The Effects of Transdermal Nicotine Patches on the Cardiorespiratory and Lactate Responses During Exercise from Light to Moderate Intensity: Implications for Exercise Prescription during Smoking Cessation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Transdermal Nicotine Patch (TNP)
2.4. Incremental Graded Exercise Test and Indirect Calorimetry Measurement
2.5. Heart Rate, Ratings of Perceived Exertion, and Blood Lactate Concentration
2.6. Statistical Analysis
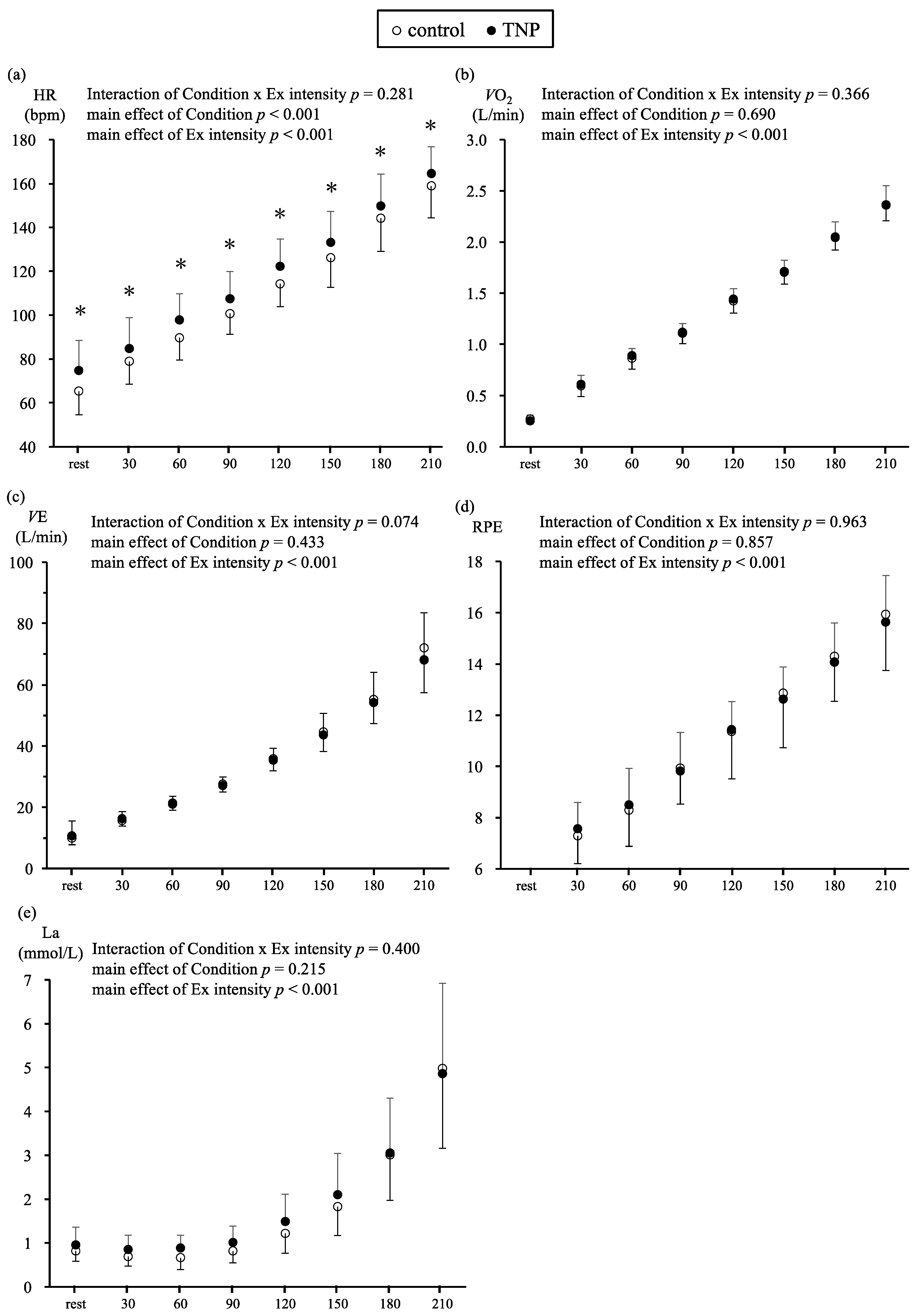
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Zong, G.; Liu, G.; Wang, M.; Rosner, B.; Pan, A.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Sun, Q. Smoking cessation, weight change, type 2 diabetes, and mortality. N. Engl. J. Med. 2018, 379, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Ramasundarahettige, C.; Landsman, V.; Rostron, B.; Thun, M.; Anderson, R.N.; McAfee, T.; Peto, R. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 2013, 368, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.J.; Thorlund, K.; Eapen, S.; Wu, P.; Prochaska, J.J. Cardiovascular events associated with smoking cessation pharmacotherapies: A network meta-analysis. Circulation 2014, 129, 28–41. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Framework Convention on Tobacco Control. In Proceedings of the sixth Session of the Conference of the Parties, Moscow, Russian, 13–18 October 2014. [Google Scholar]
- Kalkhoran, S.; Benowitz, N.L.; Rigotti, N.A. Prevention and Treatment of Tobacco Use: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Livingstone-Banks, J.; Norris, E.; Hartmann-Boyce, J.; West, R.; Jarvis, M.; Hajek, P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 2, Cd003999. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Nicotine and smokeless tobacco. CA Cancer J. Clin. 1988, 38, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Tzivoni, D.; Keren, A.; Meyler, S.; Khoury, Z.; Lerer, T.; Brunel, P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc. Drugs Ther. 1998, 12, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Pipe, A.; West, R.; Hays, J.T.; Tonstad, S.; McRae, T.; Lawrence, D.; St Aubin, L.; Anthenelli, R.M. Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch in Smokers: A Randomized Clinical Trial. JAMA Intern Med. 2018, 178, 622–631. [Google Scholar] [CrossRef]
- Joseph, A.M.; Norman, S.M.; Ferry, L.H.; Prochazka, A.V.; Westman, E.C.; Steele, B.G.; Sherman, S.E.; Cleveland, M.; Antonuccio, D.O.; Hartman, N.; et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N. Engl. J. Med. 1996, 335, 1792–1798. [Google Scholar] [CrossRef]
- Oncken, C.; Campbell, W.; Chan, G.; Hatsukami, D.; Kranzler, H.R. Effects of nicotine patch or nasal spray on nicotine and cotinine concentrations in pregnant smokers. J. Matern. Fetal Neonatal Med. 2009, 22, 751–758. [Google Scholar] [CrossRef]
- Hill, J.S. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Can. J. Public Health 1985, 76, 183–186. [Google Scholar] [PubMed]
- Roberts, V.; Maddison, R.; Simpson, C.; Bullen, C.; Prapavessis, H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology (Berl.) 2012, 222, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ussher, M.H.; Taylor, A.H.; Faulkner, G.E. Exercise interventions for smoking cessation. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Everson, E.S.; Daley, A.J.; Ussher, M. The effects of moderate and vigorous exercise on desire to smoke, withdrawal symptoms and mood in abstaining young adult smokers. Mental Health Phys. Act. 2008, 1, 26–31. [Google Scholar] [CrossRef]
- Daniel, J.; Cropley, M.; Ussher, M.; West, R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology (Berl.) 2004, 174, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.Z.; Cropley, M.; Fife-Schaw, C. The effect of exercise in reducing desire to smoke and cigarette withdrawal symptoms is not caused by distraction. Addiction 2006, 101, 1187–1192. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Hall, S.M.; Humfleet, G.; Munoz, R.F.; Reus, V.; Gorecki, J.; Hu, D. Physical activity as a strategy for maintaining tobacco abstinence: A randomized trial. Prev. Med. 2008, 47, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Robinson, B.F.; Epstein, S.E.; Beiser, G.D.; Braunwald, E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ. Res. 1966, 19, 400–411. [Google Scholar] [CrossRef]
- Cryer, P.E.; Haymond, M.W.; Santiago, J.V.; Shah, S.D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N. Engl. J. Med. 1976, 295, 573–577. [Google Scholar] [CrossRef]
- Tanus-Santos, J.E.; Toledo, J.C.; Cittadino, M.; Sabha, M.; Rocha, J.C.; Moreno, H., Jr. Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. Am. J. Hypertens 2001, 14, 610–614. [Google Scholar] [CrossRef]
- Yugar-Toledo, J.C.; Ferreira-Melo, S.E.; Sabha, M.; Nogueira, E.A.; Coelho, O.R.; Consolin Colombo, F.M.; Irigoyen, M.C.; Moreno, H., Jr. Blood pressure circadian rhythm and endothelial function in heavy smokers: Acute effects of transdermal nicotine. J. Clin. Hypertens. (Greenwich) 2005, 7, 721–728. [Google Scholar] [CrossRef]
- Perkins, K.A.; Sexton, J.E.; Solberg-Kassel, R.D.; Epstein, L.H. Effects of nicotine on perceived exertion during low-intensity activity. Med. Sci. Sports Exerc. 1991, 23, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A.; Epstein, L.H.; Marks, B.L.; Stiller, R.L.; Jacob, R.G. The effect of nicotine on energy expenditure during light physical activity. N. Engl. J. Med. 1989, 320, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; McNicol, M.W. The effect of nicotine and carbon monoxide on exercise performance in normal subjects. Respir. Med. 1993, 87, 427–431. [Google Scholar] [CrossRef]
- Urae, A.; Irie, S.; Amamoto, T.; Yoshiie, H. The Tolerability, Pharmacodynamics and Pharmacokinetics of Ba37142 (Nicotine TTS) ln a Singly Applied to Investigate. J. Clin. Ther. Med. 1994, 10, 3–34. [Google Scholar]
- Nakagata, T.; Naito, H.; Yamada, Y. Metabolic equivalents of body weight resistance training with slow movement: Implications for exercise prescription and health promotion. J. Exerc. Physiol. Online 2018, 21, 29–38. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Nakagata, T.; Yamada, Y.; Naito, H. Energy expenditure, recovery oxygen consumption, and substrate oxidation during and after body weight resistance exercise with slow movement compared to treadmill walking. Physiol. Int. 2018, 105, 371–385. [Google Scholar] [CrossRef]
- Yoshimura, E.; Kumahara, H.; Tobina, T.; Matsuda, T.; Watabe, K.; Matono, S.; Ayabe, M.; Kiyonaga, A.; Anzai, K.; Higaki, Y.; et al. Aerobic exercise attenuates the loss of skeletal muscle during energy restriction in adults with visceral adiposity. Obesity Facts 2014, 7, 26–35. [Google Scholar] [CrossRef]
- Haass, M.; Kubler, W. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 1997, 10, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Nakagata, T.; Naito, H.; Katamoto, S.; Kobayashi, H.; Sawada, S.S. Effects of transdermal nicotine patches on energy expenditure measured with a human calorimeter. Juntendo M. J. 2016, 62, 232–239. [Google Scholar] [CrossRef]
- Mundel, T.; Jones, D.A. Effect of transdermal nicotine administration on exercise endurance in men. Exp. Physiol. 2006, 91, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.J.; Galbo, H. Sympathetic nervous activity during exercise. Annu. Rev. Physiol. 1983, 45, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Tanaka, H.; Kiyonaga, A.; Terao, Y.; Ide, K.; Yamauchi, M.; Tanaka, M.; Shindo, M. Double product response is accelerated above the blood lactate threshold. Med. Sci. Sports Exerc. 1997, 29, 503–508. [Google Scholar] [CrossRef]
- Schneider, D.A.; McGuiggin, M.E.; Kamimori, G.H. A comparison of the blood lactate and plasma catecholamine thresholds in untrained male subjects. Int. J. Sports Med. 1992, 13, 562–566. [Google Scholar] [CrossRef]
- Bush, T.; Lovejoy, J.C.; Deprey, M.; Carpenter, K.M. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity 2016, 24, 1834–1841. [Google Scholar] [CrossRef]
- Aubin, H.J.; Farley, A.; Lycett, D.; Lahmek, P.; Aveyard, P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012, 345, e4439. [Google Scholar] [CrossRef]
- Sakamoto, M.; Higaki, Y.; Nishida, Y.; Kiyonaga, A.; Shindo, M.; Tokuyama, K.; Tanaka, H. Influence of mild exercise at the lactate threshold on glucose effectiveness. J. Appl. Physiol. 1999, 87, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Higaki, Y.; Tokuyama, K.; Fujimi, K.; Kiyonaga, A.; Shindo, M.; Sato, Y.; Tanaka, H. Effect of mild exercise training on glucose effectiveness in healthy men. Diabetes Care 2001, 24, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Kiyonaga, A.; Arakawa, K.; Tanaka, H.; Shindo, M. Blood pressure and hormonal responses to aerobic exercise. Hypertension 1985, 7, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Lessov-Schlaggar, C.N.; Swan, G.E.; Jacob, P., 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther. 2006, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Sweeney, C.T.; Dresler, C.M. Nicotine patch and lozenge are effective for women. Nicotine Tob. Res. 2005, 7, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ciccolo, J.T.; Williams, D.M.; Dunsiger, S.I.; Whitworth, J.W.; McCullough, A.K.; Bock, B.B.; Marcus, B.H.; Myerson, M. Efficacy of Resistance Training as an Aid to Smoking Cessation: Rationale and Design of the Strength To Quit Study. Mental Health Phys. Act. 2014, 7, 95–103. [Google Scholar] [CrossRef]
- Ciccolo, J.T.; Dunsiger, S.I.; Williams, D.M.; Bartholomew, J.B.; Jennings, E.G.; Ussher, M.H.; Kraemer, W.J.; Marcus, B.H. Resistance training as an aid to standard smoking cessation treatment: A pilot study. Nicotine Tob. Res. 2011, 13, 756–760. [Google Scholar] [CrossRef]
- Ballor, D.L.; Becque, M.D.; Marks, C.R.; Nau, K.L.; Katch, V.L. Physiological responses to nine different exercise:Rest protocols. Med. Sci. Sports Exerc. 1989, 21, 90–95. [Google Scholar] [CrossRef]
- Wilmore, J.H.; Parr, R.B.; Ward, P.; Vodak, P.A.; Barstow, T.J.; Pipes, T.V.; Grimditch, G.; Leslie, P. Energy cost of circuit weight training. Med. Sci. Sports 1978, 10, 75–78. [Google Scholar]
| Variables | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| Smokers, n = 6 | 21.7 ± 1.4 * (20.2, 23.1) | 177.8 ± 4.6 * (172.7, 182.3) | 72.3 ± 15.7 (56.0, 88.8) | 22.7 ± 4.0 (18.5, 26.9) |
| Non-smokers, n = 8 | 24.2 ± 2.3 (22.6, 25.8) | 172.2 ± 5.2 (168.7, 176.0) | 64.8 ± 5.4 (61.0, 68.7) | 21.8 ± 1.1 (21.0, 22.6) |
| Total, n = 14 | 23.3 ± 2.3 (22.0, 24.5) | 174.3 ± 5.6 (171.4, 177.1) | 67.6 ± 10.6 (62.0, 73.3) | 22.2 ± 2.5 (20.8, 23.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagata, T.; Fukao, K.; Kobayashi, H.; Katamoto, S.; Naito, H. The Effects of Transdermal Nicotine Patches on the Cardiorespiratory and Lactate Responses During Exercise from Light to Moderate Intensity: Implications for Exercise Prescription during Smoking Cessation. Medicina 2019, 55, 348. https://doi.org/10.3390/medicina55070348
Nakagata T, Fukao K, Kobayashi H, Katamoto S, Naito H. The Effects of Transdermal Nicotine Patches on the Cardiorespiratory and Lactate Responses During Exercise from Light to Moderate Intensity: Implications for Exercise Prescription during Smoking Cessation. Medicina. 2019; 55(7):348. https://doi.org/10.3390/medicina55070348
Chicago/Turabian StyleNakagata, Takashi, Kosuke Fukao, Hiroyuki Kobayashi, Shizuo Katamoto, and Hisashi Naito. 2019. "The Effects of Transdermal Nicotine Patches on the Cardiorespiratory and Lactate Responses During Exercise from Light to Moderate Intensity: Implications for Exercise Prescription during Smoking Cessation" Medicina 55, no. 7: 348. https://doi.org/10.3390/medicina55070348
APA StyleNakagata, T., Fukao, K., Kobayashi, H., Katamoto, S., & Naito, H. (2019). The Effects of Transdermal Nicotine Patches on the Cardiorespiratory and Lactate Responses During Exercise from Light to Moderate Intensity: Implications for Exercise Prescription during Smoking Cessation. Medicina, 55(7), 348. https://doi.org/10.3390/medicina55070348






