Effects of Sting Plant Extracts as Penetration Enhancers on Transdermal Delivery of Hypoglycemic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Preparation of Sting Crude Extracts
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Animal Source and Care
2.6. Diet-Induced Obesity (DIO) Model
2.7. Insulin-Glucose Tolerance Test (IGT)
2.8. Oral Glucose Tolerance Test (OGTT)
2.9. Skin Irritation Test
2.10. Histological Analysis of Skin Sections
2.11. Statistical Analysis
3. Results
3.1. Effect of Sting Crude Extracts Dm, Ut, and Ao on In Vivo Skin Irritation
3.2. Effect of Sting Crude Extracts Dm, Ut, and Ao on Mouse Skin Epidermis
3.3. Effect of Sting Crude Extracts Dm, Ut, and Ao on Human Skin Keratinocytes
3.4. Effect of Sting Crude Extracts Dm, Ut, and Ao on In Vivo Skin Permeation of Insulin and Insulin Sensitizers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef]
- Langer, R. Transdermal drug delivery: Past progress, current status, and future prospects. Adv. Drug Deliv. Rev. 2004, 56, 557–558. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Nat. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Topical pharmacology and toxicology of dimethyl sulfoxide—Part 1. JAMA 1965, 193, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Gildersleeve, N.; Bodor, N. The effect of vehicle additives on the transdermal delivery of nitroglycerin. Pharm. Res. 1987, 4, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ryu, G.R.; Ko, S.H.; Ahn, Y.B.; Song, K.H. A role of pancreatic stellate cells in islet fibrosis and beta-cell dysfunction in type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2017, 485, 328–334. [Google Scholar] [CrossRef]
- Hong, S.; Pedersen, P.L. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. MMBR 2008, 72, 590–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jang, I.S.; Yang, K.E.; Yoon, S.J.; Baek, S.; Lee, J.Y.; Suzuki, T.; Chung, K.Y.; Woo, S.H.; Choi, J.S. Effect of rutin from tartary buckwheat sprout on serum glucose-lowering in animal model of type 2 diabetes. Acta Pharm. 2016, 66, 297–302. [Google Scholar] [CrossRef]
- Tachibana, K. Transdermal delivery of insulin to alloxan-diabetic rabbits by ultrasound exposure. Pharm. Res. 1992, 9, 952–954. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Chen, S.-J.; Kuo-Huang, L.-L. Comparative study on the stinging trichomes and some related epidermal structures in the leaves of Dendrocnide meyeniana, Girardinia diversifolia, and Urtica thunbergiana. Taiwania 2003, 48, 213–223. [Google Scholar]
- Oliver, F.; Amon, E.; Breathnach, A.; Francis, D.; Sarathchandra, P.; Kobza Black, A.; Greaves, M. Contact urticaria due to the common stinging nettle (Urtica dioica)—histological, ultrastructural and pharmacological studies. Clin. Exp. Dermatol. 1991, 16, 1–7. [Google Scholar] [CrossRef]
- Taskila, K.; Saarinen, J.V.; Harvima, I.T.; Harvima, R.J. Histamine and LTC4 in stinging nettle-induced urticaria. Allergy 2000, 55, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Y.; Chen, S.J.; Chen, R.F.; Ding, W.H.; Kuo-Huang, L.L.; Huang, R.N. Identification of oxalic acid and tartaric acid as major persistent pain-inducing toxins in the stinging hairs of the nettle, Urtica thunbergiana. Ann. Bot. 2006, 98, 57–65. [Google Scholar] [CrossRef]
- Viet, L.; Houghton, P.; Forbes, B.; Corcoran, O.; Hylands, P. Wound healing activity of Alocasia odora (Roxb.) Koch. Planta Med. 2006, 72, P_024. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Liao, M.-D.; Zhang, J.-L.; Xu, H.-H. Tested on antifungal activity of Alocasia odora methanol extract. Agrochemicals 2006, 45, 744. [Google Scholar]
- Moon, J.M.; Lee, B.K.; Chun, B.J. Toxicities of raw Alocasia odora. Hum. Exp. Toxicol. 2011, 30, 1720–1723. [Google Scholar] [CrossRef]
- Chan, T.; Chan, L.; Tam, L.; Critchley, J. Neurotoxicity following the ingestion of a Chinese medicinal plant, Alocasia macrorrhiza. Hum. Exp. Toxicol. 1995, 14, 727–728. [Google Scholar] [CrossRef]
- Jorge, L.L.; Feres, C.C.; Teles, V.E. Topical preparations for pain relief: Efficacy and patient adherence. J. Pain Res. 2010, 4, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Groninger, H.; Schisler, R.E. Topical capsaicin for neuropathic pain #255. J. Palliat. Med. 2012, 15, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.A.; Wooldridge, A.A.; Stewart, A.J.; Behrend, E.N.; Kemppainen, R.J.; Zhong, Q.; Johnson, A.K. Seasonal changes in the combined glucose-insulin tolerance test in normal aged horses. J. Vet. Intern. Med. 2012, 26, 1035–1041. [Google Scholar] [CrossRef]
- Derelanko, M.J. Toxicologist’s Pocket Handbook; CRC: Boca Raton, FL, USA, 2000; p. 231. [Google Scholar]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef]
- Hadgraft, J. Modulation of the barrier function of the skin. Skin Pharmacol. Appl. Skin Physiol. 2001, 1 (Suppl. 14), 72–81. [Google Scholar] [CrossRef]
- Chang, Y.T.; Shen, J.J.; Wong, W.R.; Yen, H.R. Alternative therapy for autosensitization dermatitis. Chang Gung Med. J. 2009, 32, 668–673. [Google Scholar]
- Liesivuori, J.; Savolainen, H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- Collier, H.O.; Chesher, G.B. Identification of 5-hydroxytryptamine in the sting of the nettle (Urtica dioica). Br. J. Pharmacol. Chemother. 1956, 11, 186–189. [Google Scholar] [CrossRef]
- Emmelin, N.; Feldberg, W. The mechanism of the sting of the common nettle (urtica urens). J. Physiol. 1947, 106, 440–455. [Google Scholar] [CrossRef]
- Shah, V.P.; Maibach, H.I. Topical Drug Bioavailability, Bioequivalence, and Penetration; Plenum Press: New York, NY, USA, 1993; p. 453. [Google Scholar]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef]
- Asche, C.V.; Shane-McWhorter, L.; Raparla, S. Health economics and compliance of vials/syringes versus pen devices: A review of the evidence. Diabetes Technol. Ther. 2010, 12 (Suppl. 1), S101–S108. [Google Scholar] [CrossRef]
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474. [Google Scholar] [CrossRef]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Ultrasound-mediated transdermal protein delivery. Science 1995, 269, 850–853. [Google Scholar] [CrossRef]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef]
- Jain, S.K.; Rains, J.; Croad, J.; Larson, B.; Jones, K. Curcumin supplementation lowers TNF-α, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009, 11, 241–249. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, D.; Kang, J.; Meng, X.; Yang, J.; Yang, L.; Xue, N.; Gao, Q.; Han, S.; Gou, X. Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed. Pharmacother. 2018, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A. Treating diabetes today with gliclazide MR: A matter of numbers. Diabetes Obes. Metab. 2012, 14 (Suppl. 1), 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shisheva, A. Phosphoinositides in insulin action on GLUT4 dynamics: Not just PtdIns (3, 4, 5) P3. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E536–E544. [Google Scholar] [CrossRef] [PubMed]
- Son, N.H.; Park, T.S.; Yamashita, H.; Yokoyama, M.; Huggins, L.A.; Okajima, K.; Homma, S.; Szabolcs, M.J.; Huang, L.S.; Goldberg, I.J. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Investig. 2007, 117, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
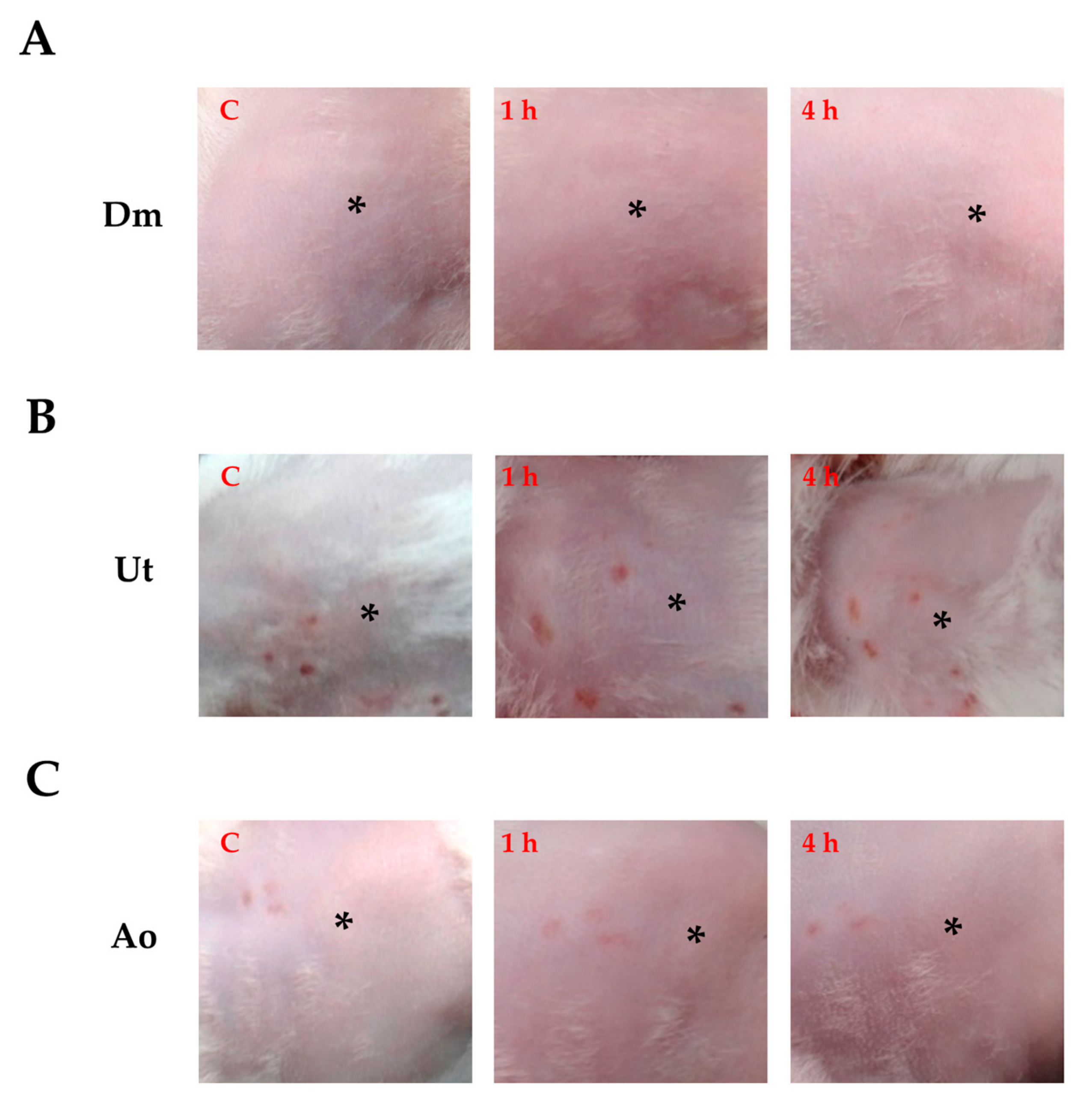
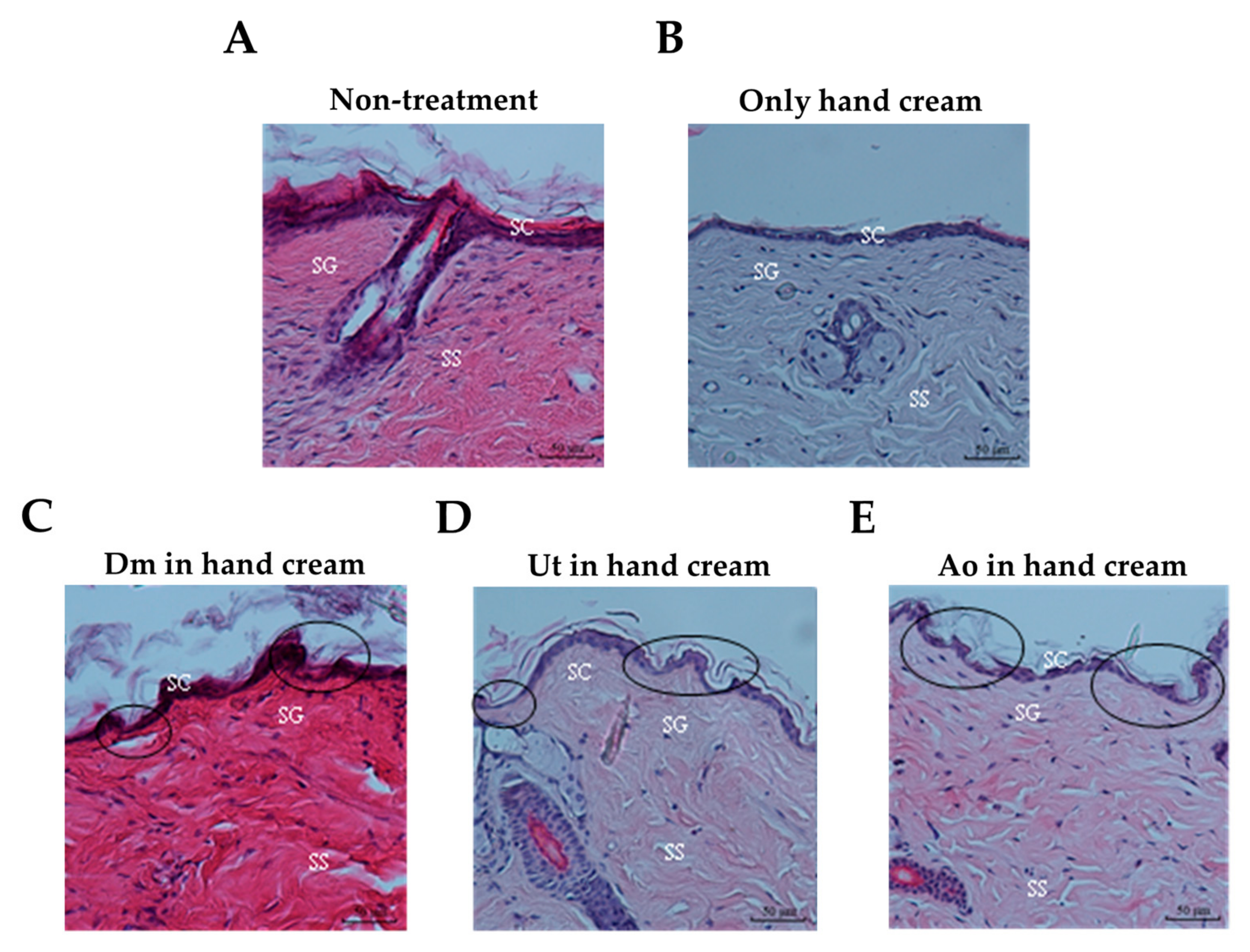
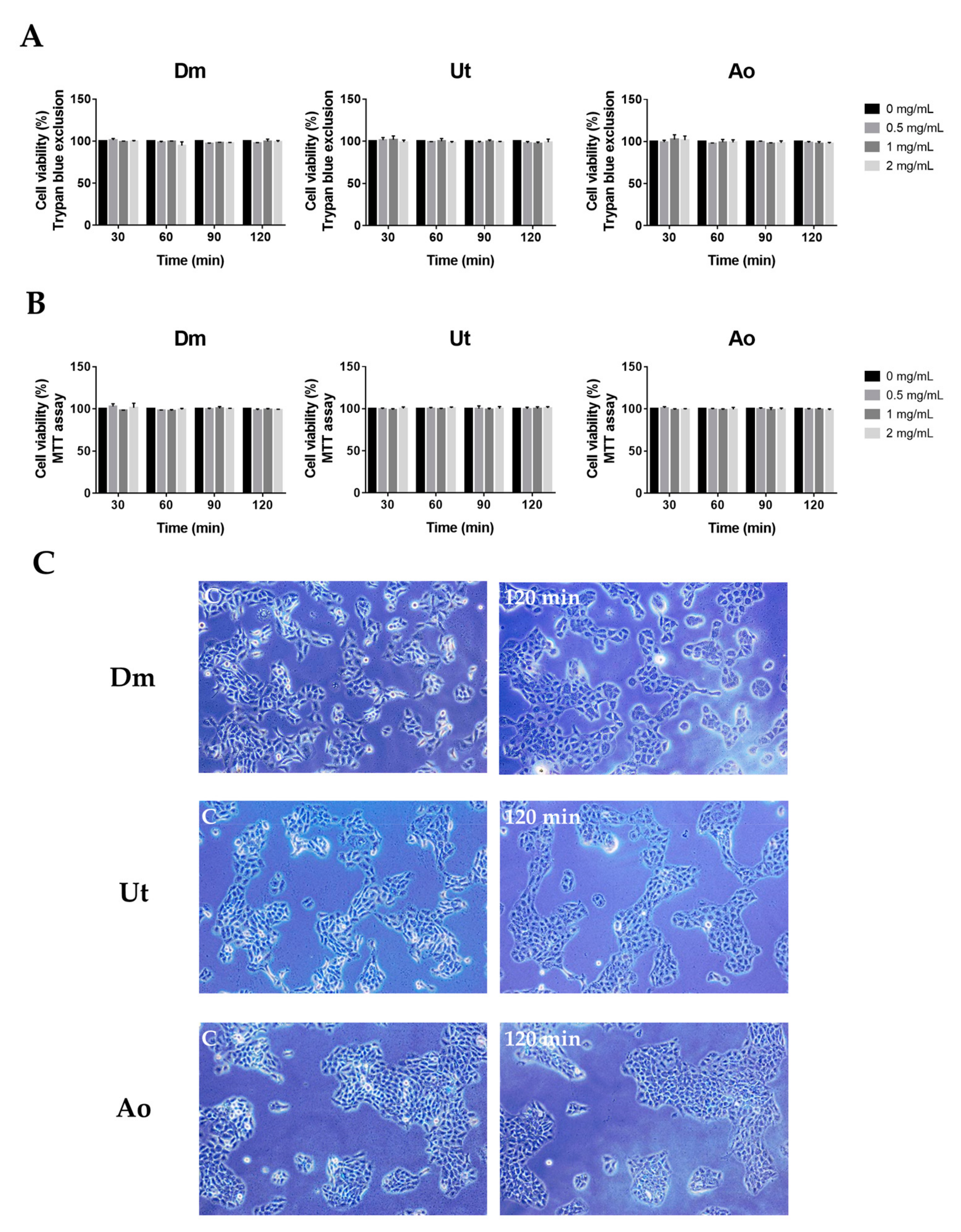
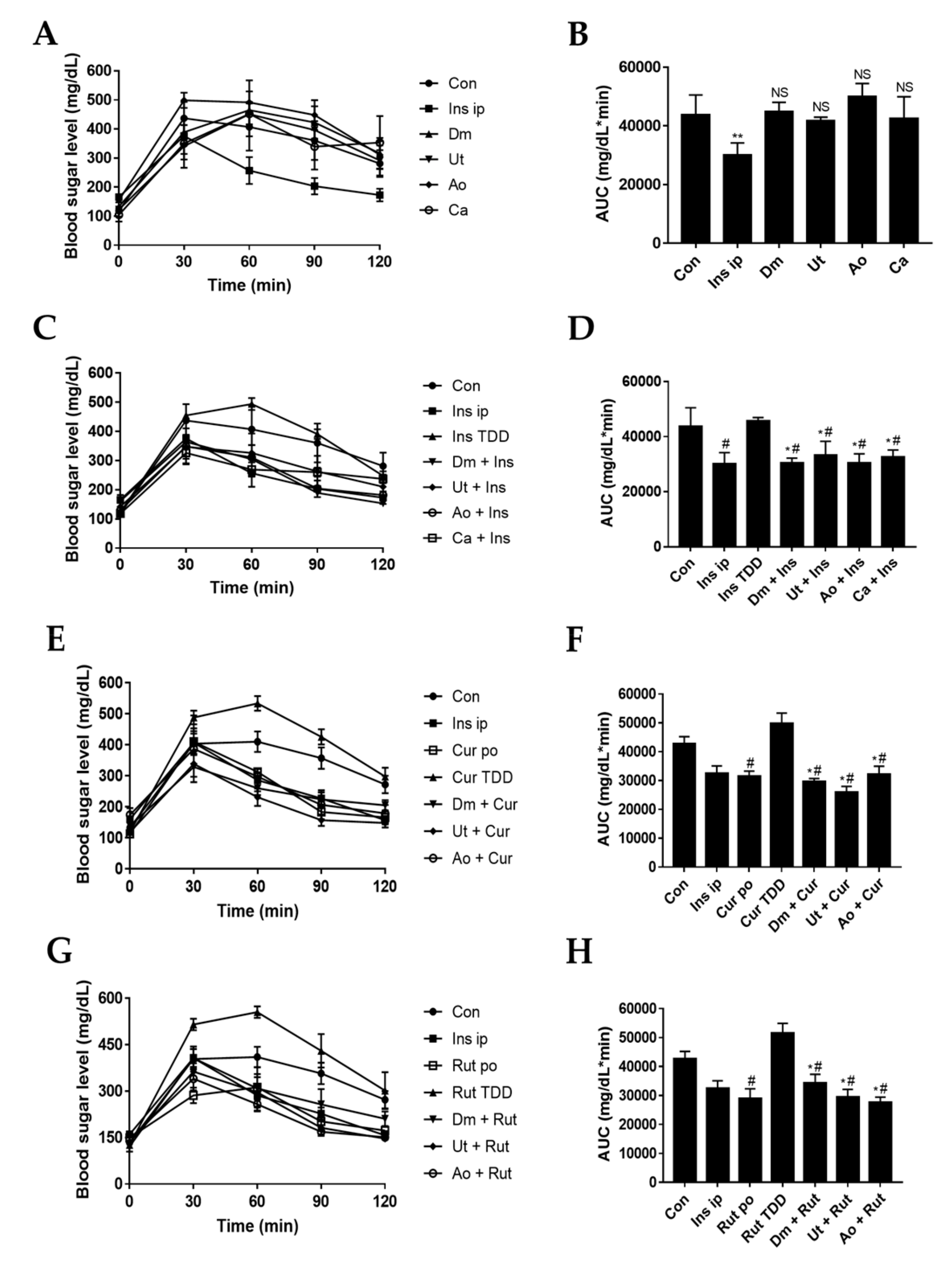
| Penetration Enhancer | Score of Skin Irritation |
|---|---|
| Dm (2 mg/mL) | 1 |
| Ut (2 mg/mL) | 1 |
| Ao (2 mg/mL) | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuh, Y.-M.; Pham, D.-C.; Weng, C.-F. Effects of Sting Plant Extracts as Penetration Enhancers on Transdermal Delivery of Hypoglycemic Compounds. Medicina 2019, 55, 121. https://doi.org/10.3390/medicina55050121
Fuh Y-M, Pham D-C, Weng C-F. Effects of Sting Plant Extracts as Penetration Enhancers on Transdermal Delivery of Hypoglycemic Compounds. Medicina. 2019; 55(5):121. https://doi.org/10.3390/medicina55050121
Chicago/Turabian StyleFuh, Yuh-Ming, Dinh-Chuong Pham, and Ching-Feng Weng. 2019. "Effects of Sting Plant Extracts as Penetration Enhancers on Transdermal Delivery of Hypoglycemic Compounds" Medicina 55, no. 5: 121. https://doi.org/10.3390/medicina55050121
APA StyleFuh, Y.-M., Pham, D.-C., & Weng, C.-F. (2019). Effects of Sting Plant Extracts as Penetration Enhancers on Transdermal Delivery of Hypoglycemic Compounds. Medicina, 55(5), 121. https://doi.org/10.3390/medicina55050121






