Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
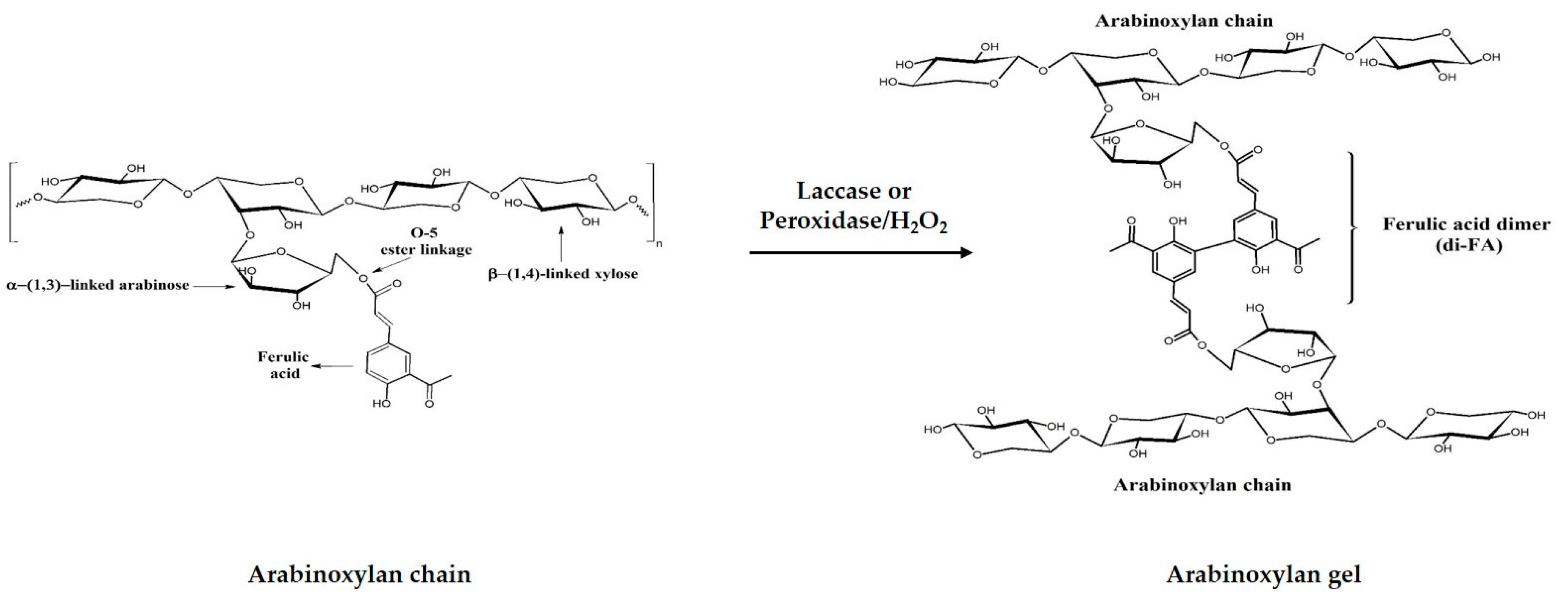
2.2.1. Gel Preparation
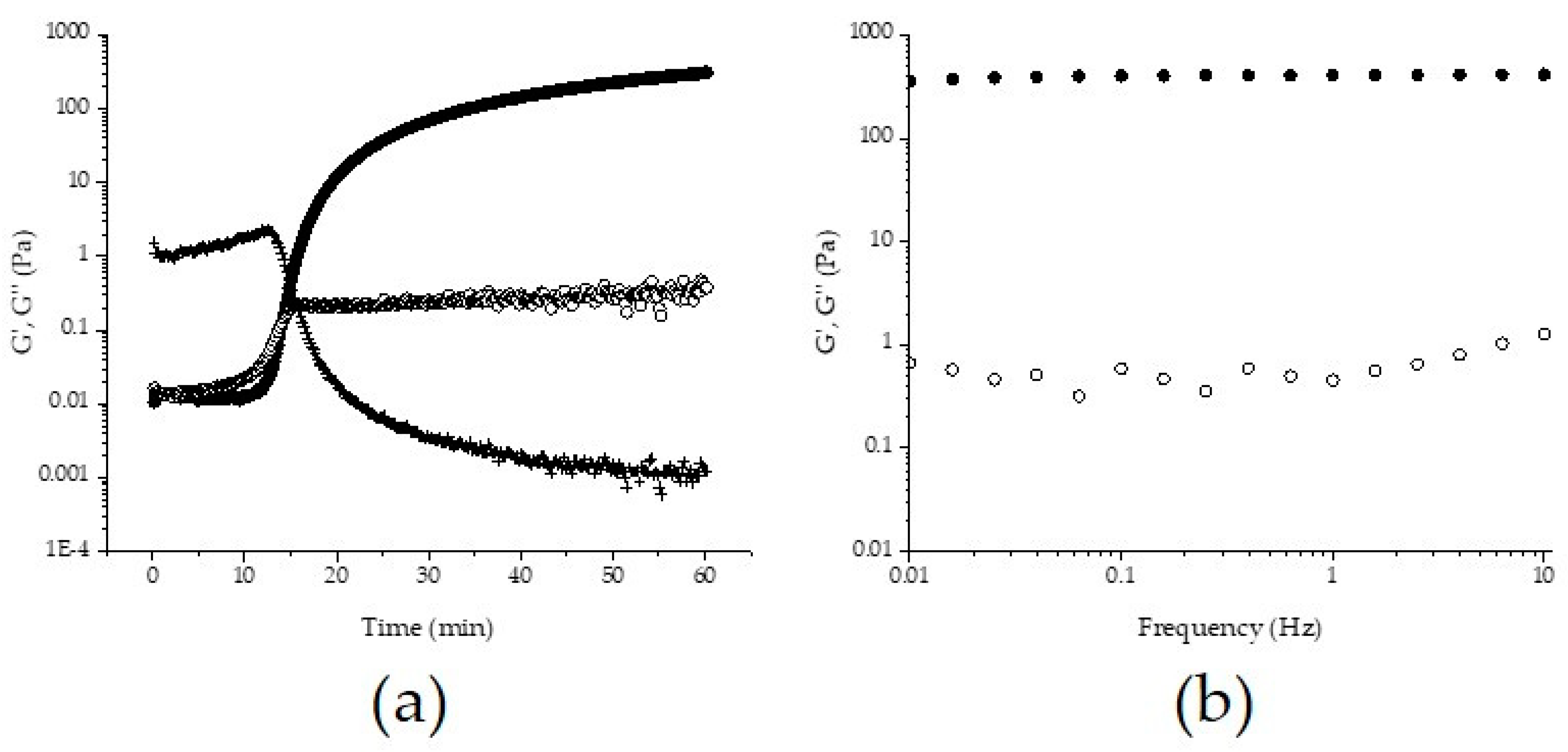
2.2.2. Rheological Measurements
2.2.3. Phenolic Acids
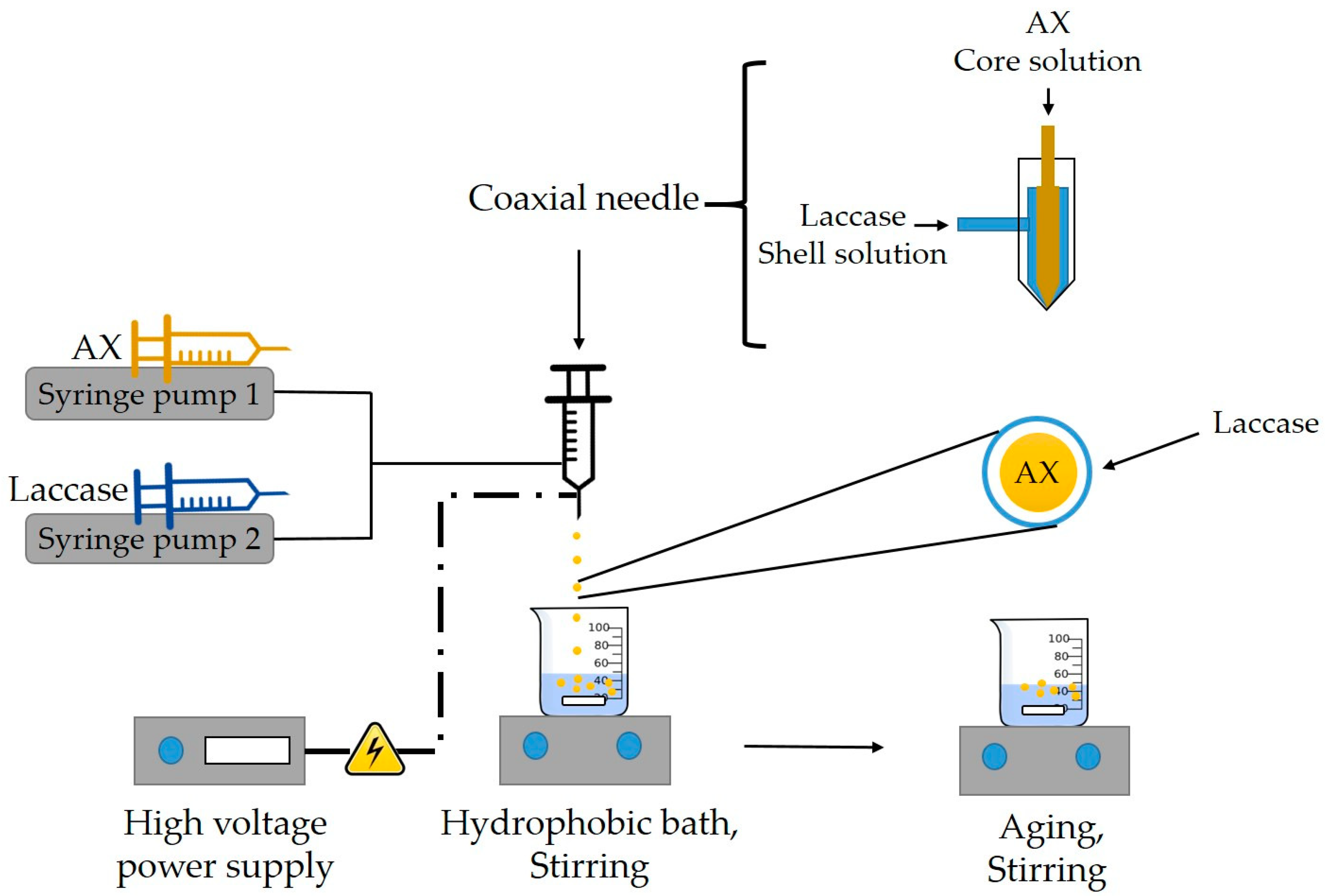
2.2.4. Preparation of AXM
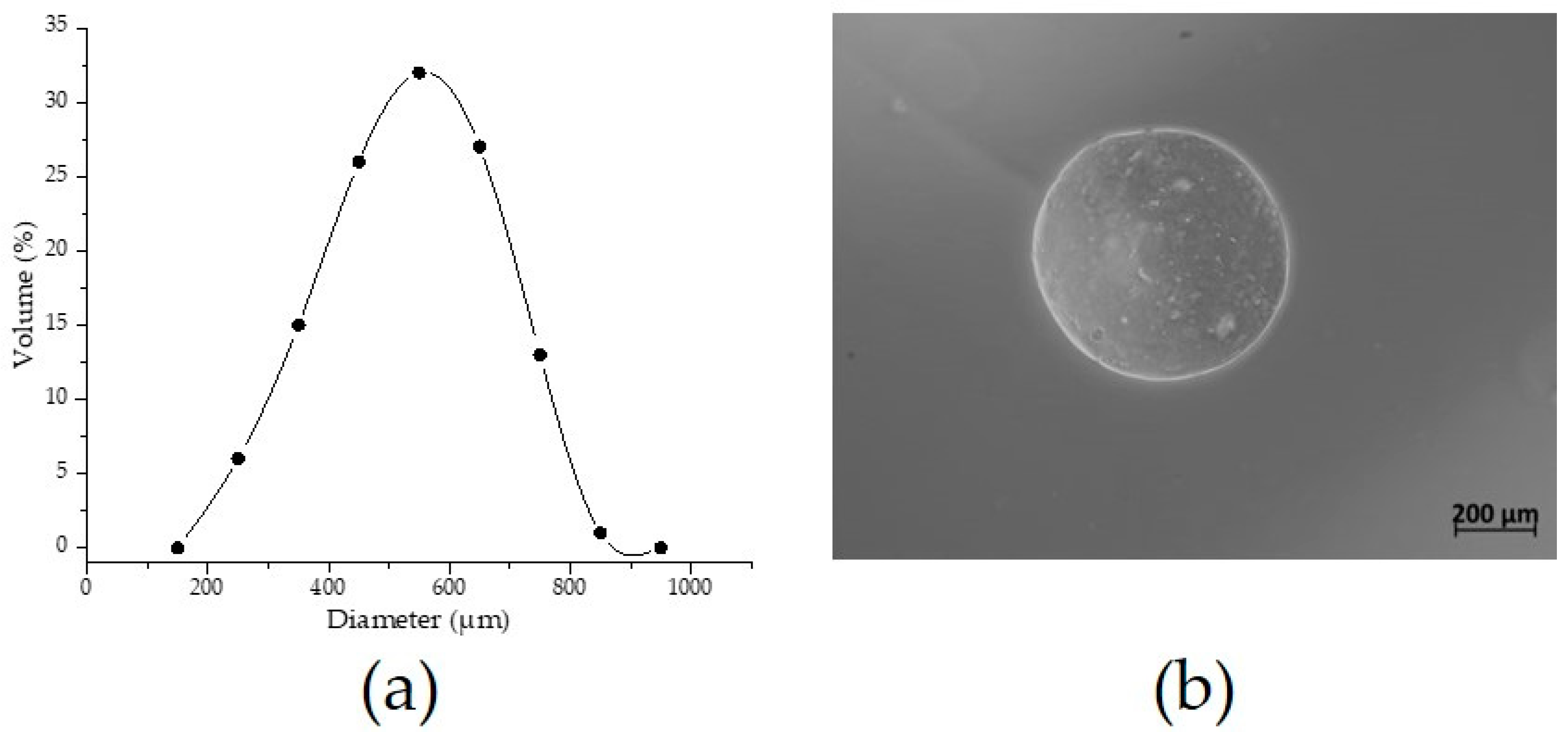
2.2.5. Optical Microscopy
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.8. Swelling Experiment
2.2.9. Structural Parameters
2.2.10. Antioxidant Activity
2.2.11. Cell Lines and Culture Conditions
2.2.12. Cytotoxicity on Normal Human Colon Cells
2.2.13. Cell morphology Analysis
2.2.14. Statistical Analysis
3. Results
3.1. Gelation and Covalent Cross-links
3.2. Morphology and Microstructure of AXM
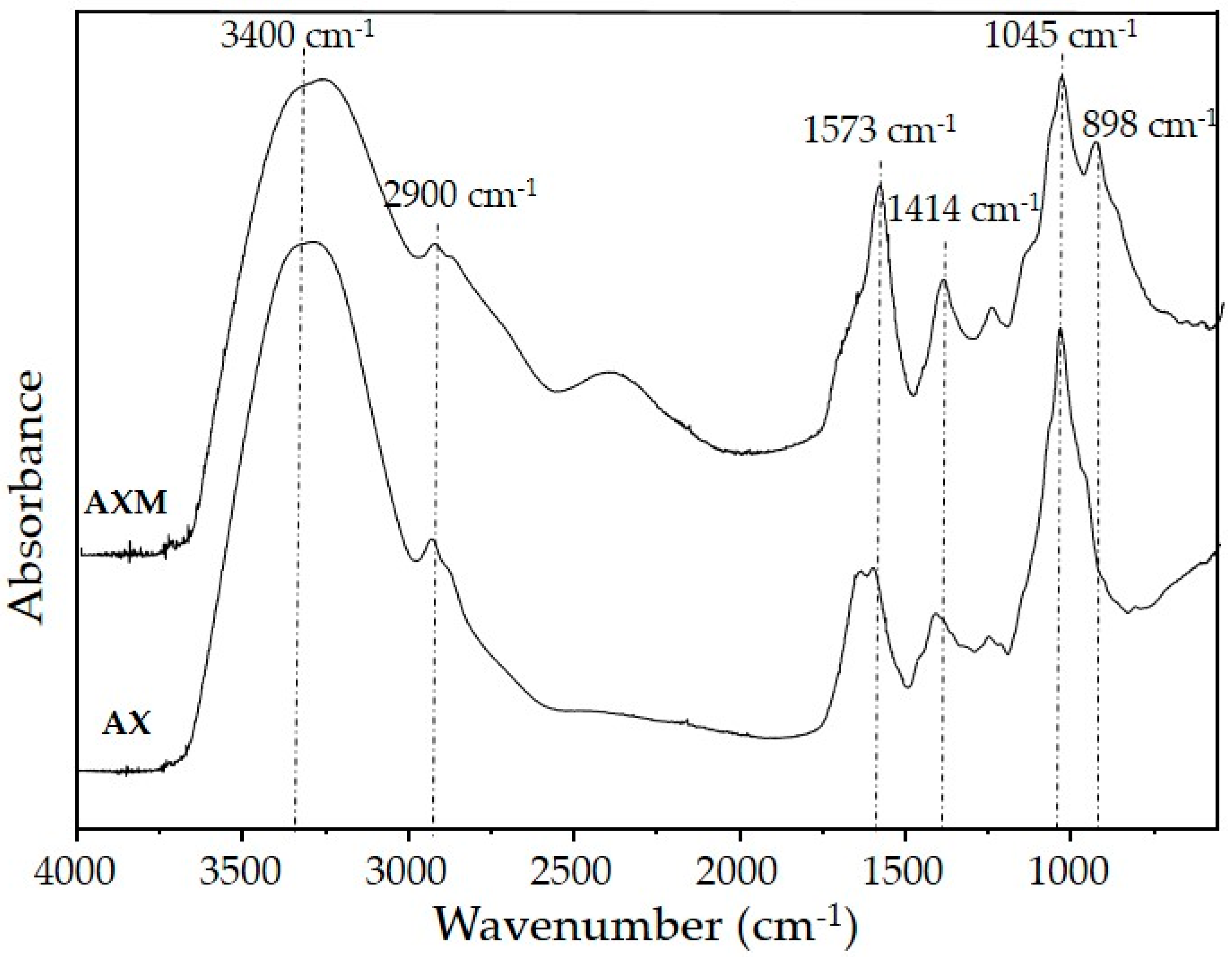
3.3. Fourier Transform Infrared Spectroscopy
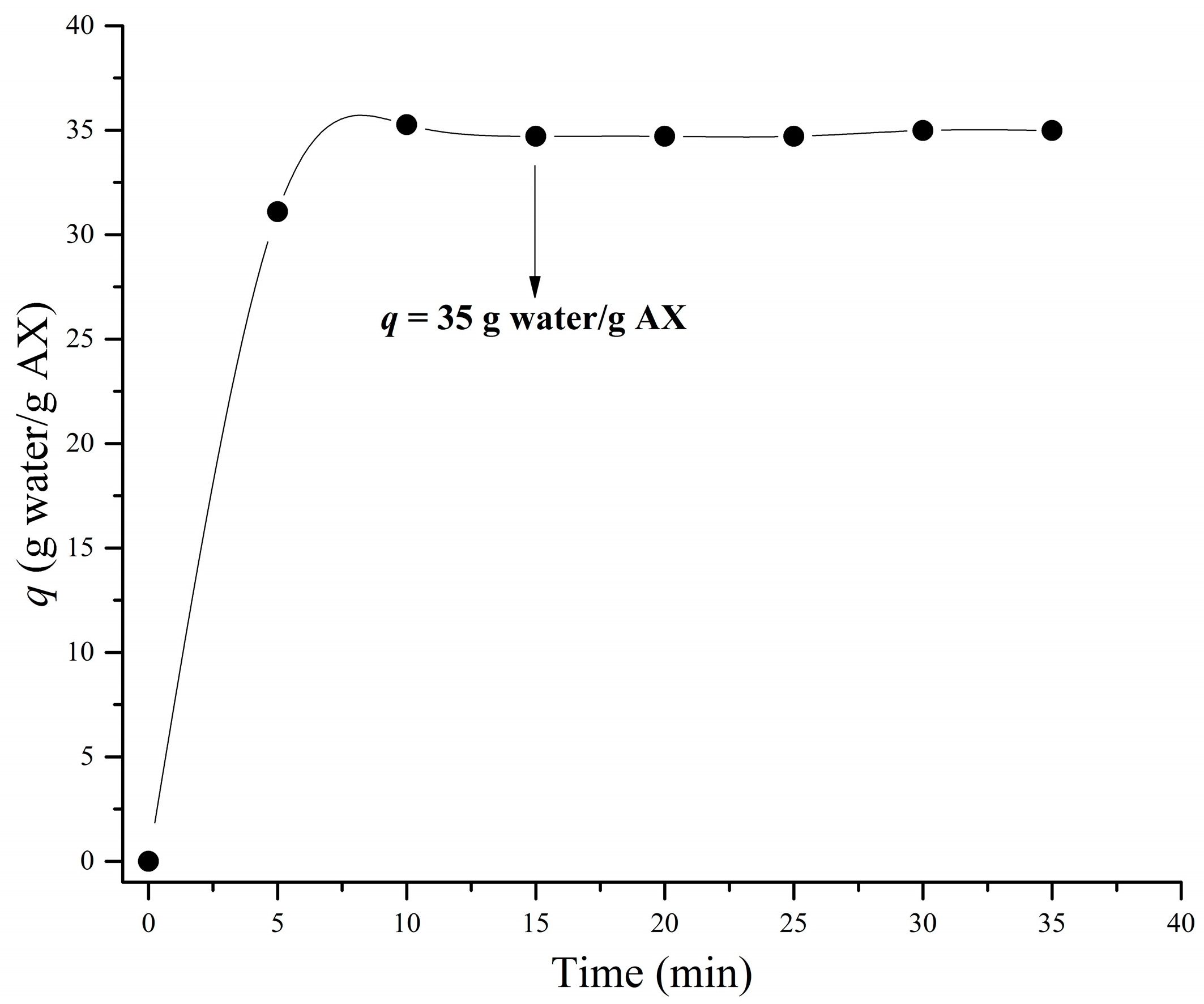
3.4. Swelling and Structure
3.5. In Vitro Antioxidant Activity
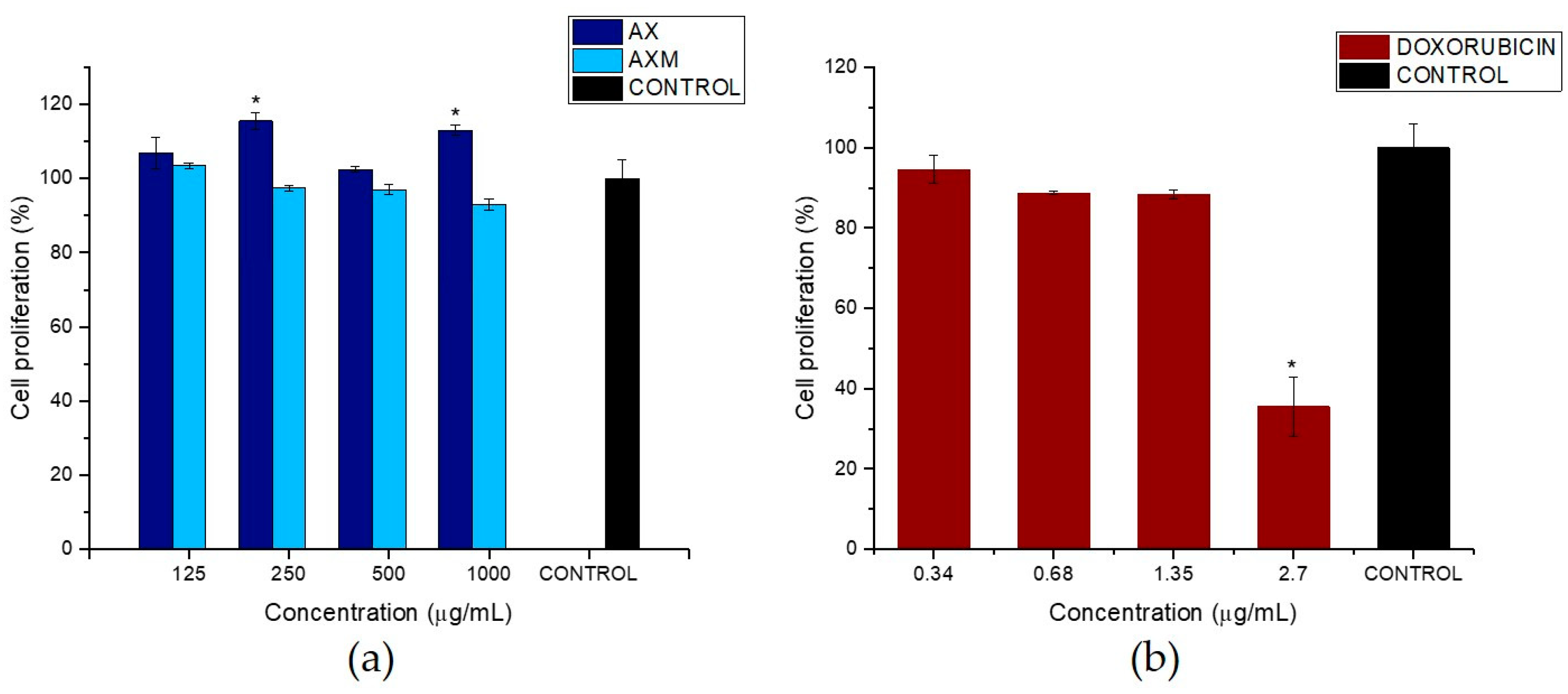
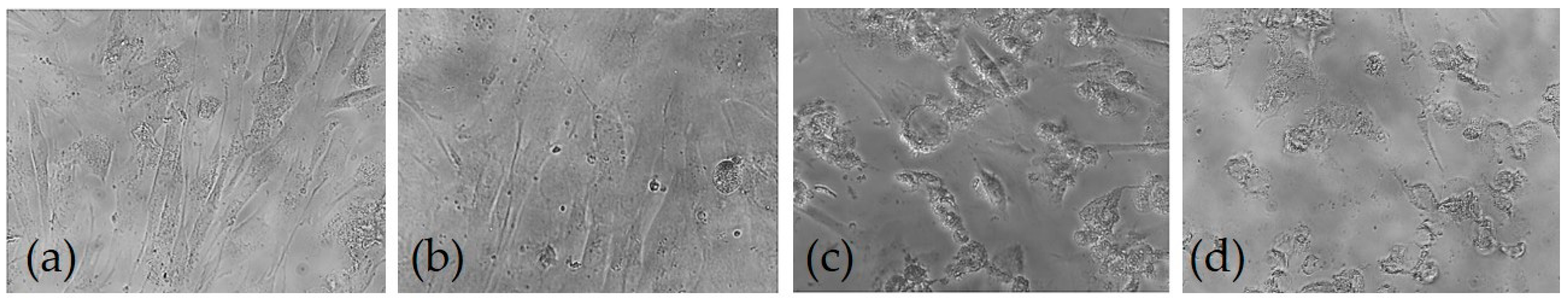
3.6. Effect of AX and AXM on Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.; Ribeiro, J.M; Vitória Figueiredo, I.; Rocha Goncalves, A.; Veiga, F. Alginate microspheres prepared by internal gelation: Development and effect on insulin stability. Int. J. Pharm. 2006, 311, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H. Budesonide-loaded guar gum microspheres for colon delivery: Preparation, characterization and in vitro/in vivo evaluation. Int. J. Mol. Sci. 2015, 16, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jain, D.; Verma, K.; Verma, S. Formulation and in vitro characterization of alginate microspheres loaded with diloxanide furoate for colon-specific drug delivery. Asian J. Pharm. 2010, 4, 199–204. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.K.; Jain, S. Metronidazole loaded pectin microspheres for colon targeting. J. Pharm. Sci. 2009, 98, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- Paz-Samaniego, R.; Rascón-Chu, A.; Brown-Bojorquez, F.; Carvajal-Millan, E.; Pedroza-Montero, M.; Silva-Campa, E.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. Electrospray-assisted fabrication of core-shell arabinoxylan gel particles for insulin and probiotics entrapment. J. Appl. Polym. Sci. 2018, 135, 46411. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Micard, V.; Rascón-Chu, A.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Canett-Romero, R. Enzymatically cross-linked arabinoxylan microspheres as oral insulin delivery system. Int. J. Biol. Macromol. 2019, 126, 952–959. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.L.; Carvajal-Millan, E.; Marquez-Escalante, J.; Campa-Mada, A.C.; Rascon-Chu, A.; Lopez-Franco, Y.L.; Lizardi-Mendoza, J. Enzymatic cross-linking of ferulated arabinoxylan: Effect of laccase or peroxidase catalysis on the gel characteristics. Food Sci. Biotechnol. 2018, 28, 311–318. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guilbert, S.; Doublier, J.L.; Micard, V. Arabinoxylan/protein gels: Structural, rheological and controlled release properties. Food Hydrocoll. 2006, 20, 53–61. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Lizardi, J.; Rascon-Chu, A.; Marquez-Escalante, J.; Gardea, A.; Martinez-Lopez, A.L.; Guerrero, V. Maize processing waste water arabinoxylans: Gelling capability and cross-linking content. Food Chem. 2009, 115, 1286–1290. [Google Scholar] [CrossRef]
- Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Hicks, K.B.; Yadav, M.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Toledo-Guillén, A.R.; Islas-Rubio, A.R. Protein/Arabinoxylans gels: Effect of mass ratio on the rheological, microstructural and diffusional characteristics. Int. J. Mol. Sci. 2014, 15, 19106–19118. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Espinoza, A.B.; Piñón-Muñiz, M.I.; Rascón-Chu, A.; Santana-Rodríguez, V.M.; Carvajal-Millan, E. Lycopene/arabinoxylan gels: Rheological and controlled release characteristics. Molecules 2012, 17, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ortega, A.; Carvajal-Millan, E.; Brown-Bojorquez, F.; Rascón-Chu, A.; Torres-Chavez, P.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Martínez-López, A.L.; Campa-Mada, A.C. Entrapment of probiotics in water extractable arabinoxylan gels: Rheological and microstructural characterization. Molecules 2014, 19, 3628–3637. [Google Scholar] [CrossRef] [PubMed]
- Paz-Samaniego, R.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Brown, F.; Rascón-Chu, A.; López-Franco, Y.L.; Lizardi-Mendoza, J. Maize processing waste water upcycling in Mexico: Recovery of arabinoxylans for probiotic encapsulation. Sustainability 2016, 8, 1104. [Google Scholar] [CrossRef]
- González-Estrada, R.; Calderón-Santoyo, M.; Carvajal-Millan, E.; Ascencio Valle, F.D.J.; Ragazzo-Sánchez, J.A.; Brown-Bojórquez, F.; Rascon-Chu, A. Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment. Molecules 2015, 20, 11373–11386. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Micard, V.; Rascón-Chu, A.; Brown-Bojorquez, F.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016, 144, 76–82. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Englyst, H.N.; Macfarlane, S.; Furrie, E.; Macfarlane, G.T.; McBain, A.J. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. Appl. Environ. Microbiol. 2003, 69, 6354–6360. [Google Scholar] [CrossRef]
- Paz-Samaniego, R.; Méndez-Encinas, M.; Fierro-Islas, J.M.; Marquez-Escalante, J.; Rascón-Chu, A.; Martinez-Lopez, A.L.; Carvajal-Millan, E. Ferulated arabinoxylans recovered from low-value maize by-products: Gelation and antioxidant capacity. In Ferulic Acid: Antioxidant Properties, Uses and Potential Health Benefits; Bryce, W., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 151–164. [Google Scholar]
- Martínez-López, A.L.; Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.; Martínez-Robinson, K. Gels of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran: Rheological and structural characteristics. CyTA-J. Food 2013, 11, 22–28. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated arabinoxylans and their gels: Functional properties and potential application as antioxidant and anticancer agent. Oxid. Med. Cell. Longev. 2018, 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Vardakou, M.; Nueno, C.; Christakopoulos, P.; Faulds, C.B.; Gasson, M.A.; Narbad, A. Evaluation of the prebiotic properties of wheat arabinoxylan fractions and induction of hydrolase activity in gut microflora. Int. J. Food Microbiol. 2008, 123, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T.; Cummings, J.H. Review article: Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Báez-González, J.G.; Carvajal-Millán, E.; Muy-Rangel, D.; Urías-Orona, V.; Martínez-López, A.L.; Márquez-Escalante, J.A.; Heredia, J.B.; Beta, T.; Niño-Medina, G. Alkali-extracted feruloylated arabinoxylans from nixtamalized maize bran byproduct: A synonymous with soluble antioxidant dietary fiber. Waste Biomass Valori. 2018, 1–7. [Google Scholar] [CrossRef]
- Katapodis, P.; Vardakou, M.; Kalogeris, E.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Enzymic production of a feruloylated oligosaccharide with antioxidant activity from wheat flour arabinoxylan. Eur. J. Nutr. 2003, 42, 55–60. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Wang, C. Wheat bran feruloyl oligosaccharides enhance the antioxidant activity of rat plasma. Food Chem. 2010, 123, 472–476. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Vargas-Albores, F.; Fierro-Islas, J.M.; Gollas-Galván, T.; Magdaleno-Moncayo, D.; Rascon-Chu, A.; Martínez-Porchas, M.; Lago-Lestón, A. Arabinoxylans and gelled arabinoxylans used as anti-obesogenic agents could protect the stability of intestinal microbiota of rats consuming high-fat diets. Int. J. Food Sci. Nutr. 2019, 1–10. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Yadav, M.P.; López-Franco, Y.L.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Brown-Bojorquez, F.; Silva-Campa, E.; Pedroza-Montero, M. Partial removal of protein associated with arabinoxylans: Impact on the viscoelasticity, crosslinking content, and microstructure of the gels formed. J. Appl. Polym. Sci. 2019, 136, 47300. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Rouau, X.; Chaynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudeu, E.; Montero, J.L.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Jonathan, A.D.; Carvajal-Millan, E.; Pérez-López, E.; Hotchkiss, A.T.; González-Ríos, H.; Balandrán-Quintana, R.; Campa-Mada, A. Electrosprayed core – shell composite microbeads based on pectin-arabinoxylans for insulin carrying. Polymers 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Carvajal-Millan, E.; Miki-Yoshida, M.; Alvarez-Contreras, L.; Rascón-Chu, A.; Lizardi-Mendoza, J.; López-Franco, Y. Arabinoxylan microspheres: Structural and textural characteristics. Molecules 2013, 18, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Morales-Burgos, A.M.; Carvajal-Millan, E.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Sotelo-cruz, N.; Brown-Bojórquez, F.; Burgara-Estrella, A.; Pedroza-Montero, M. Syneresis in gels of highly ferulated arabinoxylans: Characterization of covalent cross-linking, rheology, and microstructure. Polymers 2017, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Poly (vinyl alcohol) hydrogels: Reinforcement of radiation- crosslinked networks by crystallization. J. Polym. Sci. 1976, 14, 441–457. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. On the chain flexibility of arabinoxylans and other β-(1→4) polysaccharides. Carbohydr. Res. 2002, 337, 1781–1784. [Google Scholar] [CrossRef]
- Peppas, N.A.; Moynihan, H.J.; Lucht, L.M. The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J. Biomed. Mater. Res. 1985, 19, 397–411. [Google Scholar] [CrossRef]
- Rosa, N.N.; Barron, C.; Gaiani, C.; Dufour, C.; Micard, V. Ultra-fine grinding increases the antioxidant capacity of wheat bran. J. Cereal Sci. 2013, 57, 84–90. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Rascón-Chu, A. Antioxidant activity of maize bran arabinoxylan microspheres. In Food Composition and Analysis: Methods and Strategies; Haghi, A.K., Carvajal-Millan, E., Eds.; CRC Press: Toronto, ON, Canada, 2014; pp. 19–28. [Google Scholar]
- Malunga, L.N.; Beta, T. Antioxidant capacity of water extractable arabinoxylan from commercial barley, wheat and wheat fractions. Cereal Chem. 2015, 92, 29–36. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B.; Rieder, A.; Grimmer, S.; Michaelsen, T.E.; Knutsen, S.H. Immunomodulatory activity of dietary fiber: Arabinoxylan and mixed-linked beta-glucan isolated from barley show modest activities in vitro. Int. J. Mol. Sci. 2011, 12, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival - application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hernandez, J.; Goycoolea, F.M.; Quintero, J.; Acosta, A.; Castañeda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.F.; Astiazaran, H.; et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef]
- Liao, W.; Yu, Z.; Lin, Z.; Lei, Z.; Ning, Z.; Regenstein, J.M.; Yang, J.; Ren, J. Biofunctionalization of selenium nanoparticle with Dictyophora Indusiata polysaccharide and its antiproliferative activity through death-receptor and mitochondria-mediated apoptotic pathways. Sci. Rep. 2015, 5, 18629. [Google Scholar] [CrossRef]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef]
- Iravani, S.; Fitchett, C.S.; Georget, D.M.R. Physical characterization of arabinoxylan powder and its hydrogel containing a methyl xanthine. Carbohydr. Polym. 2011, 85, 201–207. [Google Scholar] [CrossRef]
- Morales-Ortega, A.; Carvajal-Millan, E.; López-Franco, Y.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Torres-Chavez, P.; Campa-Mada, A. Characterization of water extractable arabinoxylans from a spring wheat flour: Rheological properties and microstructure. Molecules 2013, 18, 8417–8428. [Google Scholar] [CrossRef]
- Urias-Orona, V.; Huerta-Oros, J.; Carvajal-Millán, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; Gardea, A.A. Component analysis and free radicals scavenging activity of Cicer arietinum L. husk pectin. Molecules 2010, 15, 6948–6955. [Google Scholar] [CrossRef] [PubMed]
- Egüés, I.; Stepan, A.M.; Eceiza, A.; Toriz, G.; Gatenholm, P.; Labidi, J. Corncob arabinoxylan for new materials. Carbohydr. Polym. 2014, 102, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Millan, E.; Guilbert, S.; Morel, M.H.; Micard, V. Impact of the structure of arabinoxylan gels on their rheological and protein transport properties. Carbohydr. Polym. 2005, 60, 431–438. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Méndez-Encinas, M.; Carvajal-Millan, E.; Yadav, M.; Valenzuela-Soto, E.M.; Figueroa-Soto, C.G.; Tortoledo-Ortiz, O.; García-Sánchez, G. Gels of ferulated arabinoxylans: Rheology, structural parameters and microstructure. In Advances in Physicochemical Properties of Biopolymers; Masuelli, M.A., Renard, D., Eds.; Bentham Science Publishers: Beijing, China, 2017; pp. 208–221. ISBN 978-1-68108-453-1. [Google Scholar]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of laccase induced arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Rodrigues Marcelino, H.; Eduardo da Silva, A.; Christine, M.; Salgado Gomes, M.C.; Eleamen Oliveira, E.; Nagashima-Junior, T.; Sousa Pinheiro, G.; Eduardo da Silva, A.; de Souza Timoteo, A.R.; Agnez-Lima, L.F.; et al. Leads from physical, chemical, and thermal characterization on cytotoxic effects of xylan-based microparticles. Polymers 2015, 7, 2304–2315. [Google Scholar] [CrossRef]
- Meyvis, T.K.L.; Smedt, S.C.D.; Demeester, J.; Hennink, W.E. Influence of the degradation mechanism of hydrogels on their elastic and swelling properties during degradation. Macromolecules 2000, 33, 4717–4725. [Google Scholar] [CrossRef]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; García-Lara, S.; Pérez-Carrillo, E. Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014, 35, 471–475. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Antioxidant capacity of arabinoxylan oligosaccharide fractions prepared from wheat aleurone using Trichoderma viride or Neocallimastix patriciarum xylanase. Food Chem. 2015, 167, 311–319. [Google Scholar] [CrossRef]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Pérez-Carrillo, E.; García-Lara, S. Relationship between hydroxycinnamic profile with gelation capacity and rheological properties of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014, 39, 280–285. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. Flavonoids in cancer prevention. Anticancer Agents Med. Chem. 2012, 12, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Aduba, D.C.; An, S.-S.; Selders, G.S.; Wang, J.; Andrew Yeudall, W.; Bowlin, G.L.; Kitten, T.; Yang, H. Fabrication, characterization, and in vitro evaluation of silver-containing arabinoxylan foams as antimicrobial wound dressing. J. Biomed. Mater. Res. 2016, 104, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Sardão, V.A.; Oliveira, P.J.; Holy, J.; Oliveira, C.R.; Wallace, K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biol. Toxicol. 2009, 25, 227–243. [Google Scholar] [CrossRef] [PubMed]

| Time | FA | di-FA | tri-FA | a FA Oxidized | b FA Recovered |
|---|---|---|---|---|---|
| (min) | (µg/mg AX) | (%) | |||
| 0 | 5.45 ± 0.09 | 0.35 ± 0.07 | 0.03 ± 0.00 | - | - |
| 60 | 3.52 ± 0.26 | 1.61 ± 0.14 | 0.04 ± 0.01 | 35 ± 5 | 85 ± 1 |
| Parameter | Value |
|---|---|
| qa (g water/g AX) | 35 ± 1.50 |
| Mcb × 103 (g mol−1) | 50 ± 0.10 |
| ρcc × 10−6 (mol/cm3) | 47 ± 0.01 |
| ξ d (nm) | 27 ± 3.00 |
| Antioxidant Activity a (µmol TEAC/g) | |||
|---|---|---|---|
| Sample | ABTS+ | DPPH | FRAP |
| Uncross-linked AX | 68.05 ± 0.53 | 32.23 ± 0.50 | 48.41 ± 1.07 |
| Cross-linked AX | 26.02 ± 3.82 | 12.58 ± 0.45 | 16.83 ± 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascón-Chu, A.; Astiazarán-García, H.; Valencia-Rivera, D.E.; Brown-Bojorquez, F.; Alday, E.; Velazquez, C. Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line. Medicina 2019, 55, 349. https://doi.org/10.3390/medicina55070349
Mendez-Encinas MA, Carvajal-Millan E, Rascón-Chu A, Astiazarán-García H, Valencia-Rivera DE, Brown-Bojorquez F, Alday E, Velazquez C. Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line. Medicina. 2019; 55(7):349. https://doi.org/10.3390/medicina55070349
Chicago/Turabian StyleMendez-Encinas, Mayra A., Elizabeth Carvajal-Millan, Agustín Rascón-Chu, Humberto Astiazarán-García, Dora E. Valencia-Rivera, Francisco Brown-Bojorquez, Efrain Alday, and Carlos Velazquez. 2019. "Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line" Medicina 55, no. 7: 349. https://doi.org/10.3390/medicina55070349
APA StyleMendez-Encinas, M. A., Carvajal-Millan, E., Rascón-Chu, A., Astiazarán-García, H., Valencia-Rivera, D. E., Brown-Bojorquez, F., Alday, E., & Velazquez, C. (2019). Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line. Medicina, 55(7), 349. https://doi.org/10.3390/medicina55070349






