Maternal Blood Concentration of Tadalafil and Uterine Blood Flow in Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Maternal Background, Adverse Events Due to Tadalafil, and Serum Tadalafil Concentration
2.3. Determination of Serum Tadalafil Concentration
2.4. Measurement of Uterine Artery Blood Flow
2.5. Statistical Analysis
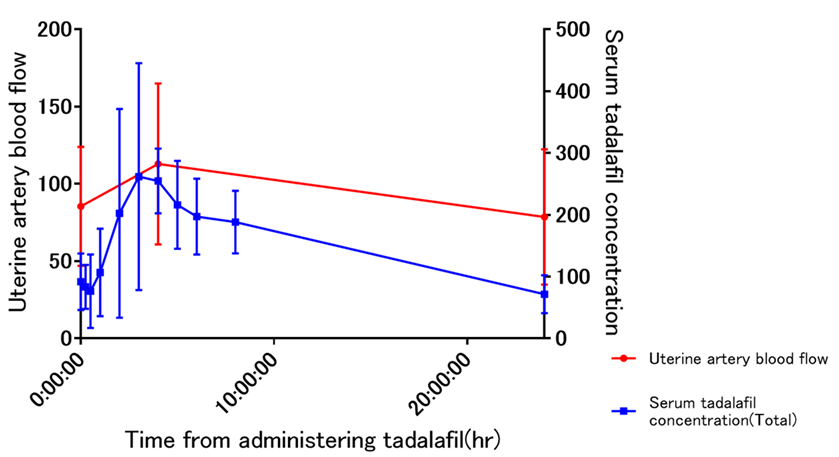
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134 fetal growth restriction. In Obstet. Gynecol.; 2013; Volume 121, pp. 1122–1133. [Google Scholar]
- Hui, L.; Challis, D. Diagnosis and management of fetal growth restriction: The role of fetal therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanaka, H.; Maki, S.; Kubo, M.; Nii, M.; Magawa, S.; Hatano, F.; Tsuji, M.; Osato, K.; Kamimoto, Y.; et al. Cardiac function and tadalafil used for treating fetal growth restriction in pregnant women without cardiovascular disease. J. Matern.-Fetal Neonatal Med. 2018, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Umekawa, T.; Maki, S.; Kubo, M.; Nii, M.; Tanaka, K.; Tanaka, H.; Osato, K.; Kamimoto, Y.; Kondo, E.; et al. Tadalafil improves L-NG-nitroarginine methyl Ester-Induced preeclampsia with fetal growth Restriction-Like symptoms in pregnant mice. Am. J. Hypertens. 2017, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Tanaka, H.; Maki, S.; Nii, M.; Murabayashi, N.; Osato, K.; Kamimoto, Y.; Umekawa, T.; Kondo, E.; Ikeda, T.; et al. Safety and dose-finding trial of tadalafil administered for fetal growth restriction: A Phase-1 clinical study. J. Obstet. Gynaecol. Res. 2017, 43, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kubo, M.; Nii, M.; Maki, S.; Umekawa, T.; Ikeda, T. Treatment using tadalafil for severe pre-eclampsia with fetal growth restriction. J. Obstet. Gynaecol. Res. 2017, 43, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Umekawa, T.; Maekawa, Y.; Tanaka, H.; Nii, M.; Murabayashi, N.; Osato, K.; Kamimoto, Y.; Ikeda, T. Retrospective study of tadalafil for fetal growth restriction: Impact on maternal and perinatal outcomes. J. Obstet. Gynaecol. Res. 2017, 43, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Heo, S.H.; Kim, G.; Chang, S.; Song, K.H.; Kim, M.G.; Jin, E.H.; Kim, J.; Lee, S.; Hong, J.H. Comparison of tadalafil pharmacokinetics after administration of a new orodispersible film versus a film-coated tablet. Drug Des. Dev. Ther. 2018, 12, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.J. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int. J. Clin. Pract. 2006, 60, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Umekawa, T.; Maki, S.; Kubo, M.; Tanaka, H.; Nii, M.; Tanaka, K.; Osato, K.; Kamimoto, Y.; Tamaru, S.; Ogura, T.; et al. TADAFER study group. TADAFER II: Tadalafil treatment for fetal growth restriction—A study protocol for a multicenter randomised controlled phase II trial. BMJ Open 2018, 8, e020948. [Google Scholar] [CrossRef] [PubMed]
- Maki, S.; Tanaka, H.; Tsuji, M.; Furuhashi, F.; Magawa, S.; Kaneda, M.K.; Nii, M.; Tanaka, K.; Kondo, E.; Tamaru, S.; et al. Safety Evaluation of Tadalafil Treatment for Fetuses with Early-Onset Growth Restriction (TADAFER): Results from the Phase II Trial. J. Clin. Med. 2019, 15, 856. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Cornforth, C.; Jackson, R.; Harrold, J.; Turner, M.A.; Kenny, L.C.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): A multicentre, randomised, placebo-controlled, Double-Blind trial. Lancet Child Adolesc. Health 2018, 2, 93–102. [Google Scholar] [CrossRef]
- Kim, Y.K.; Quadro, L. Reverse-phase High-Performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 2010, 652, 263–275. [Google Scholar] [PubMed]
- Bower, S.; Bewley, S.; Campbell, S. Improved prediction of Pre-Eclampsia by Two-Stage screening of uterine arteries using the early diastolic notch and color Doppler imaging. Obstet. Gynecol. 1993, 82, 78–83. [Google Scholar] [PubMed]
- Konje, J.C.; Kaufmann, P.; Bell, S.C.; Taylor, D.J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 2001, 185, 608–613. [Google Scholar] [CrossRef] [PubMed]
| TADAFER II Study | STRIDER Study | |
|---|---|---|
| Inclusion criteria | 20–34 gestational weeks | 22–30 gestational weeks |
| 1.5 standard deviations below the mean estimated fetal body weight according to gestational as calculated from ultrasonography | Abdominal circumference or estimated fetal weight below the 10 percentile and absent or reversed enddiastolic flow in the umbilical artery on Doppler velocimetry. | |
| The median of prolongation periods (gestational weeks) | Treatment group 47 days Control group 37 days | Treatment group 18 days Control group 18 days |
| Case | Age (Years) | Height (cm) | Weight (kg) | Gestational Age (Weeks) | Systolic BP Before Administration (mmHg) | Systolic BP After Administration (mmHg) | Obstetric Complication | Maternal Complication |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 159 | 56.3 | 32 | 110 | 108 | FGR | - |
| 2 | 33 | 160 | 60.9 | 30 | 108 | 105 | FGR | Epilepsy |
| 3 | 22 | 161 | 54.3 | 33 | 139 | 145 | Preeclampsia | - |
| 4 | 29 | 156 | 51.6 | 32 | 98 | 104 | FGR | - |
| 5 | 35 | 153.6 | 59.3 | 33 | 108 | 107 | FGR | - |
| Parameter | n = 5 |
|---|---|
| AUC0→24 (ng·h/mL) | 3638 ± 910 |
| AUC0→24/unbound (ng·h/mL) | 438 ± 65 |
| CL/F (L/min) | 4.13 ± 0.96 |
| Vdss/F (L) | 77 ± 29 |
| Cmax (ng/mL) | 329 ± 134 |
| Tmax (h) | 4 ± 1 |
| Half-life (hr) | 13 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Maki, S.; Magawa, S.; Nii, M.; Tanaka, K.; Ikemura, K.; Toriyabe, K.; Ikeda, T. Maternal Blood Concentration of Tadalafil and Uterine Blood Flow in Pregnancy. Medicina 2019, 55, 708. https://doi.org/10.3390/medicina55100708
Tanaka H, Maki S, Magawa S, Nii M, Tanaka K, Ikemura K, Toriyabe K, Ikeda T. Maternal Blood Concentration of Tadalafil and Uterine Blood Flow in Pregnancy. Medicina. 2019; 55(10):708. https://doi.org/10.3390/medicina55100708
Chicago/Turabian StyleTanaka, Hiroaki, Shintaro Maki, Shoichi Magawa, Masafumi Nii, Kayo Tanaka, Kenji Ikemura, Kuniaki Toriyabe, and Tomoaki Ikeda. 2019. "Maternal Blood Concentration of Tadalafil and Uterine Blood Flow in Pregnancy" Medicina 55, no. 10: 708. https://doi.org/10.3390/medicina55100708
APA StyleTanaka, H., Maki, S., Magawa, S., Nii, M., Tanaka, K., Ikemura, K., Toriyabe, K., & Ikeda, T. (2019). Maternal Blood Concentration of Tadalafil and Uterine Blood Flow in Pregnancy. Medicina, 55(10), 708. https://doi.org/10.3390/medicina55100708






