Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessment
2.3. Statistical Analysis
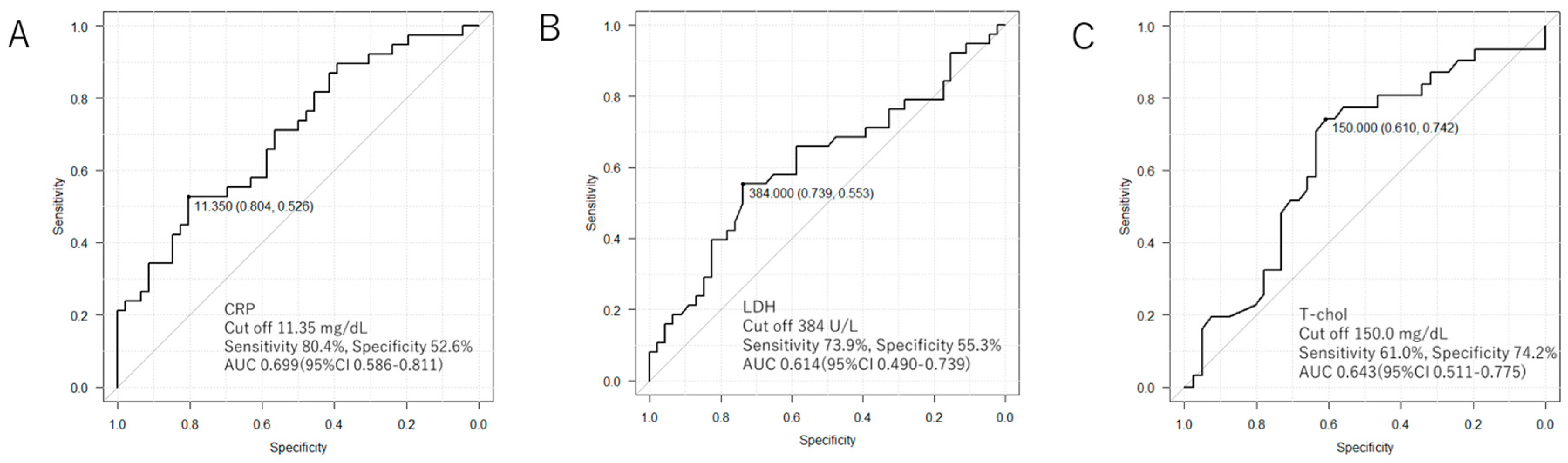
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, D.S. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur. Respir. J. 2011, 37, 356–363. [Google Scholar] [CrossRef]
- Kishaba, T.; Tamaki, H.; Shimaoka, Y.; Fukuyama, H.; Yamashiro, S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014, 192, 141–149. [Google Scholar] [CrossRef]
- Isshiki, T.; Sakamoto, S.; Kinoshita, A.; Sugino, K.; Kurosaki, A.; Homma, S. Recombinant Human Soluble Thrombomodulin Treatment for Acute Exacerbation of Idiopathic Pulmonary Fibrosis: A Retrospective Study. Respiration 2015, 89, 201–207. [Google Scholar] [CrossRef]
- Bi, Y.; Rekić, D.; Wang, Y.; Karimi-Shah, B.A.; Chowdhury, B.A.; O Paterniti, M. Acute Exacerbation and Decline in Forced Vital Capacity Are Associated with Increased Mortality in Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2017, 14, 1395–1402. [Google Scholar]
- Fujimoto, K.; Taniguchi, H.; Johkoh, T.; Kondoh, Y.; Ichikado, K.; Sumikawa, H.; Ogura, T.; Kataoka, K.; Endo, T.; Kawaguchi, A.; et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur. Radiol. 2012, 22, 83–92. [Google Scholar] [CrossRef]
- Sakamoto, S.; Shimizu, H.; Isshiki, T.; Sugino, K.; Kurosaki, A.; Homma, S. Recombinant human soluble thrombomodulin for acute exacerbation of idiopathic pulmonary fibrosis: A historically controlled study. Respir. Investig. 2018, 56, 136–143. [Google Scholar] [CrossRef]
- Zubairi, A.B.S.; Ahmad, H.; Hassan, M.; Sarwar, S.; Abbas, A.; Shahzad, T.; Irfan, M. Clinical characteristics and factors associated with mortality in idiopathic pulmonary fibrosis: An experience from a tertiary care center in Pakistan. Clin. Respir. J. 2018, 12, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef]
- Agarwal, R.; Jindal, S.K. Acute exacerbation of idiopathic pulmonary fibrosis: A systematic review. Eur. J. Intern. Med. 2008, 19, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, O.; Shimizu, M.; Ito, Y.; Kume, H.; Suzuki, R.; Yokoi, T.; Yamaki, K. Effect of prolonged low-dose methylprednisolone therapy in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Care 2001, 46, 698–701. [Google Scholar] [PubMed]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic Survey of Japanese Patients with Idiopathic Pulmonary Fibrosis and Investigation of Ethnic Differences. Am. J. Respir. Crit. Care Med. 2014, 190, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Perez, E.R.; Daniels, C.E.; Schroeder, D.R.; St Sauver, J.; Hartman, T.E.; Bartholmai, B.J.; Yi, E.S.; Ryu, J.H. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010, 137, 129–137. [Google Scholar] [CrossRef]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Taniguchi, H.; Kondoh, Y.; Nishiyama, O.; Kimura, T.; Matsuda, T.; Yokoyama, T.; Sakamoto, K.; Ando, M. Recombinant Human Thrombomodulin in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Chest 2015, 148, 436–443. [Google Scholar] [CrossRef]
- Kishaba, T.; Nei, Y.; Momose, M.; Nagano, H.; Yamashiro, S. Clinical Characteristics Based on the New Criteria of Acute Exacerbation in Patients with Idiopathic Pulmonary Fibrosis. Eurasian J. Med. 2018, 50, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Taniguchi, H.; Ebina, M.; Azuma, A.; Ogura, T.; Taguchi, Y.; Suga, M.; Takahashi, H.; Nakata, K.; Sugiyama, Y.; et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis – Extended analysis of pirfenidone trial in Japan. Respir. Investig. 2015, 53, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, Y.; Ye, Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. New Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Sakamoto, S.; Shimizu, H.; Sekiya, M.; Kinoshita, A.; Isshiki, T.; Sugino, K.; Matsumoto, K.; Homma, S. Pirfenidone for acute exacerbation of idiopathic pulmonary fibrosis: A retrospective study. Respir. Med. 2017, 126, 93–99. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821–829. [Google Scholar] [CrossRef]
- Liu, X.; Mayes, M.D.; Pedroza, C.; Draeger, H.T.; Gonzalez, E.B.; Harper, B.E.; Reveille, J.D.; Assassi, S. Does C-Reactive Protein Predict the Long-Term Progression of Interstitial Lung Disease and Survival in Patients With Early Systemic Sclerosis? Arthritis Care Res. 2013, 65, 1375–1380. [Google Scholar] [CrossRef]
- Matsumura, T.; Tsushima, K.; Abe, M.; Suzuki, K.; Yamagishi, K.; Matsumura, A.; Ichimura, Y.; Ikari, J.; Terada, J.; Tatsumi, K. The effects of pirfenidone in patients with an acute exacerbation of interstitial pneumonia. Clin. Respir. J. 2018, 12, 1550–1558. [Google Scholar] [CrossRef]
- Ishikawa, G.; Acquah, S.O.; Salvatore, M.; Padilla, M.L. Elevated serum D-dimer level is associated with an increased risk of acute exacerbation in interstitial lung disease. Respir. Med. 2017, 128, 78–84. [Google Scholar] [CrossRef]
- Tanaseanu, C.M.; Tiglea, I.A.; Marta, D.S.; Dumitrascu, A.L.; Popescu, M.; Tanaseanu, S.; Moldoveanu, E. Lactate dehydrogenase a possible marker of progressive microvasculopathy and interstitial lung disease in systemic sclerosis. Acta Med. Mediterr. 2015, 31, 941–946. [Google Scholar]
- Palmier, J.; Lanzrath, B.J. Laboratory and biometric predictors of cancer-related mortality in an insured population. J. Insur. Med. 2012, 43, 162–168. [Google Scholar]
- Chiang, C.-K.; Ho, T.-I.; Hsu, S.-P.; Peng, Y.-S.; Pai, M.-F.; Yang, S.-Y.; Hung, K.-Y.; Tsai, T.-J. Low-Density Lipoprotein Cholesterol: Association with Mortality and Hospitalization in Hemodialysis Patients. Blood Purif. 2005, 23, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Birrell, M.A.; Catley, M.C.; Hardaker, E.; Wong, S.; Willson, T.M.; McCluskie, K.; Leonard, T.; Farrow, S.N.; Collins, J.L.; Haj-Yahia, S.; et al. Novel Role for the Liver X Nuclear Receptor in the Suppression of Lung Inflammatory Responses. J. Boil. Chem. 2007, 282, 31882–31890. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Christ-Crain, M.; Stolz, D.; Keller, U.; Müller, C.; Bingisser, R.; Tamm, M.; Mueller, B.; Schuetz, P. Prognostic impact of plasma lipids in patients with lower respiratory tract infections—An observational study. Swiss Med. Wkly. 2009, 139, 166–172. [Google Scholar] [PubMed]
- Alvarez, C.; Ramos, A. Lipids, lipoproteins, and apoproteins in serum during infection. Clin. Chem. 1986, 32, 142–145. [Google Scholar]
- Mooser, V.; Berger, M.M.; Tappy, L.; Cayeux, C.; Marcovina, S.M.; Darioli, R.; Nicod, P.; Chioléro, R. Major Reduction in Plasma Lp(a) Levels During Sepsis and Burns. Arter. Thromb. Vasc. Boil. 2000, 20, 1137–1142. [Google Scholar] [CrossRef]
- Fruchter, O.; Yigla, M.; Kramer, M.R. Lipid Profile and Statin Use: The Paradox of Survival After Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Am. J. Med Sci. 2015, 349, 338–343. [Google Scholar] [CrossRef]
- Chien, Y.-F.; Chen, C.-Y.; Hsu, C.-L.; Chen, K.-Y.; Yu, C.-J. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J. Crit. Care 2015, 30, 506–510. [Google Scholar] [CrossRef] [PubMed]
| Background | n = 84 |
|---|---|
| Age | 78 (56, 95) |
| Male | 59 (70.2) |
| BMI (kg/m2) | 20.95 (13.06, 33.64) |
| BSA (m2) | 1.53 (1.1, 2.09) |
| Diagnosed IPF before AE | 50 (59.5) |
| Treatment to IPF | 17 (34) |
| Anti-fibrotic agent | 4 (8) |
| Corticosteroid | 13 (26) |
| Not treatment | 33 (66) |
| Smoking | 65 (77.4) |
| Alcohol | 30 (35.7) |
| Home oxygen therapy | 19 (22.6) |
| Medical history | |
| Diabetes mellitus | 23 (27.4) |
| Dyslipidemia | 15 (17.9) |
| Cerebral infarction | 11 (13.1) |
| Myocardial infarction | 4 (4.8) |
| Bronchial asthma | 4 (4.8) |
| Malignant tumor | 24 (28.6) |
| Serological marker | |
| WBC (/μL) | 10,900 (3500, 31,000) |
| Total protein (g/dL) | 6.65 (5.2, 9.4) |
| Albumin (g/dL) | 2.9 (1.7, 4.1) |
| LDH (U/L) | 337.5 (150, 906) |
| Calcium (mg/dL) | 9.4 (8.3, 10.3) |
| CRP (mg/dL) | 8.47 (0.1, 27.73) |
| D-dimer (μg/mL) | 4.4 (0.8, 49) |
| Blood sugar (mg/dL) | 131 (79, 477) |
| Hemoglobin A1c (%) | 5.8 (4.8, 9.9) |
| Total cholesterol (mg/dL) | 146.5 (66, 248) |
| KL-6 (U/mL) | 1032.5 (165, 5362) |
| SP-A (ng/mL) | 100.8 (27, 270.7) |
| SP-D (ng/mL) | 363 (118, 1760) |
| BGA in artery | |
| pH | 7.46 (7.03, 7.56) |
| PaCO2 (mmHg) | 36.2 (17.1, 65.5) |
| PaO2 (mmHg) | 64.5 (32.2, 188.1) |
| Base excess (mmol/L) | 1.8 (−15.50, 12.6) |
| Lactate (mmol/L) | 1.63 (0.51, 9.67) |
| AaDO2 | 130.34 (15.88, 621.42) |
| PaO2/FiO2 | 186.00 (45.15, 406.67) |
| Treatment in hospital | |
| Treatment in ICU | 3 (3.6) |
| Mechanical ventilation | 4 (4.8) |
| NPPV | 11 (13.1) |
| Nasal high flow | 28 (33.3) |
| ECMO | 0 (0) |
| Steroid pulse therapy | 50 (59.5) |
| Cyclophosphamide pulse therapy | 10 (11.9) |
| Sivelestat | 8 (9.5) |
| Recombinant thrombomodulin | 3 (3.6) |
| Anti-fibrotic agent | 4 (4.8) |
| Length of hospital day | 25 (2, 113) |
| Death during hospitalization | 38 (45.2) |
| Factor | Survival (n = 46) | Death (n = 38) | p Value |
|---|---|---|---|
| Background factor | |||
| Age | 80 (61, 91) | 77 (56, 95) | 0.316 |
| Male | 31 (67.4) | 28 (73.7) | 0.634 |
| BMI (kg/m2) | 20.45 (13.06, 33.64) | 21.94 (13.25, 30.87) | 0.128 |
| BSA (m2) | 1.52 (1.1, 1.94) | 1.55 (1.22, 2.09) | 0.267 |
| Diagnosis before AE | 30 (65.2) | 20 (52.6) | 0.271 |
| Smoking | 35 (76.1) | 30 (78.9) | 0.799 |
| Alcohol | 18 (39.1) | 12 (31.6) | 0.502 |
| Home oxygen therapy | 14 (30.4) | 5 (13.2) | 0.071 |
| Medical history | |||
| Diabetes mellitus | 14 (30.4) | 9 (23.7) | 0.624 |
| Dyslipidemia | 8 (17.4) | 7 (18.4) | 1 |
| Myocardial infarction | 2 (4.3) | 2 (5.3) | 1 |
| Cerebral infarction | 6 (13) | 5 (13.2) | 1 |
| Bronchial asthma | 3 (6.5) | 1 (2.6) | 0.623 |
| Malignant tumor | 15 (32.6) | 9 (23.7) | 0.468 |
| Treatment in hospital | |||
| Treatment in ICU | 1 (2.2) | 2 (5.3) | 0.587 |
| Mechanical ventilation | 1 (2.2) | 3 (7.9) | 0.324 |
| NPPV | 3 (6.5) | 8 (21.1) | 0.059 |
| Nasal high flow | 12 (26.1) | 16 (42.1) | 0.164 |
| Steroid pulse therapy | 24 (52.2) | 26 (68.4) | 0.181 |
| Cyclophosphamide pulse therapy | 3 (6.5) | 7 (18.4) | 0.174 |
| Sivelestat | 1 (2.2) | 7 (18.4) | 0.02 * |
| Recombinant thrombomodulin | 2 (4.3) | 1 (2.6) | 1 |
| Anti-fibrotic agent | 2 (4.3) | 2 (5.3) | 1 |
| Serological marker | |||
| WBC(/μL) | 10,300 (5800, 22,700) | 11,150 (3500, 31,000) | 0.406 |
| Total protein (g/dL) | 6.80 (5.5, 9.4) | 6.50 (5.2, 8.4) | 0.031 * |
| Albumin (g/dL) | 2.95 (2, 4.1) | 2.75 (1.7, 3.5) | 0.047 * |
| LDH (U/L) | 315.5 (150, 619) | 386.5 (157, 906) | 0.073 |
| Calcium (mg/dL) | 9.3 (8.3, 10) | 9.50 (8.7, 10.3) | 0.429 |
| CRP (mg/dL) | 6.69 (0.1, 21.84) | 1.57 (0.77, 27.73) | 0.002 ** |
| D-dimer (μg/mL) | 3.3 (0.8, 34.2) | 5.35 (0.9, 49) | 0.152 |
| Blood sugar (mg/dL) | 133 (79, 330) | 130.0 (82, 477) | 0.728 |
| Hemoglobin A1c (%) | 5.9 (4.8, 7.8) | 5.80 (5.4, 9.9) | 0.848 |
| Total cholesterol (mg/dL) | 159 (66, 222) | 133.0 (88, 248) | 0.039 * |
| KL-6 (U/mL) | 1027 (248, 3936) | 1085.0 (165, 5362) | 0.448 |
| SP-A (ng/mL) | 91.2 (27, 270.7) | 124.2 (30.4, 251) | 0.067 |
| SP-D (ng/mL) | 354 (128, 1760) | 378.0 (118, 1060) | 0.872 |
| BGA in artery | |||
| pH | 7.45 (7.23, 7.56) | 7.46 (7.03, 7.54) | 0.389 |
| PaCO2 (mmHg) | 36.4 (23.2, 57.8) | 35.3 (17.1, 65.5) | 0.909 |
| PaO2 (mmHg) | 61.3 (32.2, 188.1) | 6.25 (34.2, 91) | 0.983 |
| Base excess (mmol/L) | 1.8 (−7.1, 12.6) | 1.75 (−15.5, 10.8) | 0.628 |
| Lactate (mmol/L) | 1.74 (0.83, 7.15) | 1.6 (0.51, 9.67) | 0.754 |
| AaDO2 | 117.88 (16.2, 621.42) | 207.47 (15.88, 617.85) | 0.229 |
| PaO2/FiO2 | 191.25 (45.15, 406.67) | 146.58 (45.25, 377.14) | 0.17 |
| Factor | Survival (n = 14) | Death (n = 6) | p Value |
|---|---|---|---|
| Pulmonary function test | |||
| VC(L) | 1.99 (1.21, 3.94) | 2.74 (1.67, 3.17) | 0.232 |
| FVC(L) | 2.07 (1.26, 3.79) | 2.40 (1.85, 3.14) | 0.481 |
| FEV1.0(L) | 1.76 (1.20, 2.65) | 1.86 (1.26, 2.78) | 0.244 |
| DLCO | 8.82 (4.03, 9.72) | 8.32 (7.81, 8.82) | 0.769 |
| HR (95% CI) | p Value | |
|---|---|---|
| CRP | 1.080 (1.022–1.141) | 0.006 ** |
| LDH | 1.003 (1.000–1.006) | 0.037 * |
| T-chol | 0.985 (0.972–0.997) | 0.018 * |
| CRP ≥ 11.35 mg/dL | CRP < 11.35 mg/dL | |
|---|---|---|
| T-chol <150 mg/dL | 17/22 (77.3%) | 6/17 (35.3%) |
| T-chol ≥150 mg/dL | 1/5 (20.0%) | 7/28 (25.0%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachisu, Y.; Murata, K.; Takei, K.; Tsuchiya, T.; Tsurumaki, H.; Koga, Y.; Horie, T.; Takise, A.; Hisada, T. Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 132. https://doi.org/10.3390/medicina55050132
Hachisu Y, Murata K, Takei K, Tsuchiya T, Tsurumaki H, Koga Y, Horie T, Takise A, Hisada T. Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Medicina. 2019; 55(5):132. https://doi.org/10.3390/medicina55050132
Chicago/Turabian StyleHachisu, Yoshimasa, Keisuke Murata, Kousuke Takei, Takuma Tsuchiya, Hiroaki Tsurumaki, Yasuhiko Koga, Takeo Horie, Atsushi Takise, and Takeshi Hisada. 2019. "Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis" Medicina 55, no. 5: 132. https://doi.org/10.3390/medicina55050132
APA StyleHachisu, Y., Murata, K., Takei, K., Tsuchiya, T., Tsurumaki, H., Koga, Y., Horie, T., Takise, A., & Hisada, T. (2019). Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Medicina, 55(5), 132. https://doi.org/10.3390/medicina55050132






