Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
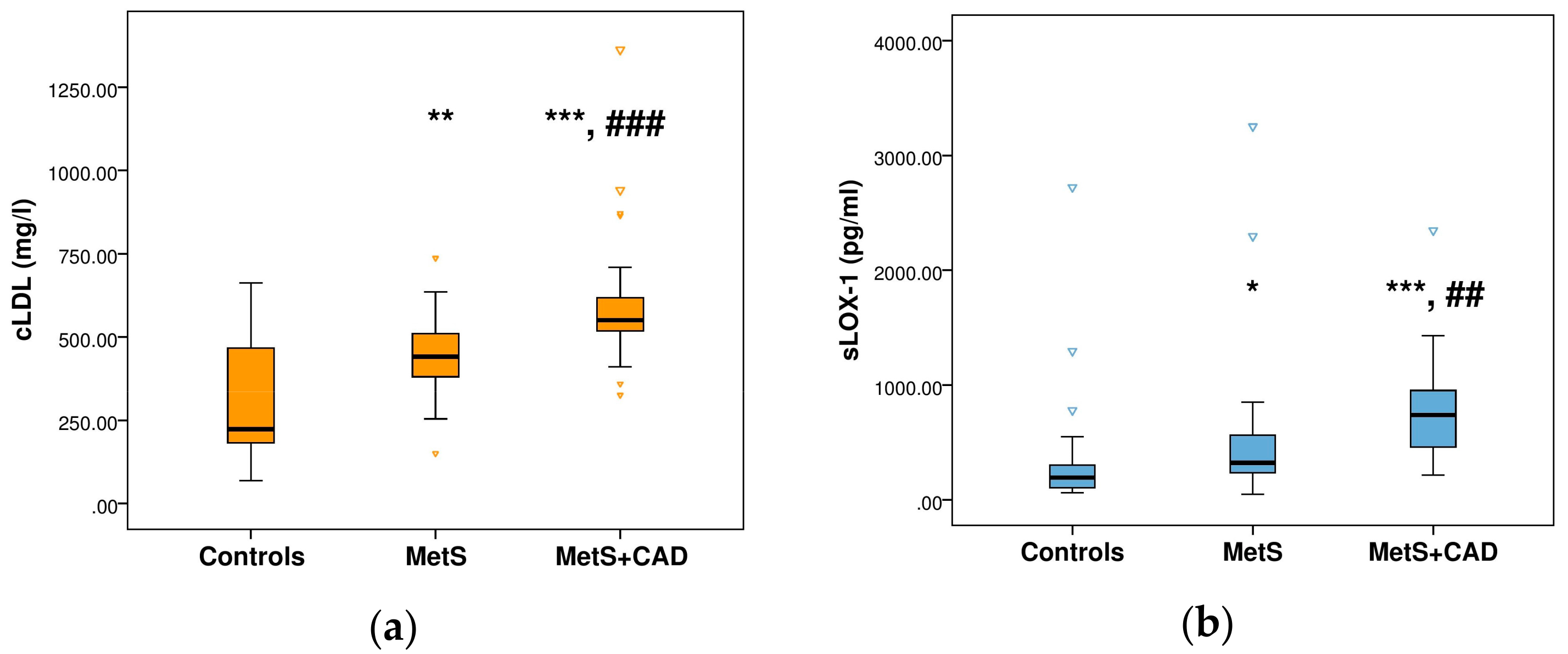
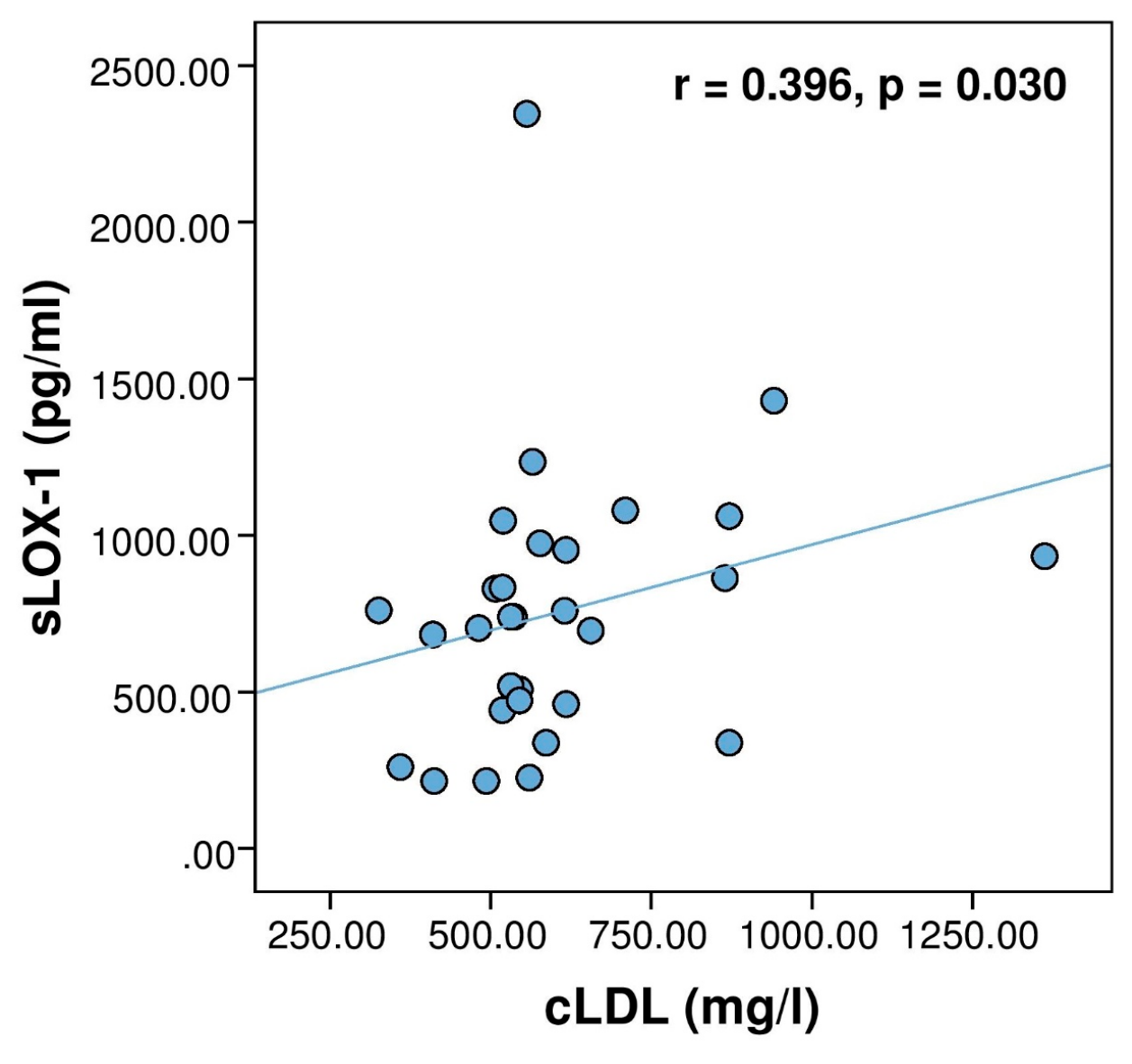
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.J. Harmonizing the Metabolic Syndrome. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gaw, A.; Scherbakova, O.; Ford, I.; O’Reilly, D.S.; Haffner, S.M.; Isles, C.; Macfarlane, P.W.; Packard, C.J.; Cobbe, S.M.; et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003, 108, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Cefalu, W.T.; Herrington, D.M. Diabetes, the metabolic syndrome, and angiographic progression of coronary arterial disease in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Wong, N.D.; Franklin, S.S.; Kamath, T.V.; L’Italien, G.J.; Pio, J.R.; Williams, G.R. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004, 110, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Urbina, A.; Benitez, S.; Perez, A.; Sanchez-Quesada, J.L. Modified low-density lipoproteins as biomarkers in diabetes and metabolic syndrome. Front Biosci. (Landmark Ed) 2018, 23, 1220–1240. [Google Scholar] [CrossRef]
- Hurtado-Roca, Y.; Bueno, H.; Fernandez-Ortiz, A.; Ordovas, J.M.; Ibañez, B.; Fuster, V.; Rodriguez-Artalejo, F.; Laclaustra, M. Oxidized LDL is associated with metabolic syndrome traits independently of central obesity and insulin resistance. Diabetes 2017, 66, 474–482. [Google Scholar] [CrossRef][Green Version]
- Tsimikas, S.; Brilakis, E.; Miller, E.; McConnell, J.; Lennon, R.; Kornman, K.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef]
- Holvoet, P.; Kritchevsky, S.B.; Tracy, R.P.; Mertens, A.; Rubin, S.M.; Butler, J.; Goodpaster, B.; Harris, T.B. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes 2004, 53, 1068–1073. [Google Scholar] [CrossRef]
- Basnakian, A.G.; Shah, S.V.; Ok, E.; Altunel, E.; Apostolov, E.O. Carbamylated LDL. Adv. Clin. Chem. 2010, 51, 25–52. [Google Scholar] [CrossRef]
- Kraus, L.M.; Kraus, A.P. Carbamoylation of amino acids and proteins in uremia. Kidney Int. Suppl. 2001, 78, 102–107. [Google Scholar] [CrossRef]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, E.O.; Shah, S.V.; Ok, E.; Basnakian, A.G. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin. Chem. 2005, 51, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Basnakian, A.G.; Apostolov, E.O.; Barri, Y.M.; Shah, S.V. Carbamylated low density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int. 2005, 68, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Asci, G.; Basci, A.; Shah, S.V.; Basnakian, A.G.; Toz, H.; Ozkahya, M.; Duman, S.; Ok, E. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 2008, 13, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, E.O.; Shah, S.V.; Ray, D.; Basnakian, A.G. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Pothineni, N.V.K.; Karathanasis, S.K.; Ding, Z.; Arulandu, A.; Varughese, K.I.; Mehta, J.L. LOX-1 in atherosclerosis and myocardial ischemia: Biology, genetics, and modulation. J. Am. Coll. Cardiol. 2017, 69, 2759–2768. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Kume, N.; Kataoka, H.; Minami, M.; Sawamura, T.; Masaki, T.; Kita, T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Kume, N.; Murase, T.; Minami, M.; Nakagawa, D.; Inada, T.; Tanaka, M.; Ueda, A.; Kominami, G.; Kambara, H.; et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome. A novel marker for early diagnosis. Circulation 2005, 112, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Balzan, S. LOX-1, a new marker of risk and prognosis in coronary artery disease? Mol. Cell Biochem. 2013, 383, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, T.E.; Kume, N.; Mitsuoka, H.; Phares, D.A.; Hagberg, J.M. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring) 2008, 16, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Shiu, S.W.; Wong, Y.; Leng, L.; Bucala, R. Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J. Lipid Res. 2008, 49, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances human macrophage LOX-1 expression: Role for LOX-1 in glucose-induced macrophage foam cell formation. Circ. Res. 2004, 94, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mehta, J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1116–1122. [Google Scholar] [CrossRef]
- Apostolov, E.O.; Basnakian, A.G.; Ok, E.; Shah, S.V. Carbamylated low-density lipoprotein: Nontraditional risk factor for cardiovascular events in patients with chronic kidney disease. J. Ren Nutr. 2012, 22, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Merino, A.; Briceno, C.; Soriano, S.; Buendía, P.; Calleros, L.; Rodriguez, M.; Martín-Malo, A.; Aljama, P.; Ramírez, R. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J. 2011, 25, 1314–1322. [Google Scholar] [CrossRef]
- Devaraj, S.; Valleggi, S.; Siegel, D.; Jialal, I. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr. Atheroscler. Rep. 2010, 12, 110–118. [Google Scholar] [CrossRef]
- Vohra, R.S.; Murphy, J.E.; Walker, J.H.; Ponnambalam, S.; Homer-Vanniasinkam, S. Atherosclerosis and the lectin-like oxidized low-density lipoprotein scavenger receptor. Trends Cardiovasc. Med. 2006, 16, 60–64. [Google Scholar] [CrossRef]
- Puttaruk, P.; Pipatsatitpong, D.; Siripurkpong, P. Soluble lectin-like oxidized low density lipoprotein receptor-1 in metabolic syndrome. Asian Biomed. (Res. Rev. News) 2015, 9, 675–682. [Google Scholar] [CrossRef]
- Hu, C.; Dandapat, A.; Sun, L.; Marwali, M.R.; Inoue, N.; Sugawara, F.; Inoue, K.; Kawase, Y.; Jishage, K.; Suzuki, H.; et al. Modulation of angiotensin II-mediated hypertension and cardiac remodeling by lectin-like oxidized low-density lipoprotein receptor-1 deletion. Hypertension 2008, 52, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances endothelial LOX-1 expression: Role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes 2003, 52, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Sawamura, T.; Inoue, K.; Mehta, J.L. Upregulation of LOX-1 expression in aorta of hypercholesterolemic rabbits: Modulation by losartan. Biochem. Biophys. Res. Commun. 2000, 276, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Nomata, Y.; Kume, N.; Sasai, H.; Katayama, Y.; Nakata, Y.; Okura, T.; Tanaka, K. Weight reduction can decrease circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels in overweight middle-aged men. Metabolism 2009, 58, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, F.; Yamashita, K.; Tasaki, H.; Kume, N.; Mitsuoka, H.; Kita, T.; Adachi, T.; Otsuji, Y. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 correlates with oxidative stress markers in stable coronary artery disease. Int. J. Cardiol. 2009, 134, 285–287. [Google Scholar] [CrossRef]
- Sayed, A.S.M.; Zhao, Z.; Guo, L.; Li, F.; Deng, X.; Deng, H.; Xia, K.; Yang, T. Serum lectin-like oxidized-low density lipoprotein receptor-1 and adiponectin levels are associated with coronary artery disease accompanied with metabolic syndrome. Iran Red. Crescent. Med. J. 2014, 16, e12106. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.H.; Yang, X.G.; Liu, X.T.; Ren, Y.G. Serum sLOX-1 levels are associated with the presence and severity of angiographic coronary artery disease in patients with metabolic syndrome. Clin. Invest. Med. 2010, 33, E398–E404. [Google Scholar] [CrossRef]
- Otsuki, T.; Maeda, S.; Mukai, J.; Ohki, M.; Nakanishi, M.; Yoshikawa, T. Association between plasma sLOX-1 concentration and arterial stiffness in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 151–155. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.H.; Yang, X.G.; Liu, Y.; Liu, X.T.; Ren, Y.G. Postprocedural serum sLOX-1 levels are associated with coronary in-stent restenosis in patients with stable coronary artery disease. Coron. Artery Dis. 2011, 22, 259–263. [Google Scholar] [CrossRef]
- Balin, M.; Celik, A.; Kobat, M.A.; Baydas, A. Circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels predict percutaneous coronary intervention-related periprocedural myocardial infarction in stable patients undergoing elective native single-vessel PCI. J. Thromb. Thrombolysis. 2012, 34, 483–490. [Google Scholar] [CrossRef]
- Skarpengland, T.; Skjelland, M.; Kong, X.Y.; Skagen, K.; Holm, S.; Otterdal, K.; Dahl, C.P.; Krohg-Sørensen, K.; Sagen, E.L.; Bjerkeli, V.; et al. Increased levels of lectin-like oxidized low-density lipoprotein receptor-1 in ischemic stroke and transient ischemic attack. J. Am. Heart Assoc. 2018, 7, e006479. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.W.; Tan, K.C.; Wong, Y.; Leng, L.; Bucala, R. Glycoxidized LDL increases lectin-like oxidized low density lipoprotein receptor-1 in diabetes mellitus. Atherosclerosis 2009, 203, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Speer, T.; Owala, F.O.; Holy, E.W.; Zewinger, S.; Frenzel, F.L.; Stähli, B.E.; Razavi, M.; Triem, S.; Cvija, H.; Rohrer, L.; et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart J. 2014, 35, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Basnakian, A.G. Interaction of carbamylated LDL with LOX-1 in the induction of endothelial dysfunction and atherosclerosis. Eur. Heart J. 2014, 35, 2996–2997. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Saad, A.H.; Kumar, A.H.; Morawietz, H. Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur. J. Pharmacol. 2010, 627, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, J.J.; Meuwese, M.C.; van Miert, J.N. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med. Sci. Monit. 2008, 14, CR406–C410. [Google Scholar] [PubMed]
- Meuwese, M.C.; Stroes, E.S.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schaub, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.; Khaw, K.T.; et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef]
- Shiu, S.W.; Xiao, S.M.; Wong, Y.; Chow, W.S.; Lam, K.S.; Tan, K.C. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin. Sci. 2014, 126, 175–181. [Google Scholar] [CrossRef]
- Stankova, T.; Delcheva, G.; Maneva, A.; Vladeva, S. Serum levels of carbamylated LDL, nitrotyrosine and soluble lectin-like oxidized low-density lipoprotein receptor-1 in poorly controlled type 2 diabetes mellitus. Folia Med. (Plovdiv) 2019. (accepted). [Google Scholar]
- Chang, H.C.; Chen, T.G.; Tai, Y.T.; Chen, T.L.; Chiu, W.T.; Chen, R.M. Resveratrol attenuates oxidized LDL-evoked LOX-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J. Cereb. Blood Flow Metab. 2011, 31, 842–854. [Google Scholar] [CrossRef]
- Guan, S.; Wang, B.; Li, W.; Guan, J.; Fang, X. Effects of berberine on expression of LOX-1 and SR-BI in human macrophage-derived foam cells induced by ox-LDL. Am. J. Chin. Med. 2010, 38, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Khan, J.A.; Ryu, S.; Shekhar, R.; Seung, K.B.; Mehta, J.L. Curcumin reduces angiotensin II mediated cardiomyocyte growth via LOX-1 inhibition. J. Cardiovasc. Pharmacol. 2010, 55, 176–183. [Google Scholar] [CrossRef] [PubMed]
| Variables | Control | MetS | MetS + CAD | p-Value |
|---|---|---|---|---|
| (n = 30) | (n = 30) | (n = 30) | ||
| Sex, Male/Female (no.) | 12/18 | 9/21 | 11/19 | 0.712 # |
| Age (years) | 45.6 ± 9.1 | 47.8 ± 6.4 | 51.1 ± 9.3 * | 0.025 |
| Body mass index (kg/m2) | 22.8 | 29.2 | 29.6 | <0.001 |
| (21.6–24.8) | (28.3–30.2) *** | (28.7–31.4) *** | ||
| Waist circumference (cm) | 83.9 ± 10.0 | 103.7 ± 10.5 *** | 100.7 ± 15.1 *** | <0.001 |
| ACEI or ARB (no.) | - | 12 | 9 | 0.589 # |
| Systolic BP (mmHg) | 115.4 ± 6.6 | 127.7 ± 8.3 *** | 128.6 ± 6.8 *** | <0.001 |
| Diastolic BP (mmHg) | 76.2 ± 4.1 | 82.1 ± 6.8 *** | 81.7 ± 6.4 *** | <0.001 |
| Fasting glucose (mmol/L) | 4.71 ± 0.46 | 5.13 ± 0.96 | 5.56 ± 1.13 * | 0.002 |
| Total cholesterol (mmol/L) | 4.3 | 5.35 | 4.91 | <0.001 |
| (3.58–4.78) | (4.70–6.00) *** | (4.30–5.40) ** | ||
| Triglycerides (mmol/L) | 1.03 | 1.35 | 1.27 | <0.001 |
| (0.80–1.41) | (0.90–1.84) | (0.88–1.97) | ||
| HDL-cholesterol (mmol/L) | 1.14 ± 0.46 | 1.19 ± 0.17 | 1.18 ± 0.22 | 0.229 |
| LDL-cholesterol (mmol/L) | 2.65 | 3.33 | 2.8 | 0.002 |
| (1.95–2.99) | (2.73–4.01) *** | (2.20–3.56) | ||
| Urea (mmol/L) | 4.93 ± 1.25 | 4.81 ± 0.95 | 4.98 ±1.17 | 0.896 |
| Creatinine (μmol/L) | 78.5 ± 11.9 | 80.8 ± 10.4 | 82.2 ± 12.5 | 0.537 |
| hs-CRP (μg/mL) | 0.61 | 1.74 | 4.14 | <0.001 |
| (0.20–1.04) | (1.03–3.67) *** | (1.38–6.02) *** |
| Variables | rs | p-Value |
|---|---|---|
| Body mass index (kg/m2) | 0.238 | 0.068 |
| Waist circumference (cm) | −0.039 | 0.765 |
| Systolic BP (mmHg) | 0.251 | 0.053 |
| Diastolic BP (mmHg) | −0.083 | 0.529 |
| Fasting glucose (mmol/L) | 0.414 | 0.001 * |
| Total cholesterol (mmol/L) | 0.074 | 0.576 |
| Triglycerides (mmol/L) | 0.031 | 0.812 |
| HDL-cholesterol (mmol/L) | −0.273 | 0.035 * |
| LDL-cholesterol (mmol/L) | 0.074 | 0.574 |
| hs-CRP (μg/mL) | 0.247 | 0.057 |
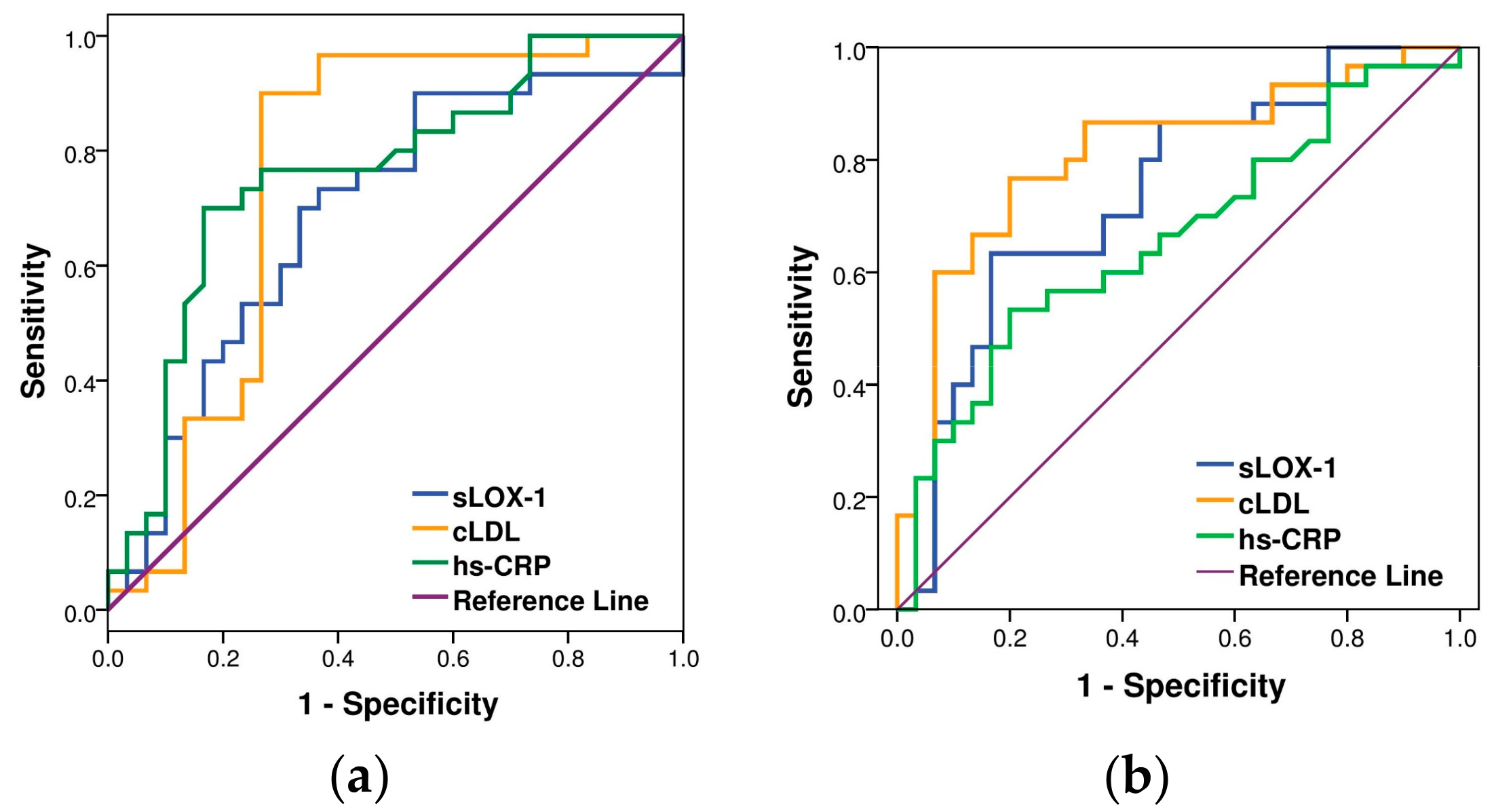
| Variables | Sensitivity (%) | Specificity (%) | Cutoff | AUC (95% CI) | p-Value |
|---|---|---|---|---|---|
| cLDL (mg/L) | 90.0 | 73.3 | 312 | 0.761 | 0.001 |
| (0.626–0.896) | |||||
| sLOX-1(pg/mL) | 73.3 | 63.3 | 244 | 0.692 | 0.011 |
| (0.555–0.829) | |||||
| hs-CRP (μg/mL) | 76.7 | 73.3 | 1 | 0.762 | <0.001 |
| (0.637–0.886) |
| Variables | Sensitivity (%) | Specificity (%) | Cutoff | AUC (95% CI) | p-Value |
|---|---|---|---|---|---|
| cLDL (mg/L) | 86.7 | 63.3 | 475 | 0.811 | <0.001 |
| (0.698–0.924) | |||||
| sLOX-1(pg/mL) | 70.0 | 63.3 | 495 | 0.739 | 0.001 |
| (0.611–0.867) | |||||
| hs-CRP (μg/mL) | 56.7 | 77.7 | 3 | 0.658 | 0.035 |
| (0.519–0.798) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankova, T.; Delcheva, G.; Maneva, A.; Vladeva, S. Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome. Medicina 2019, 55, 493. https://doi.org/10.3390/medicina55080493
Stankova T, Delcheva G, Maneva A, Vladeva S. Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome. Medicina. 2019; 55(8):493. https://doi.org/10.3390/medicina55080493
Chicago/Turabian StyleStankova, Teodora, Ginka Delcheva, Ana Maneva, and Stefka Vladeva. 2019. "Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome" Medicina 55, no. 8: 493. https://doi.org/10.3390/medicina55080493
APA StyleStankova, T., Delcheva, G., Maneva, A., & Vladeva, S. (2019). Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome. Medicina, 55(8), 493. https://doi.org/10.3390/medicina55080493




