Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Inclusion Criteria
- Age included at the time of the first visit between 1 and 24 months;
- Availability of a detailed personal history and complete clinical evaluation;
- Performing allergy tests (determination of sIgE for the main food and inhalant allergens and total IgE at 6 months, 7–12 months, 13–24 months, 2–3 years, 3–5 years, and >5 years);and
- Signed informed consent.
2.2. Clinical Assessment
2.3. Allergometric Assessment
- Monosensitized: patients with positive sIgE only to cow’s milk;
- Oligosensitized: patients sensitized to cow’s milk and another allergen; and
- Polysensitized: patients with positive sIgE to cow’s milk and at least two others allergens.
2.4. Statistical Methods
3. Results
3.1. Familial Atopy
3.2. Clinical Manifestations
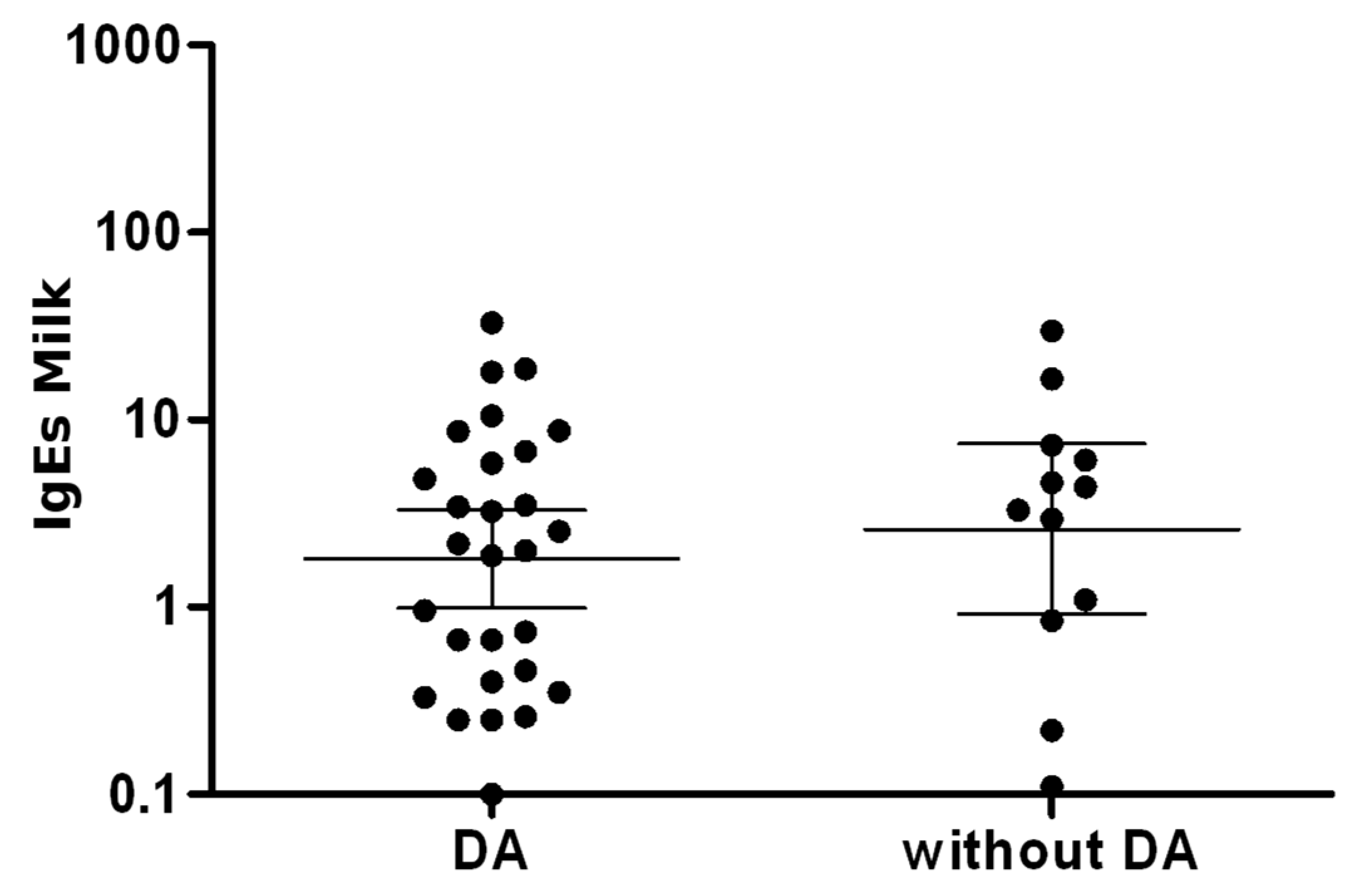
3.3. IgE Sensitization to Food and Inhalant Allergens
3.4. Atopic Dermatitis (AD) Associated with Asthma and Allergic Rhinoconjunctivitis
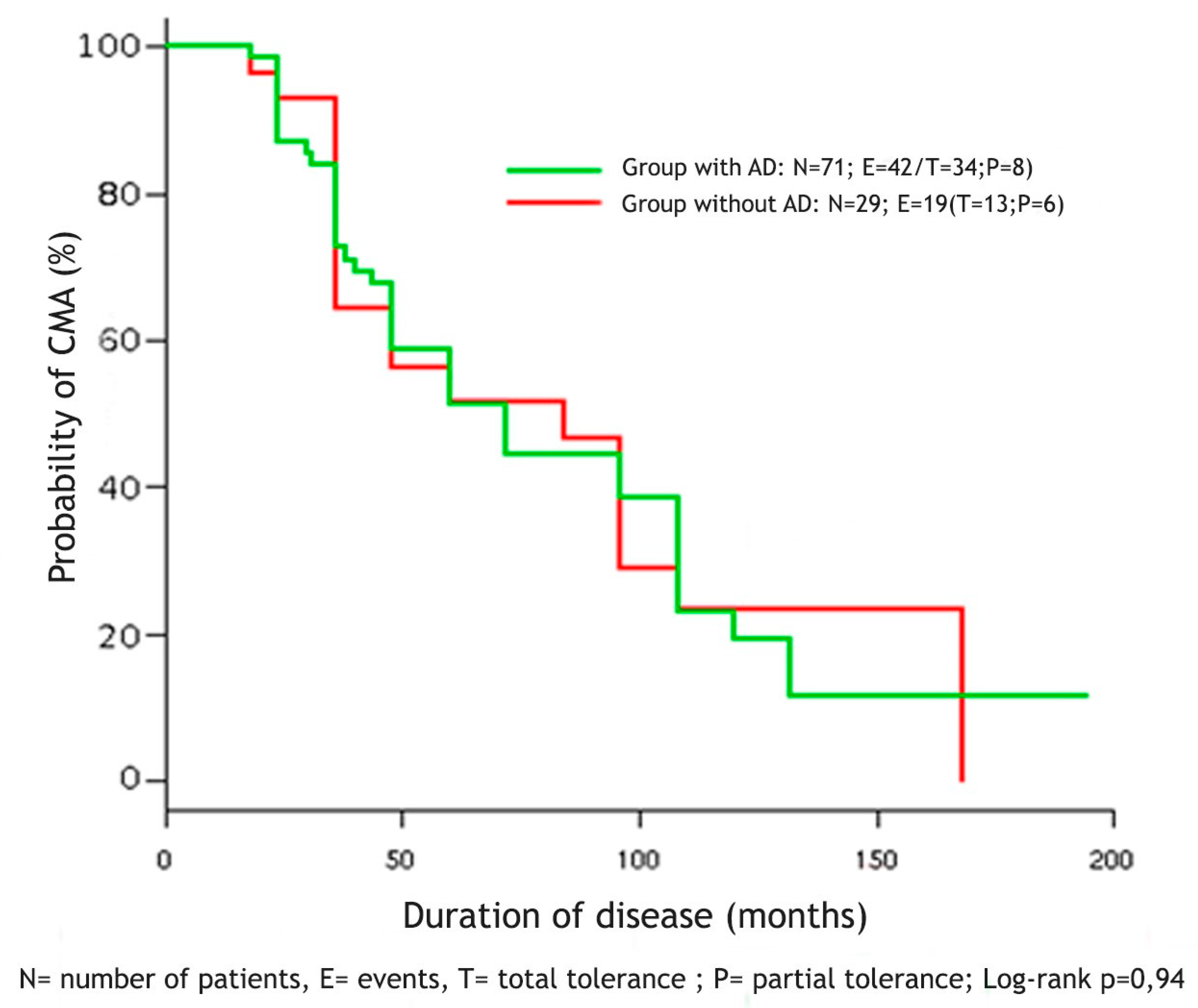
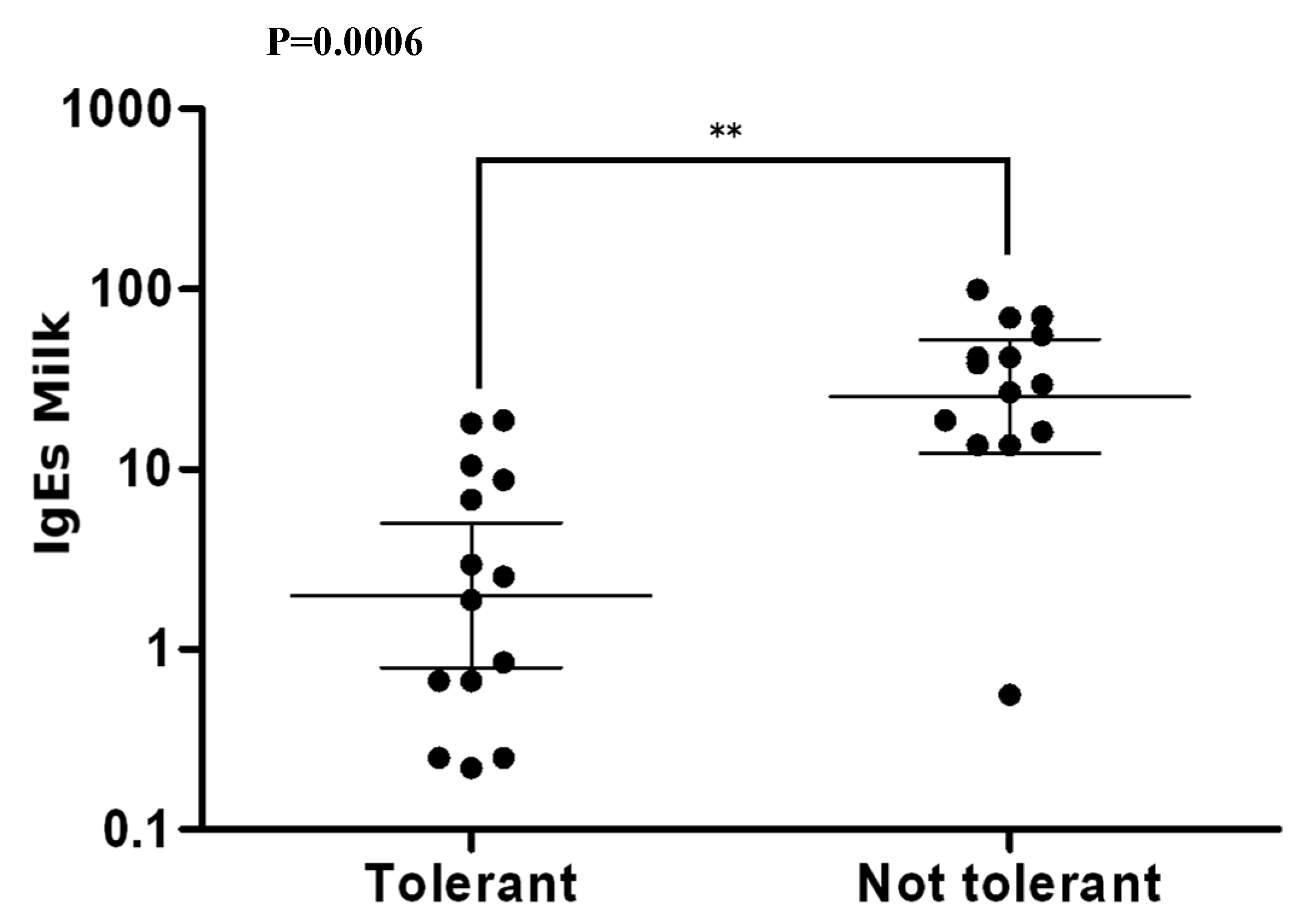
3.5. Tolerance to Cow’s Milk Proteins
4. Discussion
4.1. Sensitization to Foods
4.2. Sensitization to Inhalant Allergens
4.3. Specific IgE
4.4. Asthma and Rhinoconjunctivitis
4.5. Natural History of CMA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, D.J.; Firer, M.A.; Shelton, M.J.; Hosking, C.S. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J. Pediatr. 1986, 109, 270–276. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T.; Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI). BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R. Anaphylaxis in infants: Can recognition and management be improved? J. Allergy Clin. Immunol. 2007, 120, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R. Diagnosis and rationale for action against Cow’s milk allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12. [Google Scholar] [CrossRef] [PubMed]
- Høst, A.; Halken, S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life: Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990, 45, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, M.; Halvorsen, R.; Tambs, K.; Botten, G. Prevalence of parentally perceived adverse reactions to food in young children. Pediatr. Allergy Immunol. 1999, 10, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, M.; Botten, G.; Halvorsen, R.; Magnus, P. The prevalence of allergy to egg: A population-based study in young children. Allergy Eur. J. Allergy Clin. Immunol. 2001, 56, 403–411. [Google Scholar] [CrossRef]
- Høst, A. Frequency of cow’s milk allergy in childhood. Ann. Allergy Asthma Immunol. 2002, 89, 33–37. [Google Scholar] [CrossRef]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef]
- Vanto, T.; Helppilä, S.; Juntunen-Backman, K.; Kalimo, K.; Klemola, T.; Korpela, R.; Koskinen, P. Prediction of the development of tolerance to milk in children with cow’s milk hypersensitivity. J. Pediatr. 2004, 144, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ara, M.C.; Boyano-Martínez, M.T.; Díaz-Pena, J.M.; Martín-Muñoz, M.F.; Martin-Esteban, M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.M.; Hill, D.J.; Hosking, C.S. Natural history of cow milk allergy: Clinical outcome. J. Pediatr. 1990, 116, 862–867. [Google Scholar] [CrossRef]
- Høst, A.; Halken, S.; Jacobsen, H.P.; Christensen, A.E.; Herskind, A.M.; Plesner, K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. 2002, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Segal, N.; Garty, B.; Danon, Y.L. Lessons from the clinical course of IgE-mediated cow milk allergy in Israel. Pediatr. Allergy Immunol. 2007, 18, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Dias, A.; Pinheiro, J.A. Predictive factors for the persistence of cow’s milk allergy. Pediatr. Allergy Immunol. 2010, 21, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, K.M.; Pelkonen, A.S.; Mäkelä, M.J.; Savilahti, E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J. Allergy Clin. Immunol. 2005, 116, 869–875. [Google Scholar] [CrossRef]
- Iacono, G.; Cavataio, F.; Montalto, G.; Soresi, M.; Notarbartolo, A.; Carroccio, A. Persistent cow’s milk protein intolerance in infants: The changing faces of the same disease. Clin. Exp. Allergy 1998, 28, 817–823. [Google Scholar] [CrossRef]
- Fiocchi, A.; Terracciano, L.; Bouygue, G.R.; Veglia, F.; Sarratud, T.; Martelli, A.; Restani, P. Incremental prognostic factors associated with cow’s milk allergy outcomes in infant and child referrals: The Milan Cow’s Milk Allergy Cohort study. Ann. Allergy Asthma Immunol. 2008, 101, 166–173. [Google Scholar] [CrossRef]
- de Benedictis, F.M.; Franceschini, F.; Hill, D.; Naspitz, C.; Simons, F.E.R.; Wahn, U.; Warner, J.O.; de Longueville, M.; EPAAC Study Group. The allergic sensitization in infants with atopic eczema from different countries. Allergy 2009, 64, 295–303. [Google Scholar] [CrossRef]
- Laughter, D.; Istvan, J.A.; Tofte, S.J.; Hanifin, J.M. The prevalence of atopic dermatitis in Oregon schoolchildren. J. Am. Acad. Dermatol. 2000, 43, 649–655. [Google Scholar] [CrossRef]
- Hanifin, J.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar]
- Novembre, E.; Vierucci, A. Milk allergy/intolerance and atopic dermatitis in infancy and childhood. Allergy 2001, 56, 105–108. [Google Scholar] [CrossRef]
- Johansson, E.K.; Bergström, A.; Kull, I.; Lind, T.; Söderhäll, C.; van Hage, M.; Wickman, M.; Ballardini, N.; Wahlgren, C.F. IgE sensitization in relation to preschool eczema and filaggrin mutation. J. Allergy Clin. Immunol. 2017, 140, 1572–1579. [Google Scholar] [CrossRef]
- Hill, D.J.; Hosking, C.S.; De Benedictis, F.M.; Oranje, A.P.; Diepgen, T.L.; Bauchau, V.; EPAAC Study Group. Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: An international study. Clin. Exp. Allergy 2008, 38, 161–168. [Google Scholar] [CrossRef]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; Du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef]
- Sybilski, A.J.; Zalewska, M.; Furmańczyk, K.; Lipiec, A.; Krzych-Fałta, E.; Samoliński, B. The prevalence of sensitization to inhalant allergens in children with atopic dermatitis. Allergy Asthma Proc. 2015, 36, 81–85. [Google Scholar] [CrossRef]
- Wüthrich, B.; Schmid-Grendelmeier, P. The atopic eczema/dermatitis syndrome: Epidemiology, natural course, and immunology of the IgE-associated (“extrinsic”) and the nonallergic (“intrinsic”) AEDS. J. Investig. Allergol. Clin. Immunol. 2003, 13, 1–5. [Google Scholar]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef]
- Celakovska, J.; Bukac, J. Food allergy in patients suffering from atopic dermatitis-association with concomitant allergic diseases. Food Agric. Immunol. 2015, 26, 325–339. [Google Scholar] [CrossRef]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 2003, 48, 977–985. [Google Scholar] [CrossRef]
- Dohi, M.; Okudaira, H.; Sugiyama, H.; Tsurumachi, K.; Suko, M.; Nakagawa, T.; Morita, Y.; Ito, K.; Nakayama, H.; Miyamoto, T. Bronchial responsiveness to mite allergen in atopic dermatitis without asthma. Int. Arch. Allergy Appl. Immunol. 1990, 92, 138–142. [Google Scholar] [CrossRef]
- Petriz, N.A.; Parisi, C.A.; Busaniche, J.N.; Evangelista, P.; Mehaudy, R.; Orsi, M. Natural history of immunoglobulin E-mediated cow’s milk allergy in a population of Argentine children. Arch. Argent. Pediatr. 2017, 115, 331–335. [Google Scholar]
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–881. [Google Scholar] [CrossRef]
| Clinical Manifestations | n = 71, AD (+) | n = 29, AD (−) | P |
|---|---|---|---|
| Skin | 65 (91.6%) | 15 (51.7%) | <0.0001 |
| Urticaria | 63 | 11 | |
| Urticaria and angioedema | 2 | - | |
| Urticaria and vomiting | - | 4 | |
| Gastrointestinal | 3 (4.2%) | 8 (27.6%) | |
| Vomiting and blood streaked stools | 3 | - | |
| Vomiting | - | 3 | |
| Vomiting and diarrhea | - | 1 | |
| Diarrhea and blood streaked stools | - | 4 | |
| Anaphylaxis | 3 (4.2%) | 6 (20.7%) |
| AD (+) | AD (−) | P | ||||
|---|---|---|---|---|---|---|
| 6 months | n = 38 (53.5%) | 6 months | n = 7 (24.1%) | |||
| Mono | 6 (15.8%) | Mono | 5 (71.4%) | |||
| Oligo | 8 (21.1%) | Oligo | 1 (14.3%) | |||
| Poly | 24 (63.2%) | Poly | 1 (14.3%) | 0.006 | ||
| 7–12 months | n = 57 (80.3%) | 7–12 months | n = 25 (86.2%) | |||
| Mono | 5 (8.8%) | Mono | 8 (32%) | |||
| Oligo | 11 (19.3%) | Oligo | 11 (44%) | |||
| Poly | 41 (71.9%) | Poly | 6 (24%) | <0.0001 | ||
| 13–24 months | n = 40 (56.3%) | 13–24 months | n = 15 (51.7%) | |||
| Mono | 3 (7.5%) | Mono | 5 (33.3%) | |||
| Oligo | 3 (7.5%) | Oligo | 7 (46.7%) | |||
| Poly | 34 (85%) | Poly | 3 (20%) | <0.0001 | ||
| 2–3 years | n = 28 (39.4%) | 2–3 years | n = 13 (44.8%) | |||
| Mono | 2 (7.1%) | Mono | 8 (61.5%) | |||
| Oligo | 1 (3.6%) | Oligo | 1 (7.7%) | |||
| Poly | 25 (89.3%) | Poly | 4 (30.8%) | <0.0001 | ||
| 3–5 years | n = 30 (42.2%) | 3–5 years | n = 12 (41.4%) | |||
| Mono | 5 (16.7%) | Mono | 5 (41.7%) | |||
| Oligo | 3 (10%) | Oligo | 4 (33.3%) | |||
| Poly | 22 (73.3%) | Poly | 3 (25%) | 0.015 | ||
| >5 years | n = 20 (28.2%) | >5 years | n = 7 (24.1%) | |||
| Mono | 4 (20%) | Mono | 4 (57.1%) | |||
| Oligo | 0 (0%) | Oligo | 1 (14.3%) | |||
| Poly | 16 (80%) | Poly | 2 (28.6%) | 0.026 |
| Age | AD | Cow’s Milk N/% | Mean sIgE | Egg Yolk N/% | Mean sIgE | Egg White N/% | Mean sIgE |
|---|---|---|---|---|---|---|---|
| 6 months | + | 39/39 (100%) * | 9.90 ** | 18/31 (58%) *** | 3.33 | 29/36 (80.5%) ° | 10.05 |
| − | 6/7 (85.7%) | 2.34 | 0/7 (0%) | <0.35 | 1/7 (14%) | 0.76 | |
| 7–12 months | + | 55/57 (96.5%) | 10.89 | 37/49 (75.5%) **** | 2.39 | 51/57 (89.5%) °° | 8.99 |
| − | 24/26 (92.3%) | 9.02 | 9/24 (37.5%) | 2.48 | 13/26 (50%) | 4.19 | |
| 13–24 months | + | 40/41 (97.5%) | 9.31 | 27/38 (71%) | 3.06 | 36/40 (90%) °°° | 7.11 |
| − | 14/15 (93.3%) | 5.74 | 7/12 (58.3%) | 1.78 | 9/15 (60%) | 4.37 | |
| 2–3 years | + | 27/29 (93.1%) | 7.81 | 16/24 (66.6%) | 3.08 | 25/29 (86.2%) °°°° | 6.26 |
| − | 12/12 (100%) | 6.16 | 4/11 (36.4%) | 5.62 | 4/12 (33.3%) | 5.94 | |
| 3–5 years | + | 26/29 (89.6%) | 7.46 | 15/26 (57.7%) | 2.52 | 24/29 (82.7%) °°°°° | 3.92 |
| − | 10/12 (83.3%) | 18.10 | 4/8 (50%) | 1.64 | 6/12 (50%) | 4.30 | |
| >5 years | + | 15/19 (78.9%) | 8.73 | 7/16 (43.7%) | 2.38 | 14/19 (73.7%) | 3.10 |
| − | 6/7 (85.7%) | 16.44 | 1/4 (25%) | 0.39 | 3/7 (42.9%) | 4.11 |
| Age | AD | Dermatophagoides pteronyssinus N/% | Mean sIgE | Dermatophagoides farinae N/% | Mean sIgE | Phleum pratense N/% | Mean sIgE | Cynodon dactylon N/% | Mean sIgE |
|---|---|---|---|---|---|---|---|---|---|
| 6 months | + | 3/32 (9.4%) | 1.06 | 3/33 (9.1%) | 0.85 | 3/31 (9.7%) | 1.23 | 2/33 (6.1%) | 0.40 |
| − | 0/5 (0%) | <0.35 | 0/5 (0%) | <0.35 | 0/5 (0%) | <0.35 | 0/5 (0%) | <0.35 | |
| 7–12 months | + | 7/48 (14.6%) | 1.85 | 8/51 (15.7%) | 1.11 | 12/50 (24%) * | 1.43 | 8/49 (16.3%) ** | 1.13 |
| − | 1/21 (4.8%) | 9.92 | 1/21 (4.8%) | 3.49 | 1/22 (4.5%) | 0.36 | 0/21 (0%) | <0.35 | |
| 13–24 months | + | 7/36 (19.4%) | 3.02 | 8/39 (20.5%) | 1.53 | 16/39 (41%) | 1.75 | 10/31 (32.3%) *** | 1.78 |
| − | 2/12 (16.7%) | 1.35 | 1/12 (8.3%) | 0.95 | 3/13 (23.1%) | 1.30 | 0/12 (0%) | <0.35 | |
| 2–3 years | + | 9/21 (42.9%) | 2.81 | 7/27 (25.9%) | 3.83 | 15/27 (55.5%) ° | 4.21 | 7/17 (41.2%) °° | 4.65 |
| − | 2/10 (20%) | 3.82 | 2/11 (18.2%) | 4.46 | 1/12 (8.3%) | 0.38 | 0/9 (0%) | <0.35 | |
| 3–5 years | + | 8/21 (38.1%) | 16.54 | 11/28 (39.3%) | 5.83 | 18/28 (64.3%) ° | 8.80 | 12/21 (57.1%) °°° | 4.55 |
| − | 4/10 (40%) | 1.92 | 5/12 (41.7%) | 1.29 | 2/12 (16.7%) | 2.60 | 0/9 (0%) | <0.35 | |
| >5 years | + | 10/12 (83.3%) | 16.22 | 13/19 (68.4%) | 13.44 | 15/19 (78.9%) | 24.10 | 8/13 (61.5%) | 22.69 |
| − | 1/3 (66.6%) | 42.10 | 3/7 (42.8%) | 7.51 | 3/6 (50%) | 8.12 | 1/3 (33.3%) | 1.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, A.; Cipriani, F.; Indio, V.; Gallucci, M.; Caffarelli, C.; Ricci, G. Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children. Medicina 2019, 55, 460. https://doi.org/10.3390/medicina55080460
Giannetti A, Cipriani F, Indio V, Gallucci M, Caffarelli C, Ricci G. Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children. Medicina. 2019; 55(8):460. https://doi.org/10.3390/medicina55080460
Chicago/Turabian StyleGiannetti, Arianna, Francesca Cipriani, Valentina Indio, Marcella Gallucci, Carlo Caffarelli, and Giampaolo Ricci. 2019. "Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children" Medicina 55, no. 8: 460. https://doi.org/10.3390/medicina55080460
APA StyleGiannetti, A., Cipriani, F., Indio, V., Gallucci, M., Caffarelli, C., & Ricci, G. (2019). Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children. Medicina, 55(8), 460. https://doi.org/10.3390/medicina55080460







