Abstract
Background and objectives: Recurrence of venous thromboembolism (VTE) after a primary event is common; however, no sufficient risk scores have been widely introduced in clinical practice. The aim of this study was to assess the risk factors for VTE recurrences, as well as the effect of treatment strategies on the recurrence rate in a single-center patient cohort. Materials and Methods: The prospective cohort study included consecutive patients in a single center from June 2014 till June 2018 presenting with acute VTE confirmed by imaging tests. All patients were followed up for at least one year or till death. Statistical analyses were conducted using IBM SPSS Statistics 23 and Stata 13. Competing risk of death was considered. Results: A total of 219 eligible patients were identified during the study period. Pulmonary embolism with or without deep vein thrombosis (DVT) was present in 95.9% (n = 210), isolated DVT was present in 4.1% (n = 9) of patients. The total number of documented recurrences was 13 (5.9%). Incidence rate was 5.6 per 100 person-years. Recurrent VTE predicted significantly higher mortality rate (hazard ratio (HR) 6.64 [95% CI 2.61–16.93]). In univariate analysis, active cancer was associated with higher recurrence rate (p = 0.036). In competing-risks regression model (with death as the competing risk), active cancer (subdistribution hazard ratio (SHR) 2.11 (95% CI 0.58–7.76)) did not retain statistical significance for VTE recurrence. Discontinuation and duration of anticoagulant treatment (≤6 or >6 months), and drug class in acute or long-term therapy (parenteral, vitamin K antagonist (VKA), direct oral anticoagulant (DOAC)) were not associated with recurrences (p > 0.05). Conclusions: Patients who experienced recurrent VTE had 6.6-fold higher mortality rate than patients with no recurrences. The presence of active cancer was not a statistically significant risk factor for recurrence when taking into account the competing risk of death. Duration and drug class of anticoagulation did not seem to impact recurrence rate.
1. Introduction
Venous thromboembolism (VTE), comprised of deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cause of death from cardiovascular disease after acute myocardial infarction and stroke [1,2]. Recurrence of VTE after a primary event is common with an overall incidence rate of 4.9 per 100 person-years in non-cancer patients. Higher recurrence risk has been associated with an “unprovoked” primary VTE events and malignancy-related VTE with a 1-year risk of approximately 10% and 15%, respectively [3,4]. Previously described demographic and clinical risk factors for recurrence are male gender, body-mass index, ethnic background, lower socioeconomic status, thrombophilia, hormonal therapy, pregnancy, and cancer, among others. Several multivariate risk scores such as HERDOO2 score [5], Vienna prediction model [6], DASH score [7] have been developed but due to the lack of sufficient external validation, none of them have yet been included in international guidelines [8]. Recent studies have demonstrated the superiority of long-term direct oral anticoagulants (DOAC) over vitamin K antagonists (VKA) and low-molecular-weight heparins (LMWH) in preventing recurrent VTE [9]. The aim of this study was to assess the possible clinical risk factors for VTE recurrences, including comorbidities, cancer, VTE provoking factors, demographic features, laboratory data, and treatment approaches.
2. Materials and Methods
2.1. Study Population
The study cohort included inpatients admitted to cardiology and pulmonology clinics in the Pauls Stradiņš Clinical university hospital in Riga, Latvia from June 2014 till June 2018 (the hospital covers approximately 45,000 inpatients per year). Patients were eligible for our study if the diagnosis of acute VTE was confirmed by imaging studies (Doppler ultrasound, computed tomography angiography) (see Figure 1). All radiographic images were analyzed by experienced radiologists. All included patients had a recent onset of VTE symptoms during the previous 10 days, all patients were subsequently hospitalized. All patients provided written or oral consent for participation in the registry in accordance with local hospital ethics committee requirements (Ethics Committee of Pauls Stradiņš Clinical University Hospital Development Foundation, ID code: 110614-2L, date of approval: 11.06.2014.). Patients with previous VTE (history of any DVT or PE before the index event) were excluded from the present data analysis. We made all efforts to enrol consecutive inpatients admitted to the clinics. Data were recorded in a local computer-based registry.
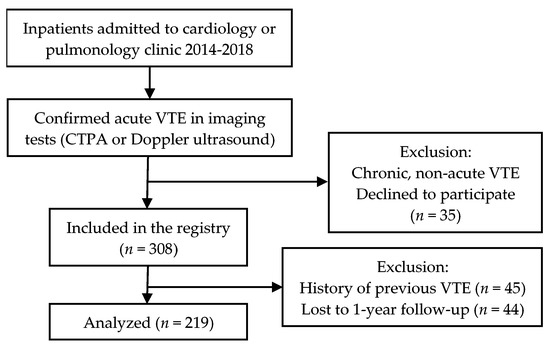
Figure 1.
Inclusion process flowchart.
2.2. Study Design
We conducted a prospective cohort study. Baseline characteristics of patients, laboratory studies, underlying conditions, and therapeutic approaches were recorded. Comorbidity burden was calculated using the Charlson Comorbidity Index. Active cancer was defined as newly diagnosed cancer, metastatic cancer (distant metastases according to the TNM classification), or cancer that is being treated. Transient risk factors were defined as surgical intervention in the past 2 months, immobility ≥ 4 days in the past 2 months, travel > 6 hours in the past 3 weeks, hormonal (estrogen, progestogen) therapy, pregnancy, or recent childbirth. Minor persistent risk factors included BMI > 30 kg/m2, family history of VTE, previous episode of VTE, inflammatory bowel disease, congestive heart failure, and thrombophilia. Unprovoked VTE was defined as an event with no transient or persistent risk factors, including concomitant active cancer according to the International Society on Thrombosis and Haemostasis (ISTH) definition [10]. All patients were followed up for at least one year after the index event or till death. The index time for follow-up was the date of the VTE diagnosis. Recurrence was defined as a repeated VTE event that was confirmed using imaging tests. Date of recurrence or death was registered. The obtained patient–time data was statistically analyzed.
2.3. Statistical Analysis
Data were expressed as absolute numbers and percentage, mean and standard deviation (SD), median and interquartile range (IQR), incidence rate (IR), hazard ratio (HR), subdistribution hazard ratio (SHR), and confidence interval (CI), where appropriate. SPSS Statistics version 23 (IBM, Chicago, Illinois, USA) and Stata version 13 (StataCorp, College Station, Texas, USA) were used for the analyses. Univariate analyses were conducted using the Mann-Whitney U test, χ2-test, and Fisher’s exact test, where appropriate. Since standard regression models overestimate HRs in high-mortality patient groups, e.g., cancer patients, further analyses were done taking the competing risk of death into consideration. To assess the independent effect on mortality of statistically significant variables in univariate analysis (p < 0.05), competing-risks regression analysis was performed and subdistribution HRs were presented. Cox regression model with time to recurrence as a time-varying covariate was used for the assessment of the impact of recurrent VTE on mortality. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Patient Cohort
Following inclusion and exclusion criteria, 219 eligible patients were identified during the study period. Pulmonary embolism with or without DVT was present in 95.9% (n = 210). Isolated DVT with no imaging evidence of PE was found in 4.1% (n = 9) of patients. Baseline clinical characteristics of the patient cohort are shown in Table 1. The total number of documented recurrences was 13 (5.9%). Incidence rate was 5.6 (95% CI 3.3–9.7) cases per 100 person-years. Of the 13 recurrences, 12 were PE with or without DVT and 1 patient had only DVT. Five of the recurrences (38.5%) were fatal (death within 10 days of recurrence and PE as the principal cause). Median time to recurrence was 130 days (IQR 29–336). Overall all-cause mortality was 32.4% (n = 71).

Table 1.
Patient characteristics.
3.2. Clinical Factors
Clinical factors in association with episodes of VTE recurrence and death are shown in Table 2. In univariate analysis, mean age was similar among patients with or without VTE recurrence, p = 0.176.

Table 2.
Univariate analysis of clinical factors in association with VTE recurrence.
Active cancer as the single variable that demonstrated a significant association with VTE recurrence (p < 0.05) was further analyzed in regression models. In a simple Cox regression model, HR of VTE recurrence for cancer was 4.43 (95% CI 1.17–16.8), p = 0.028. A competing-risks regression model (with death as a competing risk), however, did not demonstrate a statistical significance of cancer—SHR 2.11 (95% CI 0.58–7.76), p = 0.259. Unprovoked versus provoked VTE was also not a significant factor for VTE recurrence—SHR 0.93 (95% CI 0.27–3.21), p = 0.912.
Mortality according to VTE recurrence was assessed using a Cox regression with time to recurrence as a time-varying covariate. VTE recurrences were significantly associated with higher mortality—HR 6.64 (95% CI 2.61–16.93), p < 0.001.
3.3. Anticoagulation Strategies
Acute anticoagulant treatment strategy (first 10 days of treatment)—parenteral anticoagulants, VKAs or DOACs—did not influence subsequent recurrence incidence rates during follow-up, which were found to be 9.8 (n = 4, p = 0.251) for parenteral, 5.7 (n = 3, p = 0.941) for VKAs, 4.4 (n = 6, p = 0.347) per 100 person-years for DOACs. The principal drug class (LMWH, VKA, DOAC) used during long-term treatment (over 10 days after index VTE event) was also not associated with the recurrence rate—0 (p = 0.738), 7.4 (n = 4, p = 0.463), 4.9 (n = 8, p = 0.591) per 100 person-years, respectively.
4. Discussion
Our study demonstrated a significantly higher mortality among patients who experienced recurrent VTE during a follow-up period, with an approximately 6- to 7-fold higher mortality rate. Similar results were recently reported from the COMMAND VTE (COntemporary ManageMent AND outcomes in patients with Venous ThromboEmbolism) Registry [11] with an adjusted HR of 3.24 in comparison with the 6.64 in our study. These results emphasize the importance of prevention of recurrent VTE for patient survival.
Using a simple Cox regression model, active cancer seemed to be a significant predictor of VTE recurrence with a 4-fold higher risk of recurrence. Previous studies have demonstrated a significant impact of cancer on VTE recurrence [4]; however, the recurrent VTE incidence rate of 23.5 cases per 100 person-years in our cohort is an overestimation, due to the high mortality in cancer patients. When taking into account the competing risk of death, the subdistribution HR was reduced to 2.11 and the association was not statistically significant. This finding along with previous reports, e.g., by Ay et al. [12], emphasizes the importance of competing-risk analysis when assessing patient cohorts with high mortality, like that of cancer patients
In our cohort, the overall VTE recurrence rate was 5.6 cases per 100 person-years. The rate in non-cancer patients was 4.6 per 100 person-years. Martinez et al. recently reported a rate of 4.9 per 100 person-years with a peak at 11.1 per 100 person-years in the first six months in a large cohort study of non-cancer patients in United Kingdom [3]. In our results, median time to recurrence was 130 days (IQR: 29–336), thus, most of the recurrent events occurred during the first few months, after the index event. Recurrence rates in our study and most practice-based cohort studies are significantly higher than those documented in the DOAC clinical trials (2 to 3%) [13,14], possibly due to exclusion of some high-risk patient groups, a higher compliance with treatment, and better monitoring in these trials.
In contrast with previous reports, our data did not demonstrate any significant differences in the recurrence rates between the primary provoked and unprovoked VTE. This finding might be related to the characteristics of our patient cohort that largely included elderly, co-morbid patients at a relatively high baseline risk of VTE, precipitated or not by a transient provoking factor, hence, the presence of such a factor might not significantly reduce future recurrence in this group of patients. However, even analysis of patients with transient provoking factors and no persistent major or minor risk factors in comparison with the remaining individuals did not reveal lower recurrence rates, although this group of patients were considered to be at a lower risk [10].
In our study, discontinuation of anticoagulation, during follow-up, did not demonstrate a significant association with the recurrence rate. The analysis of the treatment duration (above or below 6 months) was limited by the small number of recurrences (n = 6) in patients who survived more than 6 months, since only these patients were included in order to be eligible for this analysis. Recurrence rate in these patients might have been associated with other intrinsic factors.
Principal anticoagulant drug class (parenteral, VKA, DOAC) used in acute or long-term treatment was not associated with the recurrence rate in our patient cohort. In randomized controlled trials performed during the last decade, DOACs have been demonstrated to be equally effective but a safer alternative to VKAs—a finding confirmed in a meta-analysis by Gomez-Outes et al. [15]. In our study, a possible confounding factor might be the socioeconomic status of patients, since VKAs are significantly more affordable than DOACs. In Latvia, the healthcare system is a universal health coverage, largely tax funded with co-payment from patients. However, during the time of the present study neither oral nor parenteral anticoagulants in outpatient setting were state reimbursed for the diagnosis of VTE. However, even in cancer patients, the most prevalent long-term anticoagulant class was DOACs.
Limitations
Our study was limited by the relatively modest sample size, as only 13 cases of recurrent VTE were documented in our cohort. The study population was mainly composed of elderly individuals, thereby, different demographic groups were not equally represented, thus introducing a selection bias. Another source of bias was the fact that most of the patients were followed up by telephone in person or, if not possible, by proxy or a general practitioner, hence, some cases of recurrent VTE might be undocumented because of insufficient reporting or lack of follow-up. No consistent documentation of repeated laboratory studies or tests for residual thrombosis were performed, so these potential risk factors could not be assessed in the study.
Despite limitations, our practice-based cohort study gives important insights into the risk factors for recurrent VTE events. This registry is ongoing and will produce further data as the cohort size increases. The lack of sufficient tools for prediction of VTE recurrences for directed treatment emphasize the need for further studies in this field.
5. Conclusions
In our patient cohort, VTE recurrence rate was 5.6 cases per 100 person-years. Patients who experienced recurrent VTE had 6.6-fold higher mortality rate than patients with no recurrences. The presence of active cancer was not a statistically significant risk factor for recurrence when taking into account the competing risk of death. Duration and drug class of anticoagulation did not seem to significantly impact recurrence rate.
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, data curation, and writing—original draft preparation, V.G.; investigation, data curation, and writing—review and editing, D.K.; investigation and data curation, S.S.; investigation, K.M.; investigation, V.R.K.; investigation, A.Z.; investigation, K.M.; supervision, project administration, and validation, A.L.; conceptualization, supervision, project administration, validation, resources, and writing—review and editing, A.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DeMonaco, N.A.; Dang, Q.; Kapoor, W.N.; Ragni, M.V. Pulmonary Embolism Incidence Is Increasing with Use of Spiral Computed Tomography. Am. J. Med. 2008, 121, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Burge, A.; Freeman, K.; Klapper, P.; Haramati, L. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin. Radiol. 2008, 63, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Cohen, A.T.; Bamber, L.; Rietbrock, S. Epidemiology of first and recurrent venous thromboembolism: A population-based cohort study in patients without active cancer. Thromb. Haemost. 2014, 112, 255–263. [Google Scholar] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Kahn, S.R.; Wells, P.S.; Anderson, D.A.; Chagnon, I.; Le Gal, G.; Solymoss, S.; Crowther, M.; Perrier, A.; White, R.; et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. Can. Med Assoc. J. 2008, 179, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Heinze, G.; Jandeck, L.M.; Kyrle, P.A. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: The vienna prediction model. Circulation 2010, 121, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Tosetto, A.; Iorio, A.; Marcucci, M.; Baglin, T.; Cushman, M.; Eichinger, S.; Palareti, G.; Poli, D.; Tait, R.; Douketis, J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: A proposed prediction score (DASH). J. Thromb. Haemost. 2012, 10, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Kim, E.S.H. Risk of Recurrent Venous Thromboembolism After an Initial Episode: Risk Stratification and Implications for Long-term Treatment. Curr. Cardiol. Rep. 2019, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.; Kirkilesis, G.; Tsolakis, I. Editor’s Choice—Efficacy and Safety of the New Oral Anticoagulants Dabigatran, Rivaroxaban, Apixaban, and Edoxaban in the Treatment and Secondary Prevention of Venous Thromboembolism: A Systematic Review and Meta-analysis of Phase III Trials. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.J.; Kyrle, P.A. Categorization of patients as having provoked or unprovoked venous thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yoshikawa, Y.; Morimoto, T.; Amano, H.; Takase, T.; Hiramori, S.; Kim, K.; Oi, M.; Akao, M.; Kobayashi, Y.; et al. The association of recurrence and bleeding events with mortality after venous thromboembolism: From the COMMAND VTE Registry. Int. J. Cardiol. 2019, 292, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Posch, F.; Kaider, A.; Zielinski, C.; Pabinger, I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J. Thromb. Haemost. 2015, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Einstein—PE Investigators. Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; le Maulf, F.; Peter, N.; et al. Treatment of Acute Venous Thromboembolism With Dabigatran or Warfarin and Pooled Analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Outes, A.; Terleira-Fernandez, A.I.; Lecumberri, R.; Suarez-Gea, M.L.; Vargas-Castrillon, E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: A systematic review and meta-analysis. Thromb. Res. 2014, 134, 774–782. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).