Diagnosis and Management of Left Atrium Appendage Thrombosis in Atrial Fibrillation Patients Undergoing Cardioversion
Abstract
1. Introduction
2. Risk Factors of Left Atrial Appendage Thrombosis
3. The Role of Echocardiography before Cardioversion
3.1. Transthoracic Echocardiography
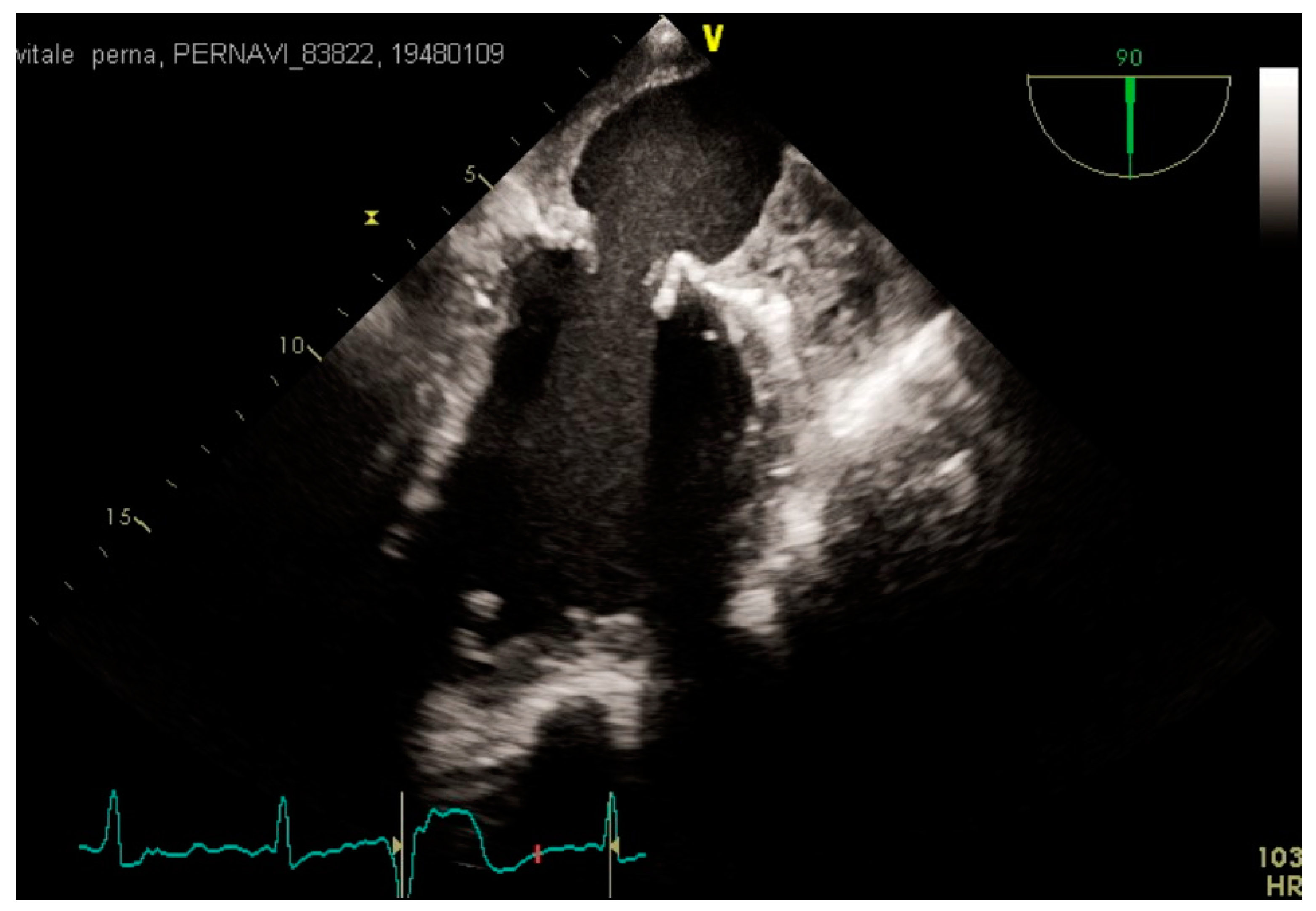
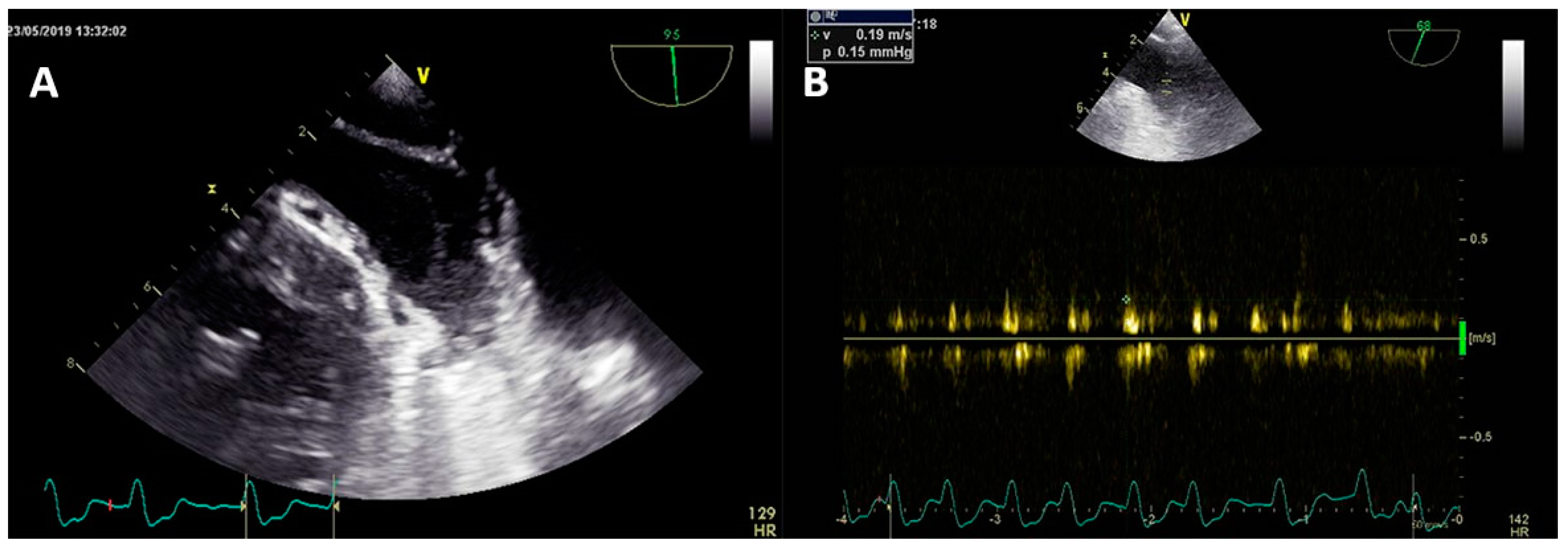
3.2. Transesophageal Echocardiography and Intracardiac Echocardiography
4. Prevention and Treatment of Left Atrial Appendage Thrombosis
5. Conclusions
- The incidence of LAA thrombosis in AF patients undergoing CV is not a negligible event despite adequate anticoagulant therapy.
- A TOE-guided CV is a mandatory strategy in order to reduce the burden of periprocedural thromboembolic events.
- Although VKAs have been historically considered the cornerstone anticoagulant therapy before CV, growing evidence show that NOACs are safe and effective alternatives in this setting.
- Further and extended data are needed to assess the efficacy and safety profile of NOACs for the treatment of LAA thrombosis.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feinberg, W.M.; Blackshear, J.L.; Laupacis, A.; Kronmal, R.; Hart, R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995, 155, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.; Engholm, G.; Johnsen, S.; Moller, H.; Henneberg, E.W.; Husted, S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch. Intern. Med. 2001, 161, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influe.nce on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.E.; Gronberg, T.; Nuotio, I.; Nikkinen, M.; Ylitalo, A.; Biancari, F.; Hartikainen, J.E. Thromboembolic complications after cardioversion of acute atrial fibrillation: The FinCV (Finnish CardioVersion) study. J. Am. Coll. Cardiol. 2013, 62, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef]
- Cappato, R.; Ezekowitz, M.D.; Klein, A.L.; Camm, A.J.; Ma, C.S.; Le Heuzey, J.Y.; Talajic, M.; Scanavacca, M.; Vardas, P.E.; Kirchhof, P.; et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur. Heart J. 2014, 35, 3346–3355. [Google Scholar] [CrossRef]
- Goette, A.; Merino, J.L.; Ezekowitz, M.D.; Zamoryakhin, D.; Melino, M.; Jin, J.; Mercuri, M.F.; Grosso, M.A.; Fernandez, V.; Al-Saady, N.; et al. Edoxaban versus enoxaparin–warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): A randomised, open-label, phase 3b trial. Lancet 2016, 388, 1995–2003. [Google Scholar] [CrossRef]
- Ezekowitz, M.D.; Pollack, C.V., Jr.; Halperin, J.L.; England, R.D.; VanPelt Nguyen, S.; Spahr, J.; Sudworth, M.; Cater, N.B.; Breazna, A.; Oldgren, J.; et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: The EMANATE trial. Eur. Heart J. 2018, 39, 2959–2971. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Cresti, A.; García-Fernández, M.A.; Sievert, H.; Mazzone, P.; Baratta, P.; Solari, M.; Geyer, A.; De Sensi, F.; Limbruno, U. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: A large Transeophageal Echo study. EuroIntervention 2019, 15, e225–e230. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Klosterman, T.; Grewal, G.; Alraies, M.C.; Varr, B.C.; Lindsay, B.; Zurick, A.O.; Shrestha, K.; Tang, W.H.; Bhargava, M.; et al. Efficacy of the CHADS₂ scoring system to assess left atrial thrombogenic milieu risk before cardioversion of non-valvular atrial fibrillation. Am. J. Cardiol. 2013, 112, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.; Pohlmeier, M.; Hess, A.; Mereles, D.; Kieser, M.; Bruckner, T.; Scholz, E.; Zitron, E.; Schweizer, P.A.; Katus, H.A.; et al. Prevalence of intracardiac thrombi under phenprocoumon, direct oral anticoagulants (dabigatran and rivaroxaban), and bridging therapy in patients with atrial fibrillation and flutter. Am. J. Cardiol. 2015, 115, 635–640. [Google Scholar] [CrossRef]
- Willens, H.J.; Gomez-Marin, O.; Nelson, K.; DeNicco, A.; Moscucci, M. Correlation of CHADS2 and CHA2DS2-VASc scores with transesophageal echocardiography risk factors for thromboembolism in a multiethnic United States population with nonvalvular atrial fibrillation. J. Am. Soc. Echocardiogr. 2013, 26, 175–184. [Google Scholar] [CrossRef]
- Sikorska, A.; Baran, J.; Pilichowska-Paszkiet, E.; Sikora-Frąc, M.; Kryński, T.; Piotrowski, R.; Stec, S.; Zaborska, B.; Kułakowski, P. Risk of left atrial appendage thrombus in patients scheduled for ablation for atrial fibrillation: Beyond the CHA2DS2VASc score. Pol. Arch. Med. Wewnętrznej 2015, 125, 921–928. [Google Scholar] [CrossRef]
- Uz, O.; Atalay, M.; Dogan, M.; Isilak, Z.; Yalcin, M.; Uzun, M.; Kardesoglu, E.; Cebeci, B.S. The CHA2DS2-VASc score as a predictor of left atrial thrombus in patients with non-valvular atrial fibrillation. Med. Princ. Pract. 2014, 23, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.B.; Dong, J.Z.; Liu, X.P.; Long, D.Y.; Yu, R.H.; Du, X.; Liu, X.H.; Ma, C.S. Is CHA2DS2-VASc score a predictor of left atrial thrombus in patients with paroxysmal atrial fibrillation? Thromb. Haemost. 2011, 105, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, H.; Varr, B.C.; Puwanant, S.; Lieber, E.; Williams, S.J.; Klostermann, T.; Jasper, S.E.; Whitman, C.; Klein, A.L. Role of CHADS2 score in evaluation of thromboembolic risk and mortality in patients with atrial fibrillation undergoing direct current cardioversion (from the ACUTE Trial Substudy). Am. J. Cardiol. 2012, 110, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ochiumi, Y.; Kagawa, E.; Kato, M.; Sasaki, S.; Nakano, Y.; Itakura, K.; Takiguchi, Y.; Ikeda, S.; Dote, K. Usefulness of brain natriuretic peptide for predicting left atrial appendage thrombus in patients with unanticoagulated nonvalvular persistent atrial fibrillation. J. Arrhythmia 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Habara, S.; Dote, K.; Kato, M.; Sasaki, S.; Goto, K.; Takemoto, H.; Hasegawa, D.; Matsuda, O. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur. Heart J. 2007, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ayirala, S.; Kumar, S.; O’Sullivan, D.M.; Silverman, D.I. Echocardiographic predictors of left atrial appendage thrombus formation. J. Am. Soc. Echocardiogr. 2011, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Marchese, P.; Bursi, F.; Delle Donne, G.; Malavasi, V.; Casali, E.; Barbieri, A.; Melandri, F.; Modena, M.G. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur. J. Echocardiogr. 2011, 12, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Doukky, R.; Khandelwal, A.; Garcia-Sayan, E.; Gage, H. External validation of a novel transthoracic echocardiographic tool in predicting left atrial appendage thrombus formation in patients with nonvalvular atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Lip, G.Y.; Galderisi, M.; Goette, A.; Shah, D.; Marwan, M.; Lederlin, M.; Mondillo, S.; Edvardsen, T.; Sitges, M.; et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Fornengo, C.; Antolini, M.; Frea, S.; Gallo, C.; Grosso Marra, W.; Morello, M.; Gaita, F. Prediction of atrial fibrillation recurrence after cardioversion in patients with left-atrial dilation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 335–341. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.S.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Card. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Cameli, M.; Lunghetti, S.; Mandoli, G.E.; Righini, F.M.; Lisi, M.; Curci, V.; Di Tommaso, C.; Solari, M.; Nistor, D.; Gismondi, A.; et al. Left Atrial Strain Predicts Pro-Thrombotic State in Patients with non-valvular Atrial Fibrillation. J. Atr. Fibrillation 2017, 10, 1641. [Google Scholar] [CrossRef]
- Costa, C.; Alujas, T.G.; Valente, F.; Aranda, C.; Rodríguez-Palomares, J.; Gutierrez, L.; Maldonado, G.; Galian, L.; Teixidó, G.; Evangelista, A. Left atrial strain: A new predictor of thrombotic risk and successful electrical cardioversion. Echo Research and practice. Echo Res. Pract. 2016, 3, 45–52. [Google Scholar] [CrossRef]
- Manning, W.J.; Weintraub, R.M.; Waksmonski, C.A.; Haering, J.M.; Rooney, P.S.; Maslow, A.D.; Johnson, R.G.; Douglas, P.S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995, 123, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Troughton, R.W.; Asher, C.R.; Klein, A.L. The role of echocardiography in atrial fibrillation and cardioversion. Heart 2003, 89, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Black, I.W. Spontaneous echo contrast: Where there’s smoke there’s fire. Echocardiography 2000, 17, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arango, A.; Landolfo, C. A novel approach to the diagnosis of left atrial appendage thrombus using contrast echocardiography and power Doppler imaging. Eur. J. Echocardiogr. 2008, 9, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Veinot, J.P.; Harrity, P.J.; Gentile, F.; Khandheria, B.K.; Bailey, K.R.; Eickholt, J.T.; Seward, J.B.; Tajik, A.J.; Edwards, W.D. Anatomy of the normal left atrial appendage: A quantitative study of age-related changes in 500 autopsy hearts—implications for echocardiographic examination. Circulation 1997, 96, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Squara, F.; Bres, M.; Baudouy, D.; Schouver, E.D.; Moceri, P.; Ferrari, E. Transesophageal echocardiography for the assessment of left atrial appendage thrombus: Study of the additional value of systematic real time 3D imaging after regular 2D evaluation. Echocardiography 2018, 35, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Seo, Y.; Ishizu, T.; Yamamoto, M.; Machino, T.; Harimura, Y.; Kawamura, R.; Sekiguchi, Y.; Tada, H.; Aonuma, K. Analysis of the left atrial appendage by three-dimensional transesophageal echocardiography. Am. J. Cardiol. 2010, 106, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Basman, C.; Parmar, Y.J.; Kronzon, I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr. Cardiol. Rep. 2017, 19, 102. [Google Scholar] [CrossRef]
- Baran, J.; Stec, S.; Pilichowska-Paszkiet, E.; Zaborska, B.; Sikora-Frąc, M.; Kryński, T.; Michałowska, I.; Łopatka, R.; Kułakowski, P. Intracardiac Echocardiography for Detection of Thrombus in the Left Atrial Appendage. Circ. Arrhythmia Electrophysiol. 2013, 6, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Sra, J.; Jordaens, L.; Kusumoto, F.; Knight, B.; Natale, A.; Kocheril, A.; Nanda, N.C.; Nagarakanti, R.; Simon, A.M.; et al. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: The intracardiac echocardiography guided cardioversion helps interventional procedures study. Circ. Arrhythmia Electrophysiol. 2010, 3, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J. The management of chronic atrial fibrillation. Prog. Cardiovasc. Dis. 1960, 2, 465–479. [Google Scholar] [CrossRef]
- Manning, W.J.; Leeman, D.E.; Gotch, P.J. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J. Am. Coll. Cardiol. 1989, 13, 617–623. [Google Scholar] [CrossRef]
- Scherr, D.; Dalal, D.; Chilukuri, K.; Dong, J.; Spragg, D.; Henrikson, C.A.; Nazarian, S.; Cheng, A.; Berger, R.D.; Abraham, T.P.; et al. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.W.; Atwater, B.D.; Daubert, J.P.; Voora, D.; Crowley, A.L.; Bahnson, T.D.; Hranitzky, P.M. Prevalence and clinical characteristics associated with left atrial appendage thrombus in fully anticoagulated patients undergoing catheter-directed ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010, 21, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M.; Garberoglio, L.; Gili, S.; Bertaglia, E.; Stabile, G.; Marazzi, R.; Themistoclakis, S.; Solimene, F.; Frea, S.; Marra, W.G.; et al. Left atrial appendage thrombi relate to easily accessible clinical parameters in patients undergoing atrial fibrillation transcatheter ablation: A multicentre study. Int. J. Cardiol. 2017, 241, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. RELY Steering Committee and Investigators, Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Russo, V.; Carbone, A.; Rago, A.; Golino, P.; Nigro, G. Direct Oral Anticoagulants in octogenarians with atrial fibrillation: it’s never too late. J. Cardiovasc. Pharmacol. 2019, 73, 207–214. [Google Scholar] [CrossRef]
- Russo, V.; Attena, E.; Mazzone, C.; Melillo, E.; Rago, A.; Galasso, G.; Riegler, L.; Parisi, V.; Rotunno, R.; Nigro, G.; et al. Real-life Performance of Edoxaban in Elderly Patients with Atrial Fibrillation: A Multicenter Propensity Score-Matched Cohort Study. Clin. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Rago, A.; Proietti, R.; Attena, E.; Rainone, C.; Crisci, M.; Papa, A.A.; Calabrò, P.; D’Onofrio, A.; Golino, P.; et al. Safety and Efficacy of Triple Antithrombotic Therapy with Dabigatran versus Vitamin K Antagonist in Atrial Fibrillation Patients: A Pilot Study. BioMed Res. Int. 2019, 2019, 5473240. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Bottino, R.; Rago, A.; Di Micco, P.; D’Onofrio, A.; Liccardo, B.; Golino, P.; Nigro, G. Atrial Fibrillation and Malignancy: The Clinical Performance of Non-Vitamin K Oral Anticoagulants-A Systematic Review. Semin. Thromb. Hemost. 2019, 45, 205–214. [Google Scholar] [PubMed]
- Russo, V.; Attena, E.; Mazzone, C.; Esposito, F.; Parisi, V.; Bancone, C.; Rago, A.; Nigro, G.; Sangiuolo, R.; D’Onofrio, A. Nonvitamin K Antagonist Oral Anticoagulants Use in Patients with Atrial Fibrillation and Bioprosthetic Heart Valves/Prior Surgical Valve Repair: A Multicenter Clinical Practice Experience. Semin. Thromb. Hemost. 2018, 44, 364–369. [Google Scholar] [PubMed]
- Russo, V.; Rago, A.; Papa, A.A.; Di Meo, F.; Attena, E.; Golino, P.; D’Onofrio, A.; Nigro, G. Use of Non-Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Malignancy: Clinical Practice Experience in a Single Institution and Literature Review. Semin. Thromb. Hemost. 2018, 44, 370–376. [Google Scholar] [PubMed]
- Russo, V.; Rago, A.; Proietti, R.; Di Meo, F.; Antonio Papa, A.; Calabrò, P.; D’Onofrio, A.; Nigro, G.; AlTurki, A. Efficacy and safety of the target-specific oral anticoagulants for stroke prevention in atrial fibrillation: The real-life evidence. Ther. Adv. Drug Saf. 2017, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Bianchi, V.; Cavallaro, C.; Vecchione, F.; De Vivo, S.; Santangelo, L.; Sarubbi, B.; Calabro, P.; Nigro, G.; D’Onofrio, A. Efficacy and safety of dabigatran in a “real-life” population at high thromboembolic and hemorrhagic risk: Data from MonaldiCare registry. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3961–3967. [Google Scholar]
- Russo, V.; Rago, A.; D’Onofrio, A.; Nigro, G. The clinical performance of dabigatran in the Italian real-life experience. J. Cardiovasc. Med. 2017, 18, 922–923. [Google Scholar] [CrossRef]
- Telles-Garcia, N.; Dahal, K.; Kocherla, C.; Lip, G.Y.H.; Reddy, P.; Dominic, P. Non-vitamin K anticoagulants oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 268, 143–148. [Google Scholar] [CrossRef]
- Gibson, C.M.; Basto, A.N.; Howard, M.L. Direct Oral Anticoagulants in Cardioversion: A Review of Current Evidence. Ann. Pharmacother. 2018, 52, 277–284. [Google Scholar] [CrossRef]
- Itäinen, S.; Lehto, M.; Vasankari, T.; Mustonen, P.; Kotamäki, M.; Numminen, A.; Lahtela, H.; Bah, A.; Hartikainen, J.; Hekkala, A.M.; et al. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients undergoing elective cardioversion. Europace 2018, 20, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, A.S.; Albertsen, A.E.; Christesen, A.M.S.; Vinter, N.; Frost, L.; Møller, D.S. Cardioversion of atrial fibrillation in a real-world setting: Non-vitamin K antagonist oral anticoagulants ensure a fast and safe strategy compared to warfarin. Europace 2018, 20, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Rago, A.; Papa, A.A.; D’Onofrio, A.; Golino, P.; Nigro, G. Efficacy and safety of dabigatran in patients with atrial fibrillation scheduled for transoesophageal echocardiogram-guided direct electrical current cardioversion: A prospective propensity score-matched cohort study. J. Thromb. Thrombolysis 2018, 45, 206–212. [Google Scholar] [CrossRef]
- Russo, V.; Di Napoli, L.; Bianchi, V.; Tavoletta, V.; De Vivo, S.; Cavallaro, C.; Vecchione, F.; Rago, A.; Sarubbi, B.; Calabrò, P.; et al. A new integrated strategy for direct current cardioversion in non-valvular atrial fibrillation patients using short term rivaroxaban administration: The MonaldiVert real life experience. Int. J. Cardiol. 2016, 224, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Rago, A.; Papa, A.A.; Cassese, A.; Arena, G.; Magliocca, M.C.G.; D’Onofrio, A.; Golino, P.; Nigro, G.; Russo, V. Clinical Performance of Apixaban vs. Vitamin K Antagonists in Patients with Atrial Fibrillation Undergoing Direct Electrical Current Cardioversion: A Prospective Propensity Score-Matched Cohort Study. Am. J. Cardiovasc. Drugs 2019, 19, 421–427. [Google Scholar] [CrossRef]
- Stabile, G.; Russo, V.; Rapacciuolo, A.; De Divitiis, M.; De Simone, A.; Solimene, F.; D’Onofrio, A.; Iuliano, A.; Maresca, G.; Esposito, F.; et al. Transesophageal echocardiograpy in patients with persistent atrial fibrillation undergoing electrical cardioversion on new oral anticoagulants: A multi center registry. Int. J. Cardiol. 2015, 184, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.; Zima, E.; Bover, R.; Karaliute, R.; Rossi, A.; Szymanski, C.; Troccoli, R.; Schneider, J.; Fagerland, M.W.; Camm, A.J.; et al. Changes in oral anticoagulation for elective cardioversion: Results from a European cardioversion registry. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 147–150. [Google Scholar] [CrossRef]
- Russo, V.; Rago, A.; Papa, A.A.; Bianchi, V.; Tavoletta, V.; DE, S.V.; Cavallaro, C.; Nigro, G.; D’Onofrio, A. Budget impact analysis of rivaroxaban vs. warfarin anticoagulation strategy for direct current cardioversion in non-valvular atrial fibrillation patients: The MonaldiVert Economic Study. Minerva Cardioangiol. 2018, 66, 1–5. [Google Scholar]
- Bertaglia, E.; Anselmino, M.; Zorzi, A.; Russo, V.; Toso, E.; Peruzza, F.; Rapacciuolo, A.; Migliore, F.; Gaita, F.; Cucchini, U.; et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int. J. Cardiol. 2017, 249, 179–183. [Google Scholar] [CrossRef]
- Hwang, J.; Park, H.S.; Jun, S.W.; Choi, S.W.; Lee, C.H.; Kim, I.C.; Cho, Y.K.; Yoon, H.J.; Kim, H.; Nam, C.W.; et al. The incidence of left atrial appendage thrombi on transesophageal echocardiography afterpretreatment with apixaban for cardioversion in the real-world practice. PLoS ONE 2018, 13, e0208734. [Google Scholar] [CrossRef]
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.A.; et al. Use of Transesophageal Echocardiography to Guide Cardioversion in Patients with Atrial Fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Tadeo, G.; Beretta, S.; Tagliagambe, L.M.; Manzillo, G.F.; Spata, M.; Santarone, M. Atrial thrombi resolution after prolonged anticoagulation in patients with atrial fibrillation. Chest 1999, 115, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.J.; Silverman, D.I.; Douglas, P.S.; Manning, W.J. Cardioversion of Nonrheumatic Atrial Fibrillation. Reduced Thromboembolic Complications with 4 Weeks of Precardioversion Anticoagulation Are Related to Atrial Thrombus Resolution. Circulation 1995, 92, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Hammerstingl, C.; Pötzsch, B.; Nickenig, G. Resolution of giant left atrial appendage thrombus with rivaroxaban. Thromb. Haemost. 2013, 109, 583–584. [Google Scholar]
- Takasugi, J.; Yamagami, H.; Okata, T.; Toyoda, K.; Nagatsuka, K. Dissolution of the left atrial appendage thrombus with rivaroxaban therapy. Cerebrovasc. Dis. 2013, 36, 322–323. [Google Scholar] [CrossRef]
- Vidal, A.; Vanerio, G. Dabigatran and left atrial appendage thrombus. J. Thromb. Thrombolysis 2012, 34, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Hammerstingl, C.; Marin, F.; Cappato, R.; Meng, I.L.; Kirsch, B.; van Eickels, M.; Cohen, A. Left atrial thrombus resolution in atrial fibrillation or flutter: Results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am. Heart J. 2016, 178, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Niku, A.D.; Shiota, T.; Siegel, R.J.; Rader, F. Prevalence and Resolution of Left Atrial Thrombus in Patients with Nonvalvular Atrial Fibrillationand Flutter with Oral Anticoagulation. Am. J. Cardiol. 2019, 123, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Fang, C.Y.; Chen, Y.L.; Fang, H.Y.; Chen, H.C.; Liu, W.H.; Fu, M.; Chen, M.C. Left Atrial or Left Atrial Appendage Thrombus Resolution After Adjustment of Oral Anticoagulant Treatment. J. Stroke Cerebrovasc. Dis. 2019, 28, 90–96. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melillo, E.; Palmiero, G.; Ferro, A.; Mocavero, P.E.; Monda, V.; Ascione, L. Diagnosis and Management of Left Atrium Appendage Thrombosis in Atrial Fibrillation Patients Undergoing Cardioversion. Medicina 2019, 55, 511. https://doi.org/10.3390/medicina55090511
Melillo E, Palmiero G, Ferro A, Mocavero PE, Monda V, Ascione L. Diagnosis and Management of Left Atrium Appendage Thrombosis in Atrial Fibrillation Patients Undergoing Cardioversion. Medicina. 2019; 55(9):511. https://doi.org/10.3390/medicina55090511
Chicago/Turabian StyleMelillo, Enrico, Giuseppe Palmiero, Adele Ferro, Paola Elvira Mocavero, Vittorio Monda, and Luigi Ascione. 2019. "Diagnosis and Management of Left Atrium Appendage Thrombosis in Atrial Fibrillation Patients Undergoing Cardioversion" Medicina 55, no. 9: 511. https://doi.org/10.3390/medicina55090511
APA StyleMelillo, E., Palmiero, G., Ferro, A., Mocavero, P. E., Monda, V., & Ascione, L. (2019). Diagnosis and Management of Left Atrium Appendage Thrombosis in Atrial Fibrillation Patients Undergoing Cardioversion. Medicina, 55(9), 511. https://doi.org/10.3390/medicina55090511




