Perfusion Index Analysis in Patients Presenting to the Emergency Department Due to Synthetic Cannabinoid Use
Abstract
:1. Introduction
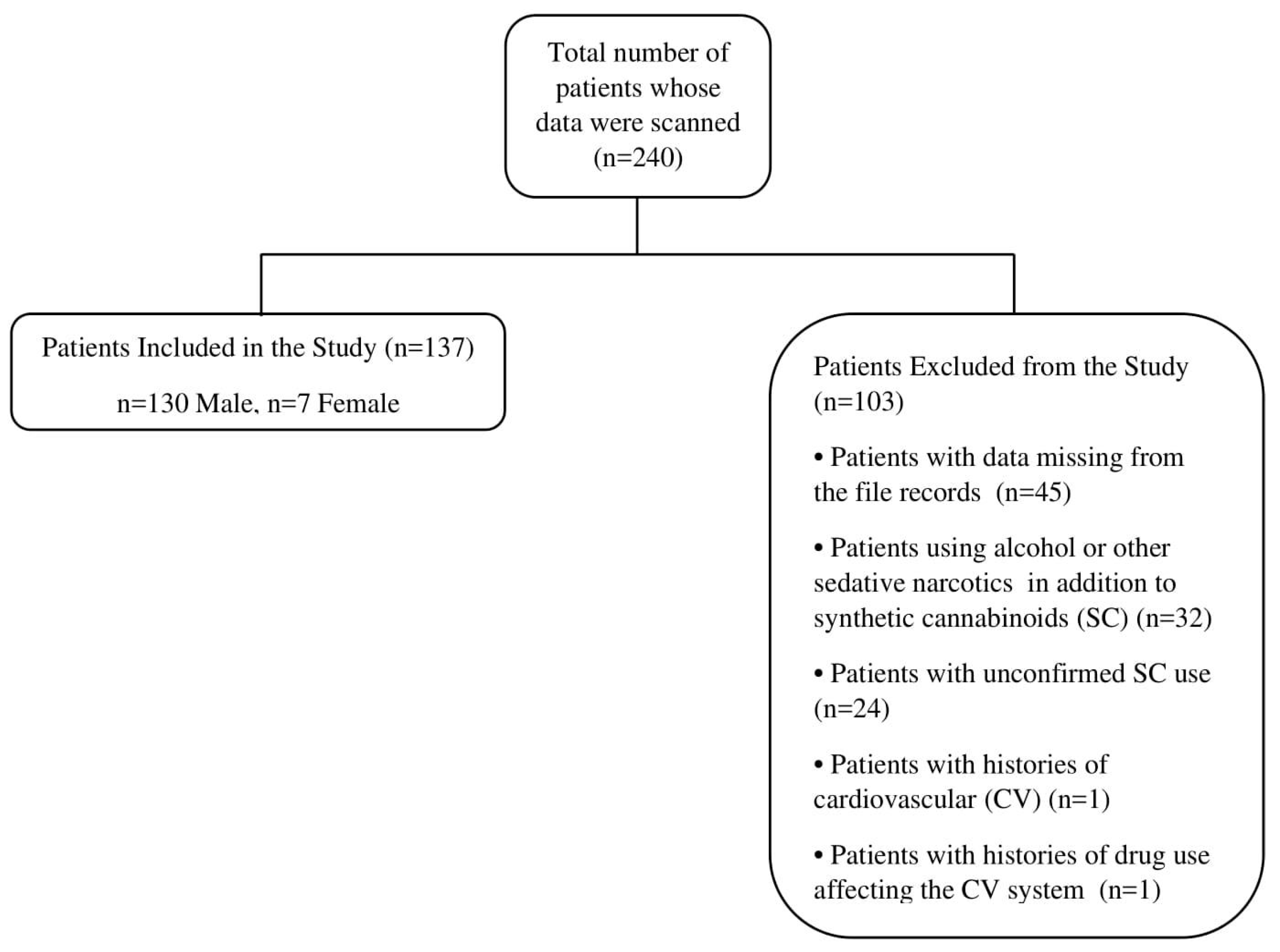
2. Materials and Methods
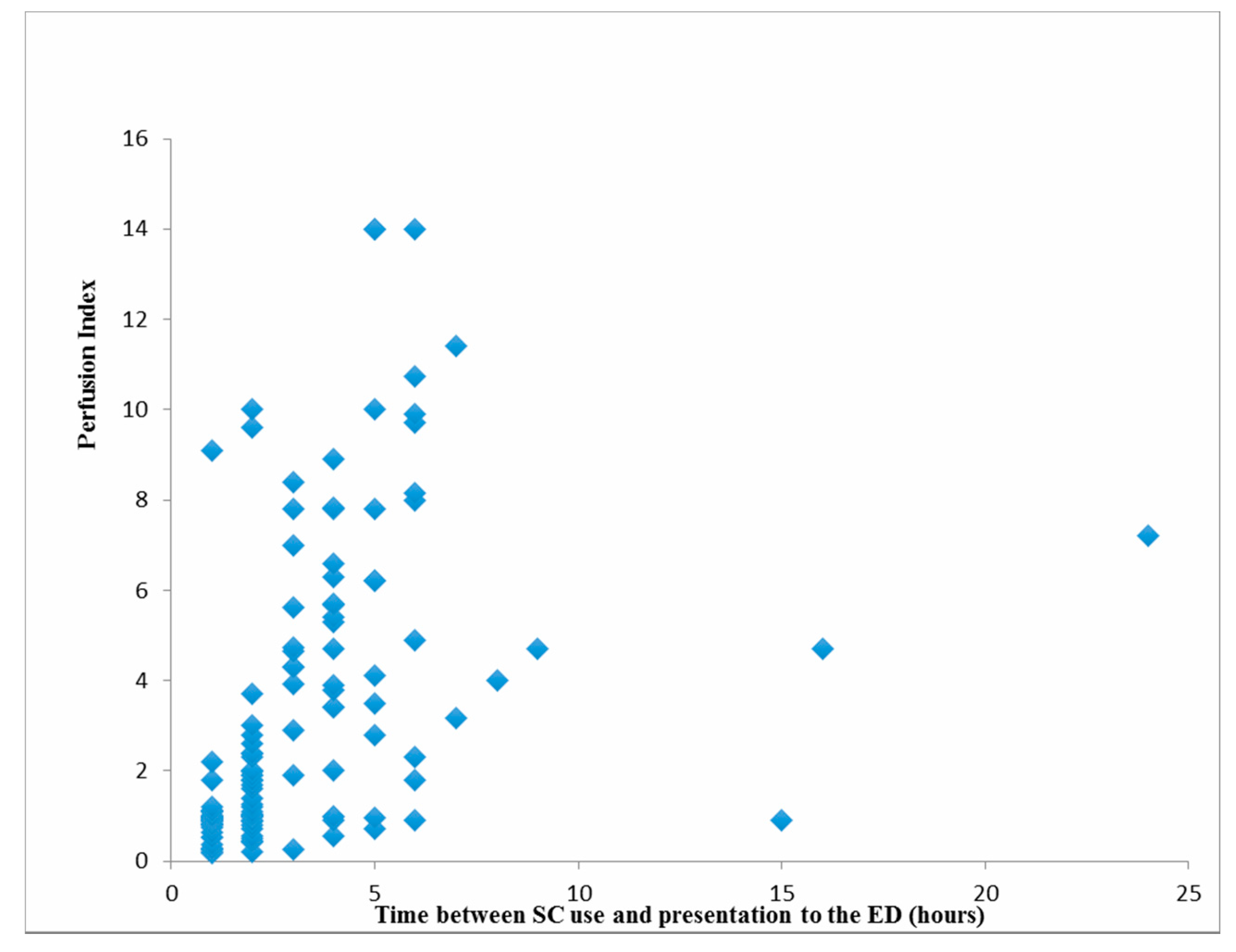
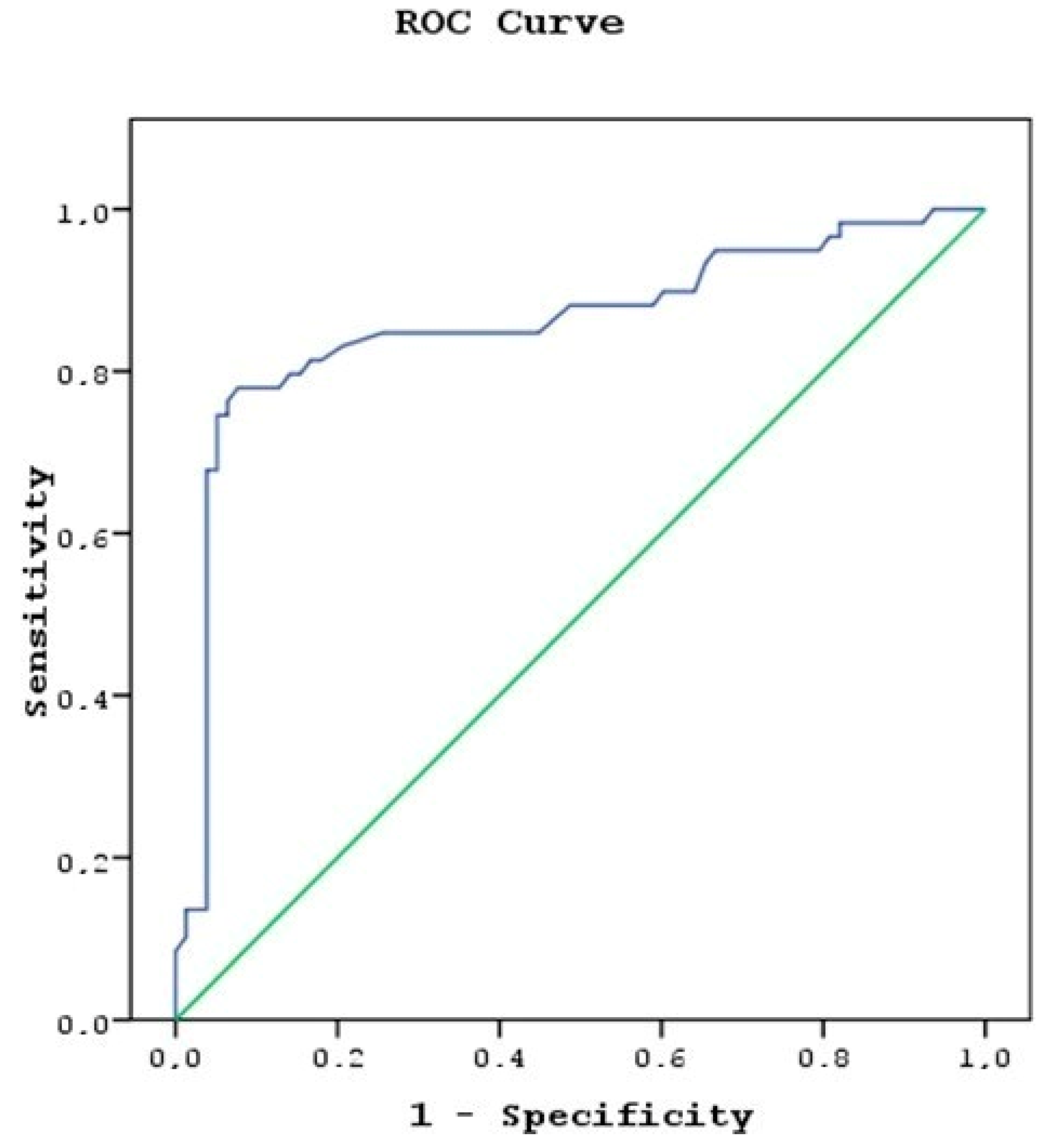
3. Results
4. Discussion
Limitations
5. Conclusions
Funding
Conflicts of Interest
References
- Türkiye Uyuşturucu Raporu. 2013. Available online: http://www.kom.gov.tr/Tr/Dosyalar/2013_TURKIYE_UYUSTURUCU_RAPORU.pdf (accessed on 15 March 2015).
- Tomiyama, K.; Funada, M. Cytotoxicity of synthetic cannabinoids found in “Spice” products: The role of cannabinoid receptors and the caspase cascade in the NG 108-15 cell line. Toxicol. Lett. 2011, 10, 12–17. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Senate Committee on Public Health and Welfare. State of Kansas supplemental note on Senate Bill No. 348. Session of 2010. Available online: http://www.kslegislature.org/supplemental/2010/SN0348.pdf (accessed on 2 June 2010).
- Guide Clinical Applications of Perfusion Index-Massimo. Available online: https://www.masimo.co.uk/siteassets/uk/documents/pdf/clinical-evidence/whitepapers/lab3410f_whitepapers_perfusion_index.pdf (accessed on 20 November 2019).
- Callahan, J.M. Pulse oximetry in emergency medicine. Emerg. Med. Clin. N. Am. 2008, 26, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Bakker, J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005, 31, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, M.C. Early identification of shock in critically ill patients. Emerg. Med. Clin. N. Am. 2010, 28, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.Y.; Rivers, E.P.; Nowak, R.M. Resuscitation of the critically ill in the ED: Responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am. J. Emerg. Med. 1996, 14, 218–225. [Google Scholar] [CrossRef]
- Lima, A.P.; Beelen, P.; Bakker, J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit. Care Med. 2002, 30, 1210–1213. [Google Scholar] [CrossRef]
- He, H.W.; Liu, D.W.; Long, Y.; Wang, X.T.; Yu, C.; Yao, B.; Zhang, R. Using peripheral perfusion index and venous-to-arterial CO(2) difference/arterial-central venous O(2) difference ratio to assess lactate clearance in septic patients after resuscitation. Zhonghua Nei Ke Za Zhi 2018, 57, 917–921. [Google Scholar]
- De Felice, C.; Latini, G.; Vacca, P.; Kopotic, R.J. The pulse oximeter perfusion index as a predictor for high illness severity in neonates. Eur. J. Pediatr. 2002, 161, 561–562. [Google Scholar] [CrossRef]
- Hiley, C.R.; Ford, W.R. Cannabinoid pharmacology in the cardiovascular system: Potential protective mechanisms through lipid signalling. Biol. Rev. Camb. Philos. Soc. 2004, 79, 187–205. [Google Scholar] [CrossRef]
- Aksel, G.; Bozan, O.; Kayacı, M.; Güneysel, Ö.; Sezgin, S.B. Rising Threat; Bonsai. Turk. J. Emerg. Med. 2015, 15, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Morrison, S.; Greenberg, J.; Saidinejad, M. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics 2012, 129, e1064–e1067. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.L.; Collins, M. A review of clinical manifestations in adolescent and young adults after use of synthetic cannabinoids. J. Spec. Pediatr. Nurs. 2014, 19, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, C.O.; Jacob, J.; Monte, A.A.; Al-Jumaan, M.; Bronstein, A.C.; Heard, K.J. A characterization of synthetic cannabinoid exposures reported to the National Poison Data System in 2010. Ann. Emerg. Med. 2012, 60, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, N.; Demirel Dengi, S.; Görükmez, S.; Arıca, S.; Yeniocak, S. Management of Bonsai intoxication at emergency service: A review of 61 cases. J. Surg. Med. 2018, 2, 278–282. [Google Scholar]
- Hu, X.; Primack, B.A.; Barnett, T.E.; Cook, R.L. College students and the use of K2: An emerging drug of abuse in young persons. Subst. Abuse Treat. Prev. Policy 2011, 6, 16. [Google Scholar] [CrossRef]
- Monte, A.A.; Bronstein, A.C.; Cao, D.J.; Heard, K.J.; Hoppe, J.A.; Hoyte, C.O.; Iwanicki, J.L.; Lavonas, E.J. An outbreak of exposure to a novel synthetic cannabinoid. N. Engl. J. Med. 2014, 370, 389–390. [Google Scholar] [CrossRef]
- Küçük, E.; Küçük, İ.; Kirazaldı, Y.Y. Acil serviste yeni bir tehlike: Sentetik kannabinoidler (Bonzai, Jameika). Genel Tıp Dergisi 2015, 25, 18–22. [Google Scholar] [CrossRef]
- Lank, P.M.; Pines, E.; Mycyk, M.B. Emergency Physicians’ Knowledge of Cannabinoid Designer Drugs. West. J. Emerg. Med. 2013, 14, 467–470. [Google Scholar] [CrossRef]
- Ashton, J.C.; Wright, J.L.; McPartland, J.M.; Tyndall, J.D. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr. Med. Chem. 2008, 15, 1428–1443. [Google Scholar] [CrossRef]
- Gurney, S.M.; Scott, K.; Kacinko, S.; Presley, B.; Logan, B. Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci. Rev. 2014, 26, 53–78. [Google Scholar] [PubMed]
- Takematsu, M.; Hoffman, R.S.; Nelson, L.S.; Schechter, J.M.; Moran, J.H.; Wiener, S.W. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin. Toxicol. 2014, 52, 973–975. [Google Scholar] [CrossRef] [PubMed]
| Total | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD | Median | Mean ± SD | Med | ||
| Age | 27.3 ± 9.1 (17–68) | 27.4 ± 8.9 | 26 | 27.2 ± 9.4 | 2 | |
| Gender n (%) | Male | 130 (94.9) | 74 (94.9) | 56 (94.9) | ||
| Female | 7 (5.1) | 4 (5.1) | 3 (5.1) | |||
| Hours elapsed since SC use (h) | 3.1 ± 3 (1–24) | 1.4 ± 0.5 | 1.13 | 5.3 ± 3.4 | 4 | |
| Systolic BP * (mmHg) | 114.1 ± 15.9 (80–195) | 111.7 ± 16.7 | 110 | 117.3 ± 14.2 | 12 | |
| Diastolic BP * (mmHg) | 70.4 ± 10.8 (40–115) | 68.3 ± 10.7 | 70 | 73.1 ± 10.5 | 7 | |
| Mean arterial BP * | 84.9 ± 11.8 (53.3–135) | 82.7 ± 12.0 | 80 | 87.8 ± 10.9 | 9 | |
| Heart rate (beats/min) | 81.9 ± 18.8 (42–136) | 82.5 ± 18.0 | 78 | 81.2 ± 20.0 | 7 | |
| GCS ** | 13.8 ± 2.1 (4–15) | 13.3 ± 2.4 | 14 | 14.3 ± 1.4 | 1 | |
| Perfusion Index (PI) | 3.16 ± 3.26 (0.19–14) | 1.49 ± 1.77 | 0.99 | 5.37 ± 3.47 | 4 | |
| Intubation n (%) | 2 (3.4) | 0 (0) | 2 (3.4) | |||
| Perfusion Index | ||
|---|---|---|
| rho | p-Value | |
| Age | −0.013 | 0.878 |
| Systolic BP * (mmHg) | 0.125 | 0.146 |
| Diastolic BP * (mmHg) | 0.187 | 0.028 |
| Mean arterial BP * (mmHg) | 0.164 | 0.056 |
| Heart rate (beats/min) | −0.091 | 0.290 |
| GCS ** Time between SC use and presentation to the ED (hours) | 0.130 0.639 | 0.134 <0.001 |
| Enter Method | B | Beta | p-Value | |
| Fixed | 2.223 | |||
| Age | −0.018 | −0.051 | 0.533 | |
| Hours since intake | 0.443 | 0.408 | <0.001 | |
| Systolic BP * (mmHg) | −0.019 | −0.094 | 0.448 | |
| Diastolic BP * (mmHg) | 0.037 | 0.124 | 0.324 | |
| Heart rate (beats/min) | −0.007 | −0.041 | 0.615 | |
| GCS ** | 0.116 | 0.074 | 0.360 | |
| Sex | −1.232 | −0.084 | 0.301 | |
| Backward Method | Fixed | 1.775 | ||
| Hours since intake | 0.464 | 0.428 | <0.001 | |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeniocak, S. Perfusion Index Analysis in Patients Presenting to the Emergency Department Due to Synthetic Cannabinoid Use. Medicina 2019, 55, 752. https://doi.org/10.3390/medicina55120752
Yeniocak S. Perfusion Index Analysis in Patients Presenting to the Emergency Department Due to Synthetic Cannabinoid Use. Medicina. 2019; 55(12):752. https://doi.org/10.3390/medicina55120752
Chicago/Turabian StyleYeniocak, Selman. 2019. "Perfusion Index Analysis in Patients Presenting to the Emergency Department Due to Synthetic Cannabinoid Use" Medicina 55, no. 12: 752. https://doi.org/10.3390/medicina55120752
APA StyleYeniocak, S. (2019). Perfusion Index Analysis in Patients Presenting to the Emergency Department Due to Synthetic Cannabinoid Use. Medicina, 55(12), 752. https://doi.org/10.3390/medicina55120752





