Expression of Sigma-Class Glutathione-S-Transferase in Fetal and Pediatric Filum Terminale Samples: A Comparative Study
Abstract
1. Introduction
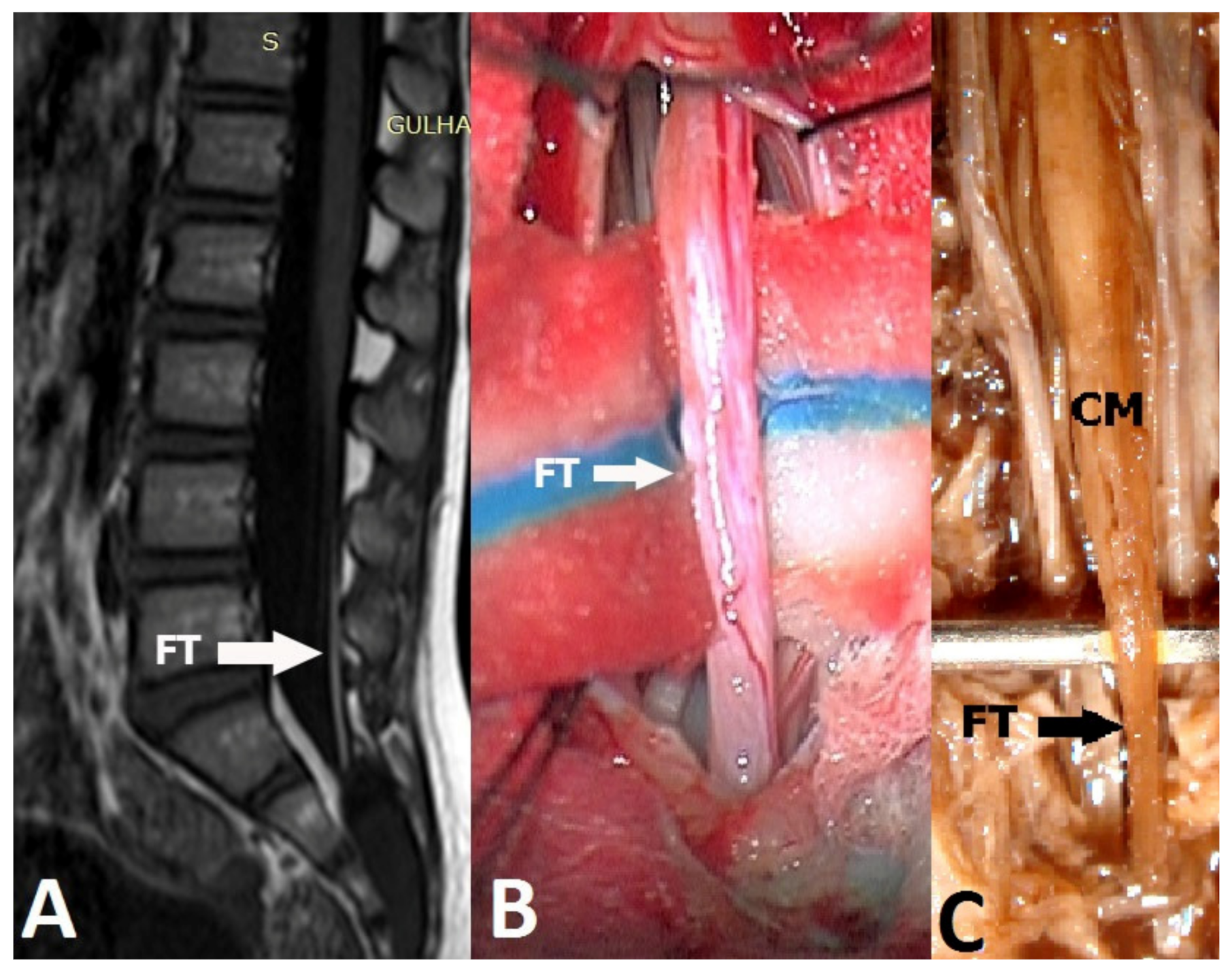
2. Materials and Methods
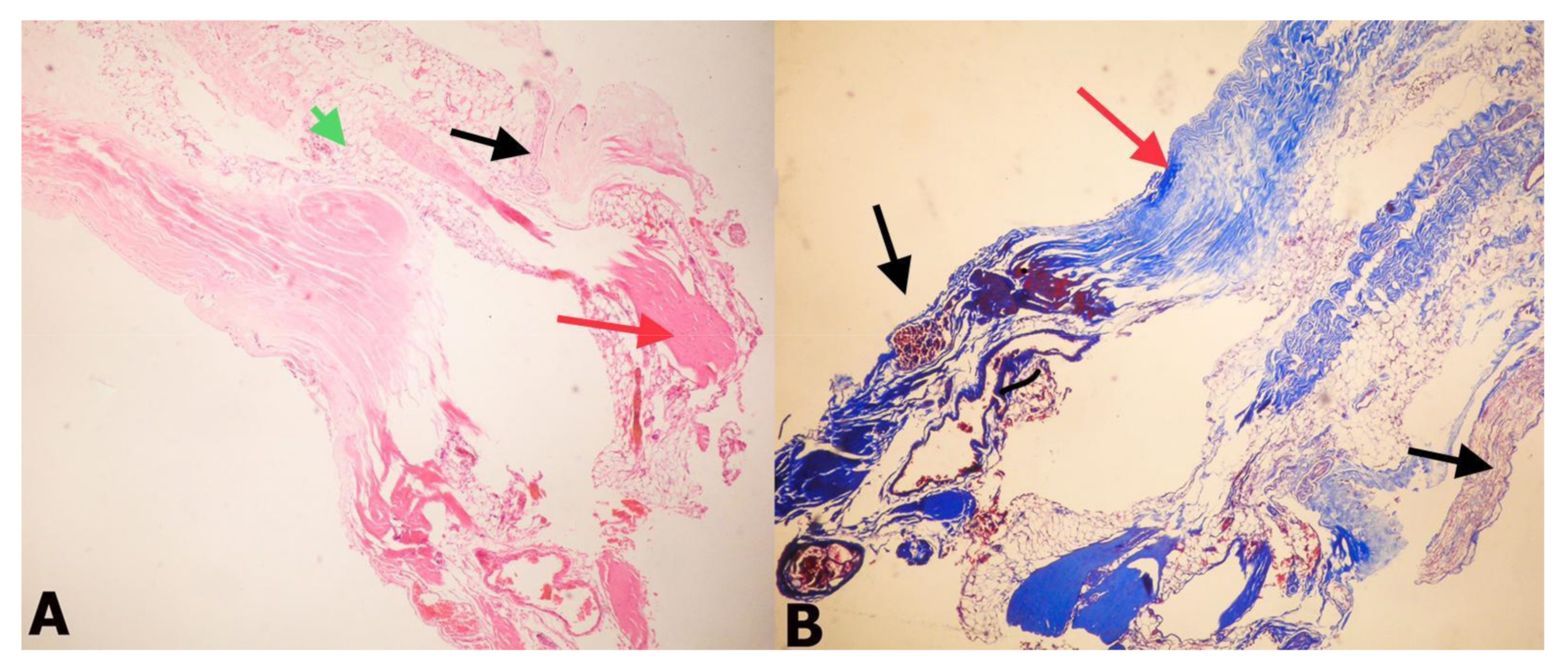
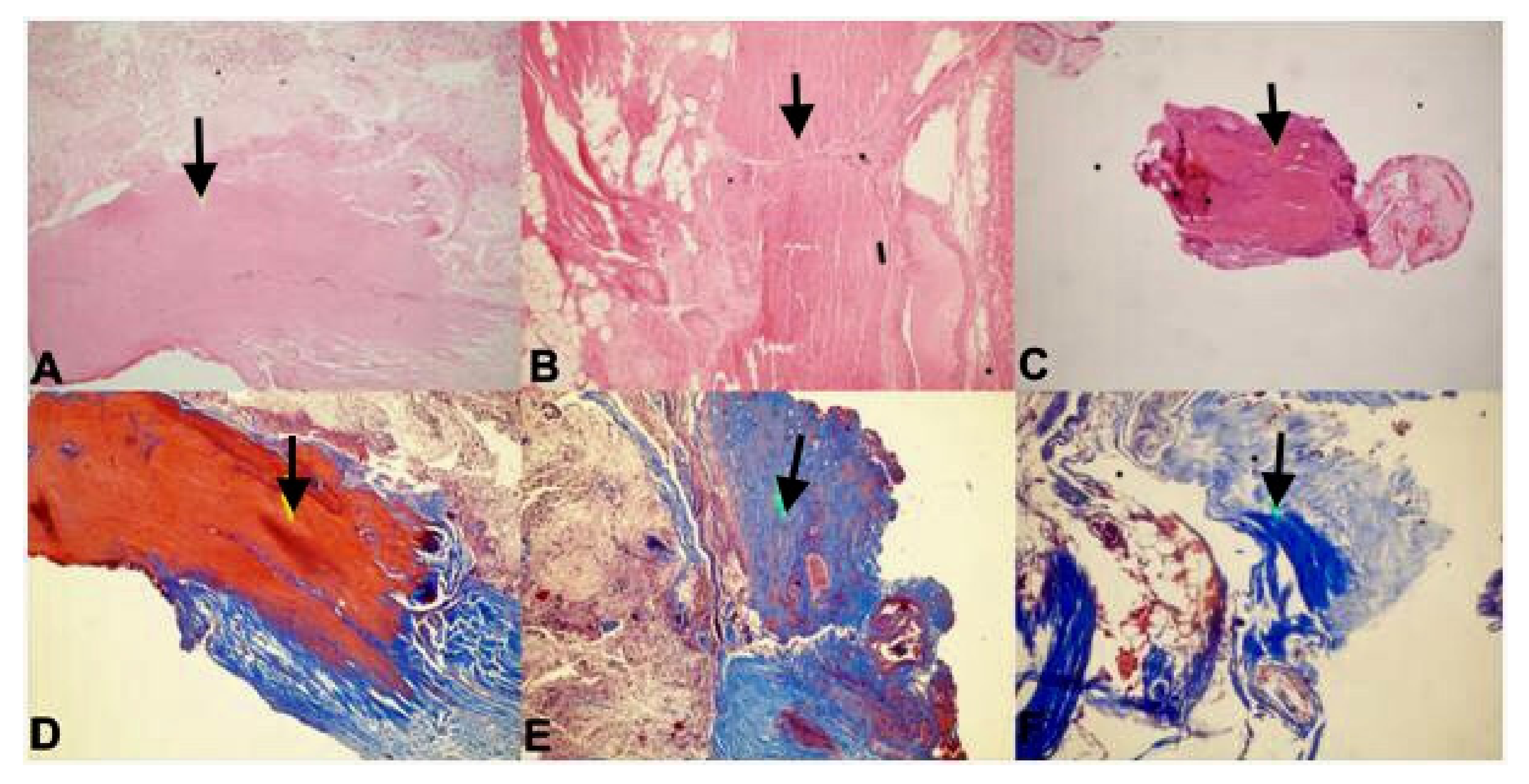
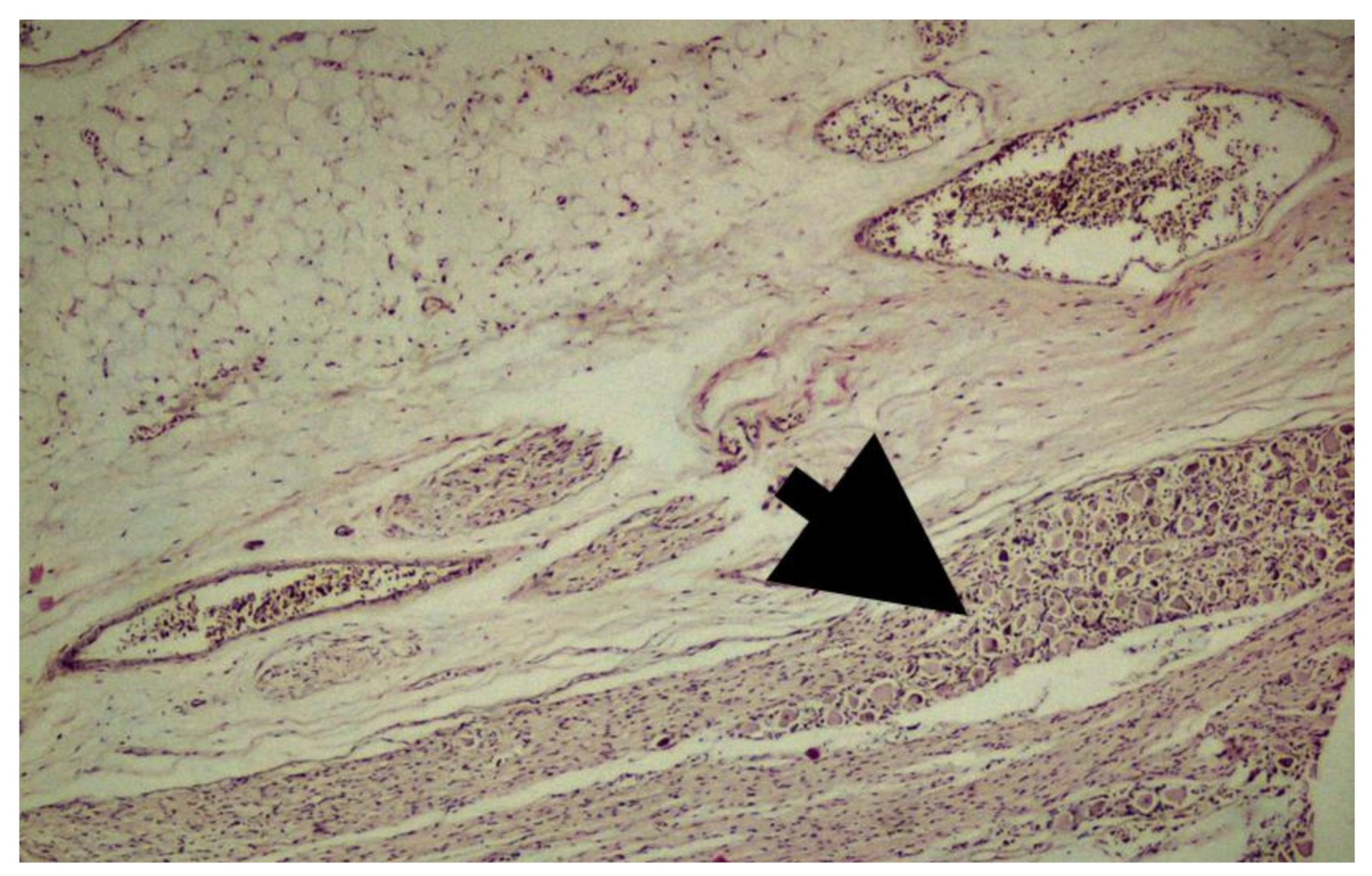
Immunohistochemical Staining
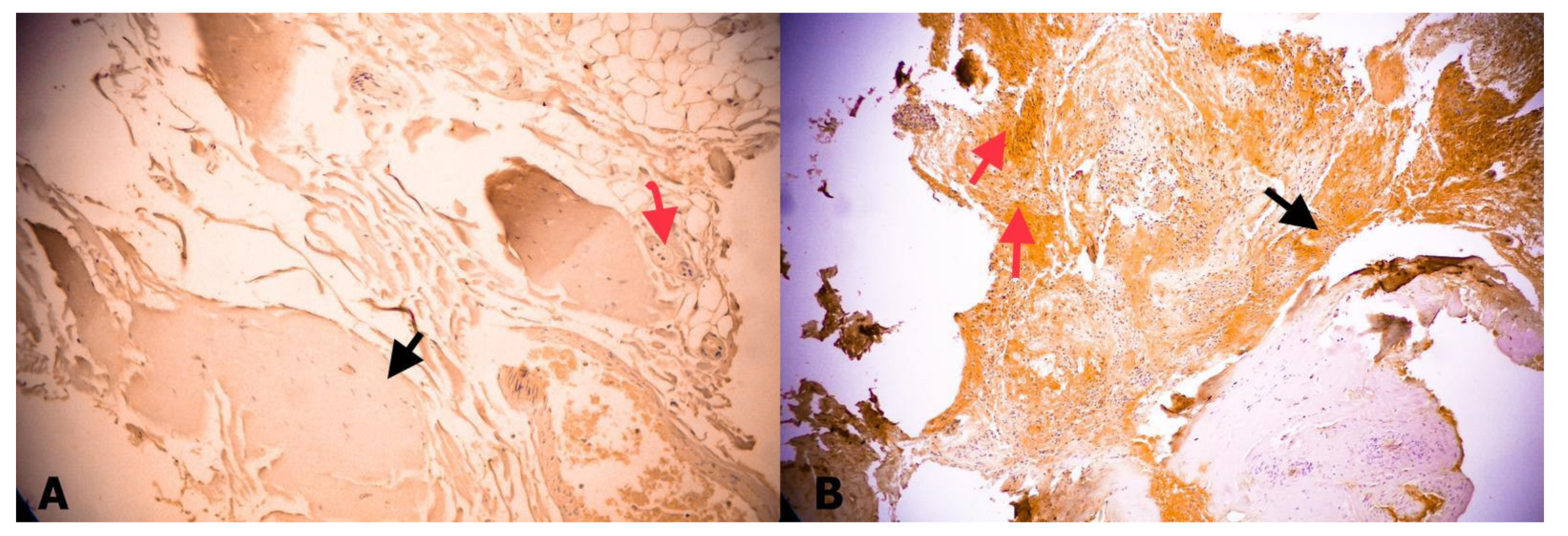
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fontes, R.B.; Saad, F.; Soares, M.S.; de Oliveira, F.; Pinto, F.C.; Liberti, E.A. Ultrastructural study of the filum terminale and its elastic fibers. Neurosurgery 2006, 58, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Geyik, M.; Alptekin, M.; Erkutlu, I.; Geyik, S.; Erbas, C.; Pusat, S.; Kural, C. Tethered cord syndrome in children: A single-center experience with 162 patients. Childs Nerv. Syst. 2015, 31, 1559–1563. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Hendrick, E.B.; Humphreys, R.P. The tethered spinal cord: Its protean manifestations, diagnosis and surgical correction. Childs Brain 1976, 2, 145–155. [Google Scholar] [CrossRef]
- Yamada, S.; Zinke, D.E.; Sanders, D.C. Pathophysiology of “tethered cord syndrome”. J. Neurosurg. 1981, 54, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Knerium, D.S.; Mandybur, G.M.; Schultz, R.L.; Yamada, B.S. Pathophysiology of tethered cord syndrome and other complex factors. Neurol. Res. 2004, 26, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.Y.; Hussein, A.M.; Zaghloul, S.A. Morphometric parameters and histological study of the filum terminale of adult human cadavers and MR images. Folia Morphol. (Warsz.) 2018, 77, 609–619. [Google Scholar] [CrossRef]
- Pinto, F.C.; Fontes, R.B.; Leonhardt Mde, C.; Amodio, D.T.; Porro, F.F.; Machado, J. Anatomic study of the filum terminale and its correlations with the tethered cord syndrome. Neurosurgery 2002, 51, 725–729. [Google Scholar] [CrossRef]
- Saker, E.; Henry, B.M.; Tomaszewski, K.A.; Loukas, M.; Iwanaga, J.; Oskouian, R.J.; Tubbs, R.S. The filum terminale internum and externum: A comprehensive review. J. Clin. Neurosci. 2017, 40, 6–13. [Google Scholar] [CrossRef]
- Solmaz, I.; Izci, Y.; Albayrak, B.; Cetinalp, E.; Kural, C.; Sengul, G.; Gocmez, C.; Pusat, S.; Tuzun, Y. Tethered cord syndrome in childhood: special emphasis on the surgical technique and review of the literature with our experience. Turk. Neurosurg. 2011, 21, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P.; MacNeily, A.E.; Hengel, A.R.; Afshar, K.; Landgraf, J.M.; Hader, W.; Pugh, J. Filum section for urinary incontinence in children with occult tethered cord syndrome: A randomized, controlled pilot study. J. Urol. 2016, 195, 1183–1188. [Google Scholar] [CrossRef]
- Yörükoğlu, A.G.; Tahta, A.; Akçakaya, M.O.; Sabancı, P.A.; Aras, Y.; Aydoseli, A.; Dolgun, M.; Sencer, A; Hepgül, K. Percutaneous fully endoscopic interlaminar approach to the filum terminale: A cadaveric study. World Neurosurg. 2016, 92, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Tehli, O.; Hodaj, I.; Kural, C.; Solmaz, I.; Onguru, O.; Izci, Y. A comparative study of histopathological analysis of filum terminale in patients with tethered cord syndrome and in normal human fetuses. Pediatr. Neurosurg. 2011, 47, 412–416. [Google Scholar] [CrossRef]
- De Vloo, P.; Monea, A.G.; Sciot, R.; van Loon, J.; Van Calenbergh, F. The filum terminale: A cadaver study of anatomy, histology, and elastic properties. World Neurosurg. 2016, 90, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kural, C.; Guresci, S.; Simsek, G.G.; Arslan, E.; Tehli, O.; Solmaz, I.; Izci, Y. Histological structure of filum terminale in human fetuses. J. Neurosurg. Pediatr. 2014, 13, 362–367. [Google Scholar] [CrossRef]
- Durdag, E.; Börcek, P.B.; Öcal, Ö.; Börcek, A.Ö.; Emmez, H.; Baykaner, M.K. Pathological evaluation of the filum terminale tissue after surgical excision. Childs Nerv. Syst. 2015, 31, 759–763. [Google Scholar] [CrossRef]
- Hendson, G.; Dunham, C.; Steinbok, P. Histopathology of the filum terminale in children with and without tethered cord syndrome with attention to the elastic tissue within the filum. Childs Nerv. Syst. 2016, 32, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Mannervik, B.; Board, P.G.; Hayes, J.D.; Listowsky, I.; Pearson, W.R. Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol. 2005, 401, 1–8. [Google Scholar] [CrossRef]
- Sherratt, P.J.; Hayes, J.D. Glutathione S-transferases. In Enzyme Systems that Metabolise Drugs and Other Xenobiotics; Ioannides, C., Ed.; John Wiley and Sons Ltd.: West Sussex, UK, 2001; pp. 319–352. [Google Scholar]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D. Theroleof glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef]
- Flanagan, J.U.; Smythe, M.L. Sigma-class glutathione transferases. Drug Metab. Rev. 2011, 43, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, J.; Janphen, K.; Saisawang, C.; Ketterman, A.J. Interaction of Omega, Sigma, and Theta glutathione transferases with p38b mitogen-activated protein kinase from the fruit fly, Drosophila melanogaster. J. Insect Sci. 2014, 14, 60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, J.A.; el Barbary, A.; Kornguth, S.E.; Brugge, J.F.; Siegel, F.L. Glutathione S-transferase isoenzymes in rat brain neurons and glia. J. Neurosci. 1993, 13, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Jowsey, I.R.; Thomson, A.M.; Flanagan, J.U.; Murdock, P.R.; Moore, G.B.; Meyer, D.J.; Murphy, G.J.; Smith, S.A.; Hayes, J.D. Mammalian class Sigma glutathione S-transferases: Catalytic properties and tissue-specific expression of human and rat GSH-dependent prostaglandin D2 synthases. Biochem. J. 2001, 359, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, Y.; Takamori, R.; Koshihara, Y. Prostaglandin D2 metabolite stimulates collagen synthesis by human osteoblasts during calcification. Prostaglandins 1991, 41, 303–313. [Google Scholar] [CrossRef]
- Ayabe, S.; Kida, T.; Hori, M.; Ozaki, H.; Murata, T. Prostaglandin D2 inhibits collagen secretion from lung fibroblasts by activating the DP receptor. J. Pharmacol. Sci. 2013, 121, 312–317. [Google Scholar] [CrossRef]
- Patel, M.; Vetter, M.; Simonds, E.; Schumacher, M.; Laws, T.; Iwanaga, J.; Oskouian, R.; Tubbs, R.S. Mechanical relationship of filum terminaleexternum and filum terminaleinternum: Is it possible to detether the spinal cord extradurally? Childs Nerv. Syst. 2018, 34, 1767–1770. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef]
- Listowsky, I.; Abramovitz, M.; Homma, H.; Niitsu, Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab. Rev. 1988, 19, 305–318. [Google Scholar] [CrossRef]
- Doré, S.; Takahashi, M.; Ferris, C.D.; Zakhary, R.; Hester, L.D.; Guastella, D.; Snyder, S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 1999, 96, 2445–2450. [Google Scholar] [CrossRef]
- Da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Vasconcelos, V.; Antunes, A. Molecular evolution and the role of oxidative stress in the expansion and functional diversification of cytosolic glutathione transferases. BMC Evol. Biol. 2010, 10, 281. [Google Scholar] [CrossRef]
- Herlong, J.L.; Scott, T.R. Positioning prostanoids of the D and J series in the immunopathogenic scheme. Immunol. Lett. 2006, 102, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Urade, Y.; Ujihara, M.; Horiguchi, Y.; Igarashi, M.; Nagata, A.; Ikai, K.; Hayaishi, O. Mast cells contain spleen-type prostaglandin D synthetase. J. Biol. Chem. 1990, 265, 371–375. [Google Scholar] [PubMed]
- McMillan, D.H.; van der Velden, J.L.; Lahue, K.G.; Qian, X.; Schneider, R.W.; Iberg, M.S; Nolin, J.D.; Abdalla, S.; Casey, D.T.; Tew, K.D.; et al. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase π. JCI Insight 2016, 1, e85717. [Google Scholar] [CrossRef] [PubMed]
| Patient No | Age | Sex | Complaints | Associated Pathology | Level of Conus Medullaris | Structure of Filum Terminale | Fibrosis | GST Expression |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 years | F | Backache, walking disturbance | Fatty filum terminale | L5 | Thick and fatty | + | + |
| 2 | 3 years | F | Unable to walk | Type 1 SCM+Syringomyelia | L4 | Normal | + | − |
| 3 | 16 years | F | Backache | Syringomyelia | L4 | Fatty | + | ++ |
| 4 | 16 years | M | Back pain, left leg pain | None | L4 | Normal | ++ | − |
| 5 | 7 months | F | Hypertrichosis, scoliosis | Type 1 SCM | L4 | Thick and fatty | + | + |
| 6 | 6 years | F | Walking disturbance | Previously operated for myelomeningocele | L4 | Normal | +++ | ++ |
| 7 | 2 years | F | Walking disturbance | Syringomyelia | L3 | Normal | + | ++ |
| 8 | 5 years | F | Urinary incontinence | None | L2 | Normal | + | + |
| 9 | 11 months | F | Walking disturbance | Type 1 SCM | L4 | Normal | + | + |
| 10 | 6 months | M | Motor weakness in left leg | Type 1 SCM | L4 | Normal | ++ | + |
| Fetus No | Fibrosis | GST-Expression |
|---|---|---|
| 1 | − | ++ |
| 2 | − | + |
| 3 | − | + |
| 4 | ++ | ++ |
| 5 | − | ++ |
| 6 | +++ | − |
| 7 | + | − |
| 8 | + | − |
| 9 | ++ | + |
| 10 | + | − |
| 11 | + | − |
| 12 | − | + |
| 13 | − | + |
| 14 | − | + |
| 15 | + | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kural, C.; Oguztuzun, S.; Şimşek, G.G.; Guresci, S.; Kaygın, P.; Yasar, S.; Tehli, O.; Izci, Y. Expression of Sigma-Class Glutathione-S-Transferase in Fetal and Pediatric Filum Terminale Samples: A Comparative Study. Medicina 2019, 55, 133. https://doi.org/10.3390/medicina55050133
Kural C, Oguztuzun S, Şimşek GG, Guresci S, Kaygın P, Yasar S, Tehli O, Izci Y. Expression of Sigma-Class Glutathione-S-Transferase in Fetal and Pediatric Filum Terminale Samples: A Comparative Study. Medicina. 2019; 55(5):133. https://doi.org/10.3390/medicina55050133
Chicago/Turabian StyleKural, Cahit, Serpil Oguztuzun, Gülçin Güler Şimşek, Servet Guresci, Pınar Kaygın, Soner Yasar, Ozkan Tehli, and Yusuf Izci. 2019. "Expression of Sigma-Class Glutathione-S-Transferase in Fetal and Pediatric Filum Terminale Samples: A Comparative Study" Medicina 55, no. 5: 133. https://doi.org/10.3390/medicina55050133
APA StyleKural, C., Oguztuzun, S., Şimşek, G. G., Guresci, S., Kaygın, P., Yasar, S., Tehli, O., & Izci, Y. (2019). Expression of Sigma-Class Glutathione-S-Transferase in Fetal and Pediatric Filum Terminale Samples: A Comparative Study. Medicina, 55(5), 133. https://doi.org/10.3390/medicina55050133





