Matrix Metalloproteinases System and Types of Fibrosis in Rat Heart during Late Pregnancy and Postpartum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histology
2.3. Matrix Metalloproteinase-1 (MMP-1) Measurement by ELISA
2.4. Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Transcript Levels by Quantitative Polymerase Chain Reaction (PCR)
- F: 5′-CAGCGAGGAGTTTCTCATCG-3′
- R: 5′-GGCTGAACAGGGAAACACTG-3′
- F: 5′-TCCCTCAAGATTGTCAGCAA-3′
- R: 5′-GCAGTGATGGCATGGACT-3′
2.5. Inmunoblot Analysis
2.6. Statistical Analysis
3. Results
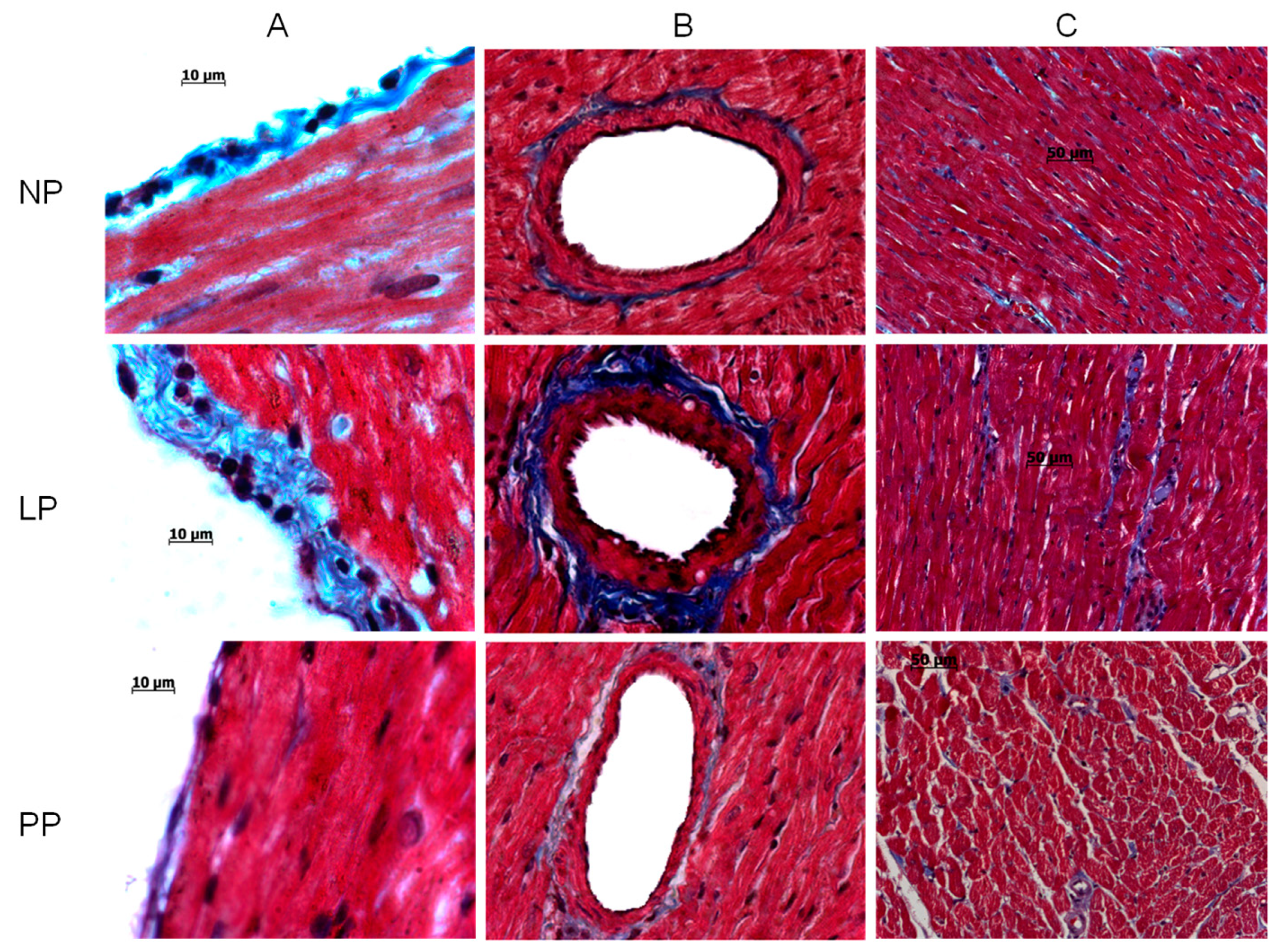
3.1. Cardiac Hypertrophy and Fibrosis
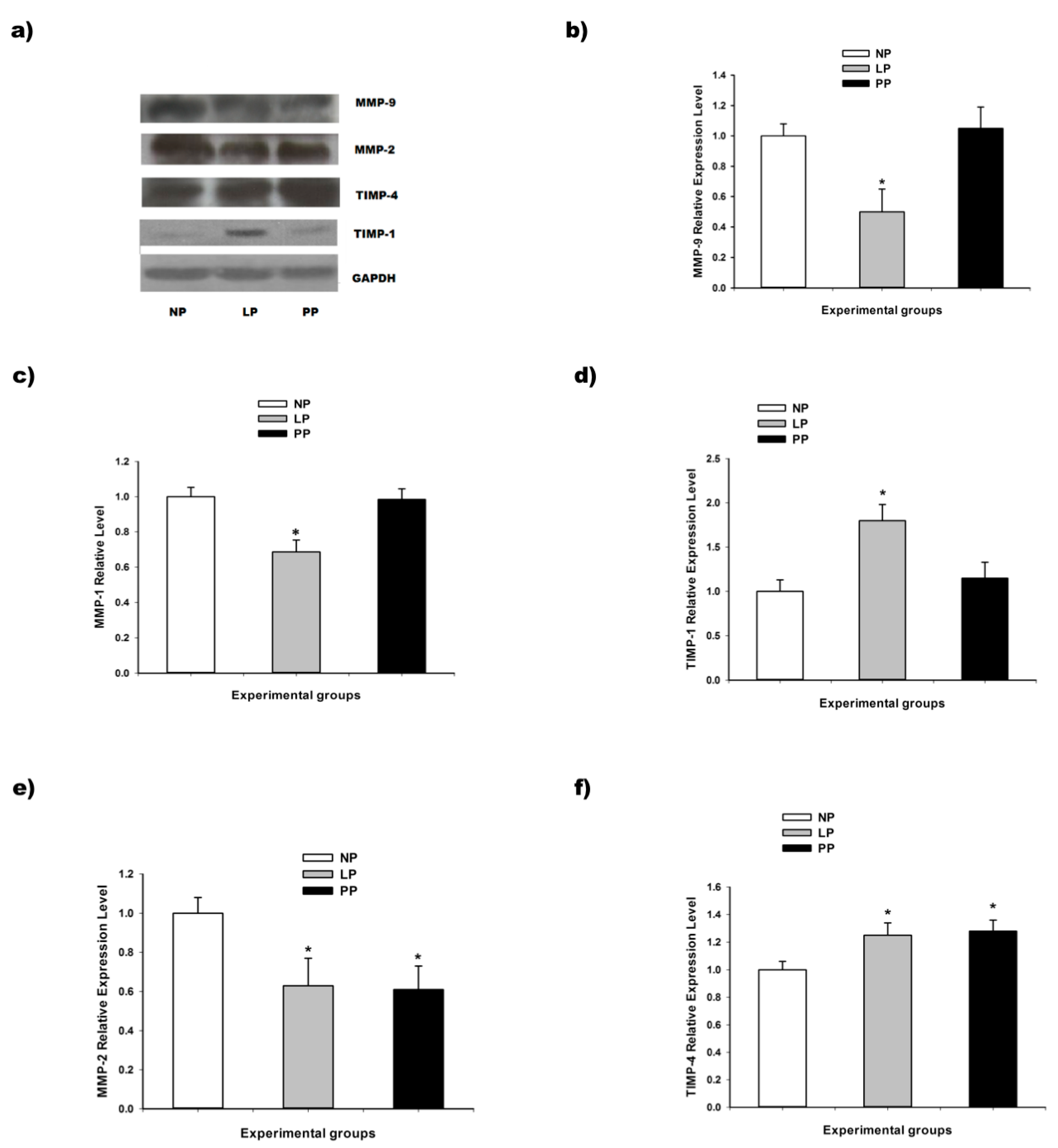
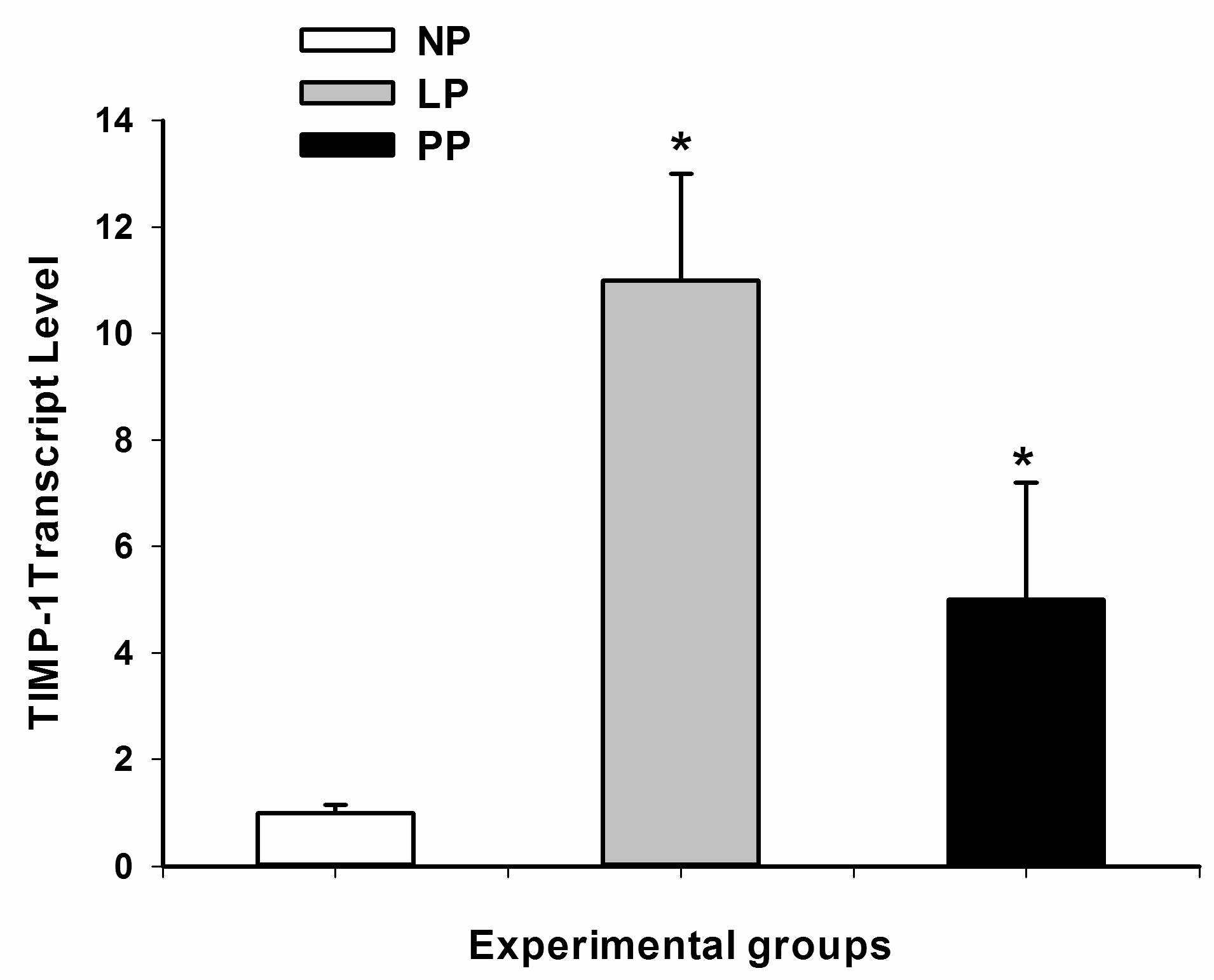
3.2. Metalloproteinases and Tissue Inhibitor of Metalloproteinases (TIMPs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osol, G.; Ko, N.L.; Mandalà, M. Plasticity of the Maternal Vasculature During Pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Savu, O.; Jurcuţ, R.; Giuşcă, S.; van Mieghem, T.; Gussi, I.; Popescu, B.A.; Ginghină, C.; Rademakers, F.; Deprest, J.; Voigt, J.U. Morphological and functionaladaptation of the maternal heart during pregnancy. Circ. Cardiovasc. Imaging 2012, 5, 289–297. [Google Scholar]
- Eghbali, M.; Deva, R.; Alioua, A.; Minosyan, T.Y.; Ruan, H.; Wang, Y.; Toro, L.; Stefani, E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ. Res. 2005, 96, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortiz, A.; Marin, J.L.; Elizalde, A.; Castro, E.; Estefani, E.; Toro, L.; Muñiz, J. Passive mechanical properties of cardiac tissues in heart hypertrophy during pregnancy. J. Physiol. Sci. 2009, 59, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.M.; Acquistapace, G.; Perrucci, G.L.; Nicolini, P.; Toffetti, L.; Braidotti, P.; Ferrero, S.; Zucca, I.; Aquino, D.; Busca, G.; et al. Immunohistochemical expression of oncological proliferation markers in the hearts of rats during normal pregnancy. Biomark. Med. 2013, 7, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Nadadur, R.; Iorga, A.; Amjedi, M.; Matori, H.; Eghbali, M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J. Appl. Physiol. 2012, 113, 1253–1259. [Google Scholar] [CrossRef]
- Soñanez-Organis, J.G.; Godoy-Lugo, J.A.; Hernández-Palomares, M.L.; Rodríguez-Martínez, D.; Rosas-Rodríguez, J.A.; González-Ochoa, G.; Virgen-Ortiz, A.; Ortiz, R.M. HIF-1α and PPARγ during physiological cardiac hypertrophy induced by pregnancy: Transcriptional activities and effects on target genes. Gene 2016, 591, 376–381. [Google Scholar] [CrossRef]
- Redondo-Angulo, I.; Mas-Stachurska, A.; Sitges, M.; Tinahones, F.J.; Giralt, M.; Villarroya, F.; Planavila, A. Fgf21 is required for cardiac remodeling in pregnancy. Cardiovasc. Res. 2017, 113, 1574–1584. [Google Scholar] [CrossRef]
- Sampaolesi, M.; Van Calsteren, K. Physiological and pathological gestational cardiac hypertrophy: What can we learn from rodents? Cardiovasc. Res. 2017, 113, 1533–1535. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Kass, D.A. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc. Med. 2006, 16, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miranda, S.; Salazar-Enriquez, D.G.; Muñiz, J.; Ramirez-Archila, M.V.; Sanchez-Pastor, E.A.; Andrade, F.; Soñanez-Organis, J.G.; Moran-Palacio, E.F.; Virgen-Ortiz, A. Pregnancy differentially regulates the collagens types I and III in left ventricle from rat heart. Biomed. Res. Int. 2014, 2014, 984785. [Google Scholar] [CrossRef]
- Jiménez-Maldonado, A.; de Álvarez-Buylla, E.R.; Montero, S.; Melnikov, V.; Castro-Rodríguez, E.; Gamboa-Domínguez, A.; Rodríguez-Hernández, A.; Lemus, M.; Murguía, J.M. Chronic exercise increases plasma brain-derived neurotrophic factor levels, pancreatic islet size, and insulin tolerance in a TrkB-dependent manner. PLoS ONE 2014, 9, e115177. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Osorio, J.C.; Manlhiot, C.; Gruber, D.; Homma, S.; Mital, S. Hypertrophy signaling during peripartum cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3008–H3013. [Google Scholar] [CrossRef]
- Jankowski, M.; Wang, D.; Mukaddam-Daher, S.; Gutkowska, J. Pregnancy alters nitric oxide synthase and natriuretic peptide systems in the rat left ventricle. J. Endocrinol. 2005, 184, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.; Doggen, K.; De Keulenaer, G.W. Activation of the neuregulin/ErbB system during physiological ventricular remodeling inpregnancy. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H931–H942. [Google Scholar] [CrossRef]
- Iorga, A.; Dewey, S.; Partow-Navid, R.; Gomes, A.V.; Eghbali, M. Pregnancy is associated with decreased cardiac proteasome activity and oxidative stress inmice. PLoS ONE 2012, 7, e48601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Umar, S.; Amjedi, M.; Iorga, A.; Sharma, S.; Nadadur, R.D.; Regitz-Zagrosek, V.; Eghbali, M. New frontiers in heart hypertrophy during pregnancy. Am. J. Cardiovasc. Dis. 2012, 2, 192–207. [Google Scholar] [PubMed]
- Aljabri, M.B.; Songstad, N.T.; Lund, T.; Serrano, M.C.; Andreasen, T.V.; Al-Saad, S.; Lindal, S.; Sitras, V.; Acharya, G.; Ytrehus, K. Pregnancy protects against antiangiogenic and fibrogenic effects of angiotensin II in rat hearts. Acta Physiol. 2011, 201, 445–456. [Google Scholar] [CrossRef]
- Kuroda, K.; Kato, T.S.; Amano, A. Hypertensive cardiomyopathy: A clinical approach and literature review. World J. Hypertens. 2015, 5, 41–52. [Google Scholar] [CrossRef]
- Chung, E.; Leinwand, L.A. Pregnancy as a cardiac stress model. Cardiovasc. Res. 2014, 101, 561–570. [Google Scholar] [CrossRef]
- Chung, E.; Heimiller, J.; Leinwand, L.A. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS ONE 2012, 7, e42297. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgen-Ortiz, A.; Limón-Miranda, S.; Salazar-Enríquez, D.G.; Melnikov, V.; Sánchez-Pastor, E.A.; Castro-Rodríguez, E.M. Matrix Metalloproteinases System and Types of Fibrosis in Rat Heart during Late Pregnancy and Postpartum. Medicina 2019, 55, 199. https://doi.org/10.3390/medicina55050199
Virgen-Ortiz A, Limón-Miranda S, Salazar-Enríquez DG, Melnikov V, Sánchez-Pastor EA, Castro-Rodríguez EM. Matrix Metalloproteinases System and Types of Fibrosis in Rat Heart during Late Pregnancy and Postpartum. Medicina. 2019; 55(5):199. https://doi.org/10.3390/medicina55050199
Chicago/Turabian StyleVirgen-Ortiz, Adolfo, Saraí Limón-Miranda, Diana Guadalupe Salazar-Enríquez, Valery Melnikov, Enrique Alejandro Sánchez-Pastor, and Elena Margarita Castro-Rodríguez. 2019. "Matrix Metalloproteinases System and Types of Fibrosis in Rat Heart during Late Pregnancy and Postpartum" Medicina 55, no. 5: 199. https://doi.org/10.3390/medicina55050199
APA StyleVirgen-Ortiz, A., Limón-Miranda, S., Salazar-Enríquez, D. G., Melnikov, V., Sánchez-Pastor, E. A., & Castro-Rodríguez, E. M. (2019). Matrix Metalloproteinases System and Types of Fibrosis in Rat Heart during Late Pregnancy and Postpartum. Medicina, 55(5), 199. https://doi.org/10.3390/medicina55050199





