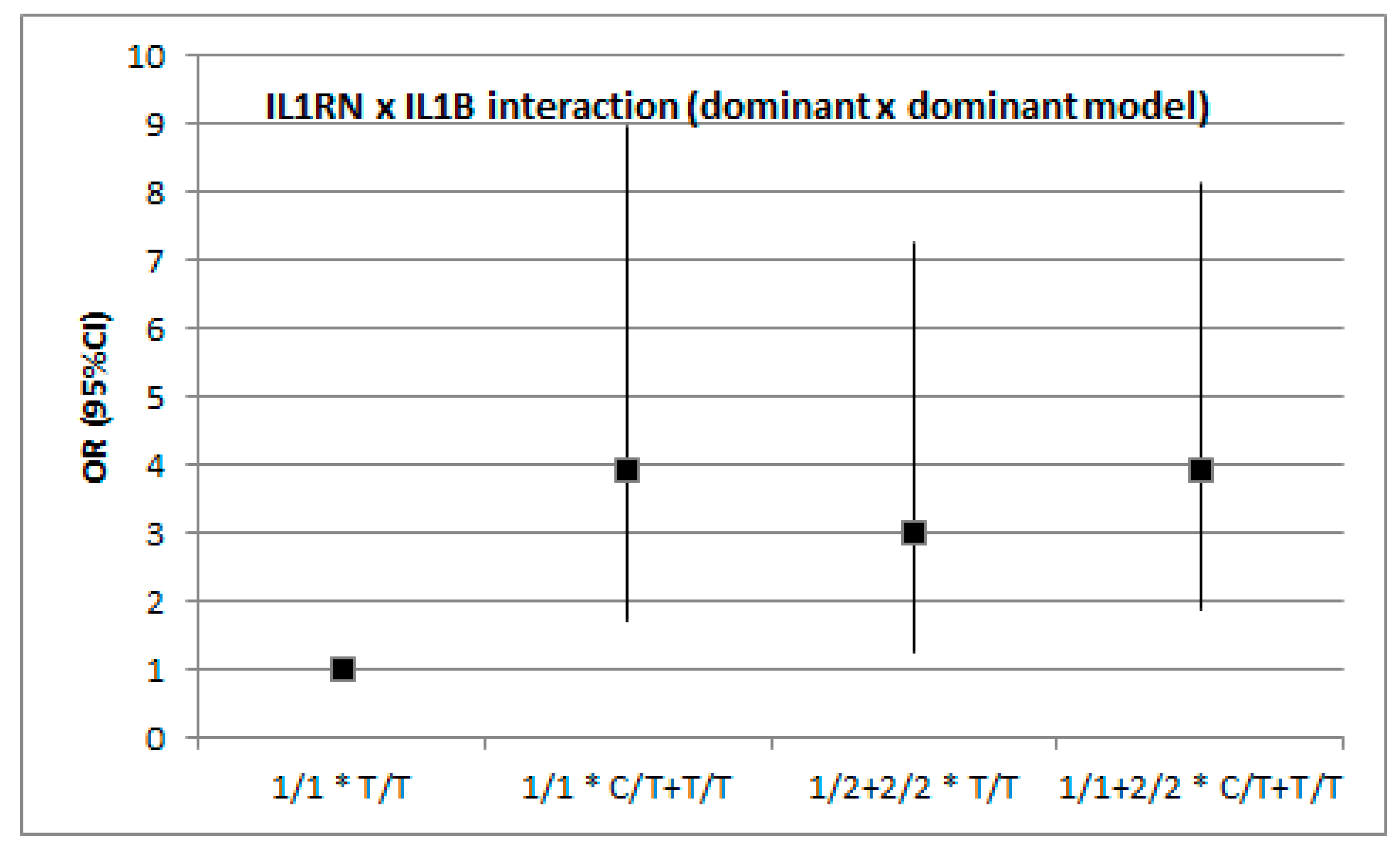
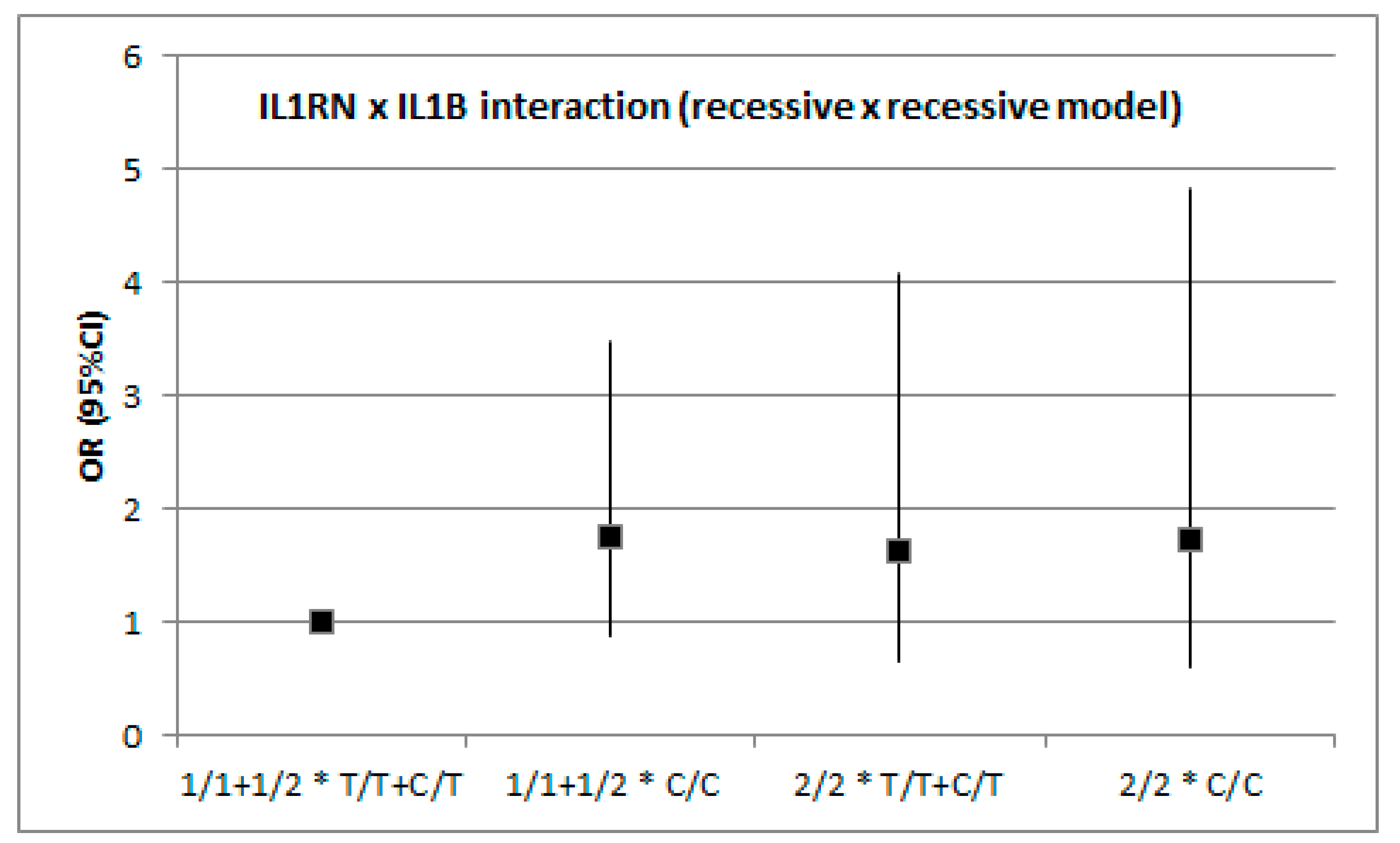
3.2. Association between IS and Genetic Variation in IL1B and IL1RN by Stroke Subtype (TOAST)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bochner, B.S.; Luscinskas, F.W.; Gimbrone, M.A., Jr.; Newman, W.; Sterbinsky, S.A.; Derse-Anthony, C.P.; Klunk, D.; Schleimer, R.P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin-1 activated endothelial cells: Contributions of endothelial adhesion molecules. J. Exp. Med. 1991, 173, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Marculescu, R.; Endler, G.; Schillinger, M.; Iordanova, N.; Exner, M.; Hayden, E.; Huber, K.; Wagner, O.; Mannhalter, C. Interleukin-1 receptor antagonist genotype is associated with coronary atherosclerosis in patients with type 2 diabetes. Diabetes 2002, 51, 3582–3585. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.L.; Cerqueira, C.C.; Bonfim-Silva, R.; Araújo, L.J.; Pereira, J.F.; Gadelha, S.R.; Barbosa, A.A. Interleukin-1 beta and interleukin-6 polymorphism associations with angiographically assessed coronary artery disease in Brazilians. Cytokine 2010, 50, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhong, L.J.; He, B.X.; Li, W.C.; Nie, J.; Wang, X.; Chen, X.T. The correlation between polymorphism at position—511 C/T in the promoter region of interleukin 1B and the severity of coronary heart disease. Zhonhua Yi Xue Yi Chuan Xue Za Zhi 2006, 3, 86–88. [Google Scholar]
- Santilla, S.; Savinainen, K.; Hurme, M. Presence of the IL-1RA allele2(IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scan. J. Immunol. 1998, 47, 195–198. [Google Scholar] [CrossRef]
- Blakemore, A.I.; Tarlow, J.K.; Cork, M.J.; Gordon, C.; Emery, P.; Duff, G.W. Interleukin-1 receptor antagonist gene polymorphism as a disease severity factor in systemic lupus erythematosus. Artritis Rheum. 1994, 37, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.A.; Loddick, S.A.; Toulmond, S.; Stroemer, R.P.; Hunt, J.; Rothwell, N.J. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 87–98. [Google Scholar] [CrossRef]
- Touzani, O.; Boutin, H.; Chuquet, J.; Rothwell, N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J. Neuroimmunol. 1999, 100, 203–215. [Google Scholar] [CrossRef]
- Touzani, O.; Boutin, H.; LeFeuvre, R.; Parker, L.; Miller, A.; Luheshi, G.; Rothwell, N. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 receptor. J. Neurosc. 2002, 22, 38–43. [Google Scholar] [CrossRef]
- Tarlow, J.K.; Blakemore, A.I.; Lennard, A.; Solari, R.; Hughes, H.N.; Steinkasserer, A.; Duff, G.W. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum. Genet. 1993, 91, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Hurme, M.; Santila, S. IL-1 receptor antagonist (IL-Ra) plasma levels are co-ordinately regulated by both IL-Ra and IL-1beta genes. Eur. J. Immunol. 1998, 47, 195–198. [Google Scholar]
- Worrall, B.B.; Azhar, S.; Nyquist, P.A.; Ackerman, R.H.; Hamm, T.L.; DeGraba, T.J. Interleukin-1 receptor antagonist gene polymorphisms in carotid atherosclerosis. Stroke 2003, 34, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, J.M.; Delgadillo, H.; Llorente, L.; Chuquiure, E.; Juárez-Cedillo, T.; Vallejo, M.; Lima, G.; Furuzawa-Carballeda, J.; Peña-Duque, M.A.; Martínez-Ríos, M.A.; et al. Interleukin 1 receptor antagonist polymorphisms are associated with the risk of developing acute coronary syndrome in Mexicans. Immunol. Lett. 2010, 133, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.E.; Camp, N.J.; Dewberry, R.M.; Gunn, J.; Syrris, P.; Carter, N.D.; Jeffery, S.; Kaski, J.C.; Cumberland, D.C.; Duff, G.W.; et al. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation 1999, 99, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P.S.; Sheikine, Y.; Jatta, K.; Ghaderi, M.; Samnegård, A.; Eriksson, P.; Sirsjö, A. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1 beta: Interleukin-1 receptor antagonist balance in atherosclerosis. Circ. J. 2009, 73, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, Z.Y.; Chen, J.Q.; Chen, H.M. Association between the interleukin-1B gene—511C/T polymorphism and ischemic stroke: An update meta-analysis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Li, N.; He, Z.; Xu, J.; Liu, F.; Deng, S.; Zhang, H. Association of PDE4D and IL-1 gene polymorphism with ischemic stroke in a Han Chinese population. Brain Res. Bull. 2010, 81, 38–42. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Wang, L.; Ding, Y. Lack of association between interleukin-1β receptor antagonist gene 86-bp VNTR polymorphism and ischemic stroke: A meta-analysis. Medicine 2018, 97, e11750. [Google Scholar] [CrossRef]
- Noha, A.R.; Hanan, S.M. Influence of interleukin-1 gene cluster polymorphism on the susceptibility and outcomes of acute stroke in Egyptian patients. Cell Biochem. Biophys. 2015, 71, 637–647. [Google Scholar]
- Nemetz, A.; Nosti-Escanilla, M.P.; Molnár, T.; Köpe, A.; Kovács, A.; Fehér, J.; Tulassay, Z.; Nagy, F.; García-González, M.A.; Peña, A.S. IL-1B gene polymorphism influence the course and severity of inflammatory bowel disease. Immunogenetics 1999, 49, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Di Castelnuovo, A.; Gattone, M.; Pezzini, A.; Assanelli, D.; Lorenzet, R.; Del Zotto, E.; Colombo, M.; Napoleone, E.; Amore, C.; et al. Polymorphisms of the interleukin-1beta gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Nishioka, T.; Soemantri, A.; Ishida, T. Cis-acting effect of the IL-1B C-31T polymorphism on IL-1beta mRNA expression. Genes Immun. 2005, 5, 571–575. [Google Scholar]
- Hall, S.K.; Perregaux, D.G.; Gabel, C.A.; Woodworth, T.; Durham, L.K.; Huizinga, T.W.; Breedveld, F.C.; Seymour, A.B. Correction of polymorphic variation in the promoter region of the interleukin-1β gene with secretion of interleukin 1β protein. Arthritis Reum. 2004, 50, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.K.; Menon, S.; Griffiths, L.R.; Gan, S.H. Signaling pathway genes for blood pressure, folate and cholesterol levels among hypertensives: An epistasis analysis. J. Hum. Hypertens. 2015, 29, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cadenas, I.; Del Río-Espínola, A.; Giralt, D.; Domingues-Montanari, S.; Quiroga, A.; Mendióroz, M.; Ruíz, A.; Ribó, M.; Serena, J.; Obach, V.; et al. IL1B and VWF variants are associated with fibrynolitic early recanalization in patients with ischemic stroke. Stroke 2012, 43, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Manso, H.; Krug, T.; Sobral, J.; Albergaria, I.; Gaspar, G.; Ferro, J.M.; Oliveira, S.A.; Vicente, A.M. Variants in the inflammatory IL6 and MPO genes modulate stroke susceptibility through main effects and gene–gene interactions. J. Cereb. Blood Flow Metab. 2011, 31, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Rechciński, T.; Grebowska, A.; Kurpesa, M.; Sztybrych, M.; Peruga, J.Z.; Trzos, E.; Rudnicka, W.; Krzemińska-Pakuła, M.; Chmiela, M. Interleukin-1b and interleukin-1 receptor inhibitor gene cluster polymorphisms in patients with coronary artery disease after percutaneous angioplasty or coronary artery bypass grafting. Kardiol. Pol. 2009, 67, 601–610. [Google Scholar]
- Gorący, J.; Gorący, I.; Safranow, K.; Taryma, O.; Adler, G.; Ciechanowicz, A. Lack of association of interleukin-1 gene cluster polymorphisms with angiographically documented coronary artery disease: Demonstration of association with hypertension in the Polish population. Arch. Med. Res. 2011, 42, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Ulfhammer, E.; Karlsson, L.; Bokarewa, M.; Wåhlander, K.; Jern, S. Effects of IL-1beta and IL-6 on tissue-type plasminogen activator expression in vascular endothelial cells. Thromb. Res. 2008, 123, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Neuroinflammatory changes negatively impact on LTP: A focus on IL-1beta. Brain Res. 2015, 1621, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.K.; Bechlioullis, A.; Marini, A.; Sionis, D.; Vakalis, K.; Triantis, G.; Wilkins, L.; Rogus, J.; Kornman, K.S.; Witztum, J.L.; et al. Interleukin-1 genotypes modulate the long-term effect of lipoprotein (a) on cardiovascular events: The Ioannina study. J. Clin. Lipidol. 2018, 12, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Flex, A.; Gaetani, E.; Papaleo, P.; Straface, G.; Proia, A.S.; Pecorini, G.; Tondi, P.; Pola, P.; Pola, R. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke 2004, 35, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Koukkou, E.; Watts, G.F.; Mazurkiewicz, J.; Lowy, C. Ethnic differences in lipid and lipoprotein metabolism in pregnant women of African and Caucasian origin. J. Clin. Pathol. 1994, 47, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Cases (n = 147) | Control (n = 117) | p |
|---|---|---|---|
| Age (years) | 66.9 ± 12.1 | 56.8 ± 9.8 | <0.0001 |
| BMI (kg/m2) | 27.6 ± 4.8 | 26.9 ± 4.2 | 0.286 |
| Sex (Males) | 54% (80) | 56% (65) | 0.854 |
| Smoking | 32% (47) | 24% (28) | 0.150 |
| Diabetes mellitus | 27% (40) | 13% (15) | 0.004 |
| Hypertension | 60% (88) | 51% (60) | 0.163 |
| Dyslipidemia | 16% (23) | 76% (89) | <0.0001 |
| TOAST | |||
| Large-vessel atherosclerosis | 46% (68) | ||
| Cardioembolism | 17% (25) | ||
| Small-vessel | 26% (38) | ||
| Others | 11% (16) |
| Model | Control (n = 117) | % | Cases (n = 147) | % | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|
| Codominant | ||||||||
| 1/1 | 54 | 46.2 | 49 | 33.3 | 1.00 | 0.647 * | ||
| 1/2 | 49 | 41.9 | 73 | 49.7 | 1.64 | 0.97 | 2.79 | |
| 2/2 | 14 | 12.0 | 25 | 17.0 | 1.97 | 0.92 | 4.21 | |
| Dominant | ||||||||
| 1/1 | 54 | 46.2 | 49 | 33.3 | 1.00 | 0.358 * | ||
| 1/2–2/2 | 63 | 53.8 | 98 | 66.7 | 1.71 | 1.04 | 2.83 | |
| Recessive | ||||||||
| 1/1–1/2 | 103 | 88.0 | 122 | 83.0 | 1.00 | 0.650 * | ||
| 2/2 | 14 | 12.0 | 25 | 17.0 | 1.51 | 0.75 | 3.05 | |
| Model | Control (n = 117) | % | Cases (n = 147) | % | OR | 95% CI | p * | |
|---|---|---|---|---|---|---|---|---|
| Codominant | ||||||||
| T/T | 51 | 43.6 | 37 | 25.2 | 1.00 | 0.065 * | ||
| C/T | 45 | 38.5 | 71 | 48.3 | 2.17 | 1.24 | 3.82 | |
| C/C | 21 | 17.9 | 39 | 26.5 | 2.56 | 1.30 | 5.05 | |
| Dominant | ||||||||
| T/T | 51 | 43.6 | 37 | 25.2 | 1.00 | 0.020 * | ||
| C/T-C/C | 66 | 56.4 | 110 | 74.8 | 2.30 | 1.36 | 3.87 | |
| Recessive | ||||||||
| T/T-C/T | 96 | 82.1 | 108 | 73.5 | 1.00 | 0.322 * | ||
| C/C | 21 | 17.9 | 39 | 26.5 | 1.65 | 0.91 | 3.00 | |
| Model | Control (n = 117) | CEI (n = 25) | OR (95% CI) | p * | SVI (n = 38) | OR (95% CI) | p * | LVI (n = 68) | OR (95% CI) | p * |
|---|---|---|---|---|---|---|---|---|---|---|
| Codominant | ||||||||||
| 1/1 | 54 (46.2) | 6 (24.0) | 1.00 | 13 (34.2) | 1.00 | 25 (36.8) | 1.00 | |||
| 1/2 | 49 (41.9) | 14 (56.0) | 2.57 (0.92–7.21) | 19 (50.0) | 1.61 (0.72–3.60) | 34 (50.0) | 1.50 (0.79–2.86) | |||
| 2/2 | 14 (12.0) | 5 (20.0) | 3.21 (0.85–12.09) | 0.388 | 6 (15.8) | 1.78 (0.57–5.52) | 0.947 | 9 (13.2) | 1.39 (0.53–3.63) | 0.974 |
| Dominant | ||||||||||
| 1/1 | 54 (46.2) | 6 (24.0) | 1.00 | 13 (34.2) | 1.00 | 25 (36.8) | 1.00 | |||
| 1/2–2/2 | 63 (53.8) | 19 (76.0) | 2.71 (1.01–7.28) | 0.261 | 25 (65.8) | 1.65 (0.77–3.53) | 0.854 | 43 (63.2) | 1.47 (0.80–2.72) | 0.982 |
| Recessive | ||||||||||
| 1/1–1/2 | 103 (88.0) | 20 (80.0) | 1.00 | 32 (84.2) | 1.00 | 59 (86.8) | 1.00 | |||
| 2/2 | 14 (12.0) | 5 (20.0) | 1.84 (0.60–5.68) | 0.695 | 6 (15.8) | 1.38 (0.49–3.88) | 0.752 | 9 (13.2) | 1.12 (0.46–2.75) | 0.823 |
| Model | Control (n = 117) | CEI (n = 25) | OR (95% CI) | p * | SVI (n = 38) | OR (95% CI) | p * | LVI (n = 68) | OR (95% CI) | p * |
|---|---|---|---|---|---|---|---|---|---|---|
| Codominant | ||||||||||
| T/T | 51 (43.6) | 5 (20.0) | 1.00 | 8 (21.1) | 1.00 | 19 (27.9) | 1.00 | |||
| C/T | 45 (38.5) | 14 (56.0) | 3.17 (1.06–9.50) | 19 (50.0) | 2.69 (1.07–6.74) | 33 (48.5) | 1.97 (0.99–3.93) | |||
| C/C | 21 (17.9) | 6 (24.0) | 2.91 (0.80–10.60) | 0.074 | 11 (28.9) | 3.34 (1.18–9.48) | 0.236 | 16 (23.5) | 2.05 (0.89–4.72) | 0.069 |
| Dominant | ||||||||||
| T/T | 51 (43.6) | 5 (20.0) | 1.00 | 8 (21.1) | 1.00 | 19 (27.9) | 1.00 | |||
| C/T-C/C | 66 (56.4) | 20 (80.0) | 3.09 (1.09–8.80) | 0.305 | 30 (78.9) | 2.90 (1.22–6.86) | 0.106 | 49 (72.1) | 1.99 (1.05–3.79) | 0.036 |
| Recessive | ||||||||||
| T/T-C/T | 96 (82.1) | 19 (76.0) | 1.00 | 27 (71.1) | 1.00 | 52 (76.5) | 1.00 | |||
| C/C | 21 (17.9) | 6 (24.0) | 1.44 (0.51–4.05) | 0.225 | 11 (28.9) | 1.86 (0.80–4.34) | 0.874 | 16 (23.5) | 1.41 (0.68–2.93) | 0.993 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorący, I.; Kaczmarczyk, M.; Ciechanowicz, A.; Lewandowska, K.; Jakubiszyn, P.; Bodnar, O.; Kopijek, B.; Brodkiewicz, A.; Cyryłowski, L. Polymorphism of Interleukin 1B May Modulate the Risk of Ischemic Stroke in Polish Patients. Medicina 2019, 55, 558. https://doi.org/10.3390/medicina55090558
Gorący I, Kaczmarczyk M, Ciechanowicz A, Lewandowska K, Jakubiszyn P, Bodnar O, Kopijek B, Brodkiewicz A, Cyryłowski L. Polymorphism of Interleukin 1B May Modulate the Risk of Ischemic Stroke in Polish Patients. Medicina. 2019; 55(9):558. https://doi.org/10.3390/medicina55090558
Chicago/Turabian StyleGorący, Iwona, Mariusz Kaczmarczyk, Andrzej Ciechanowicz, Klaudyna Lewandowska, Paweł Jakubiszyn, Oksana Bodnar, Bartosz Kopijek, Andrzej Brodkiewicz, and Lech Cyryłowski. 2019. "Polymorphism of Interleukin 1B May Modulate the Risk of Ischemic Stroke in Polish Patients" Medicina 55, no. 9: 558. https://doi.org/10.3390/medicina55090558
APA StyleGorący, I., Kaczmarczyk, M., Ciechanowicz, A., Lewandowska, K., Jakubiszyn, P., Bodnar, O., Kopijek, B., Brodkiewicz, A., & Cyryłowski, L. (2019). Polymorphism of Interleukin 1B May Modulate the Risk of Ischemic Stroke in Polish Patients. Medicina, 55(9), 558. https://doi.org/10.3390/medicina55090558






