Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats
Abstract
1. Introduction
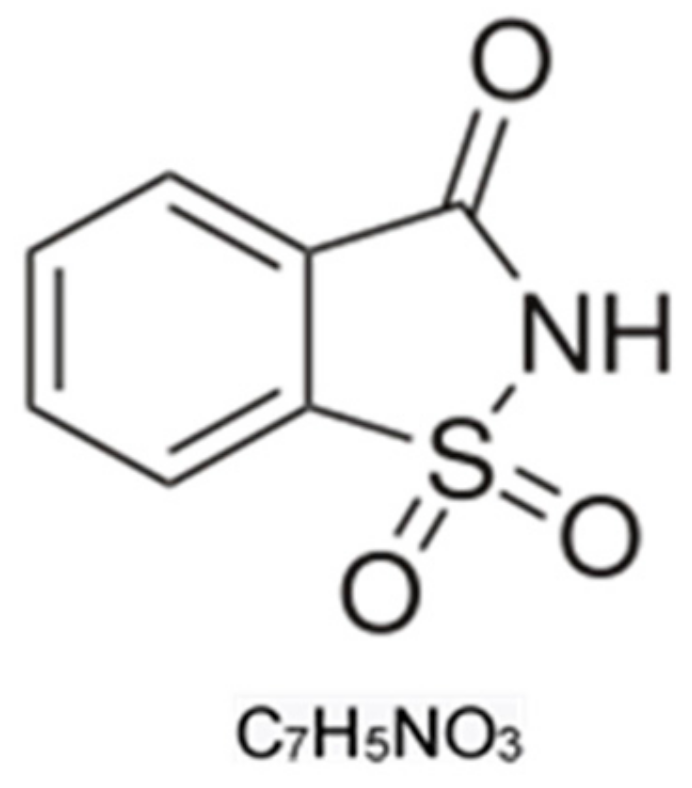
1.1. Saccharin Chemical Characteristics and Uses
1.2. Pharmacokinetic Characteristics of Saccharin
1.3. Regulations Concerning Saccharin
1.4. Saccharin and Toxicity
1.5. Saccharin and Oxidative Stress
2. Materials and Methods
2.1. Animals
2.2. Biochemical Assays
2.2.1. Chemicals
2.2.2. Experimental Design
2.2.3. Collection of Samples
2.2.4. Methods
2.3. Statistical Analysis
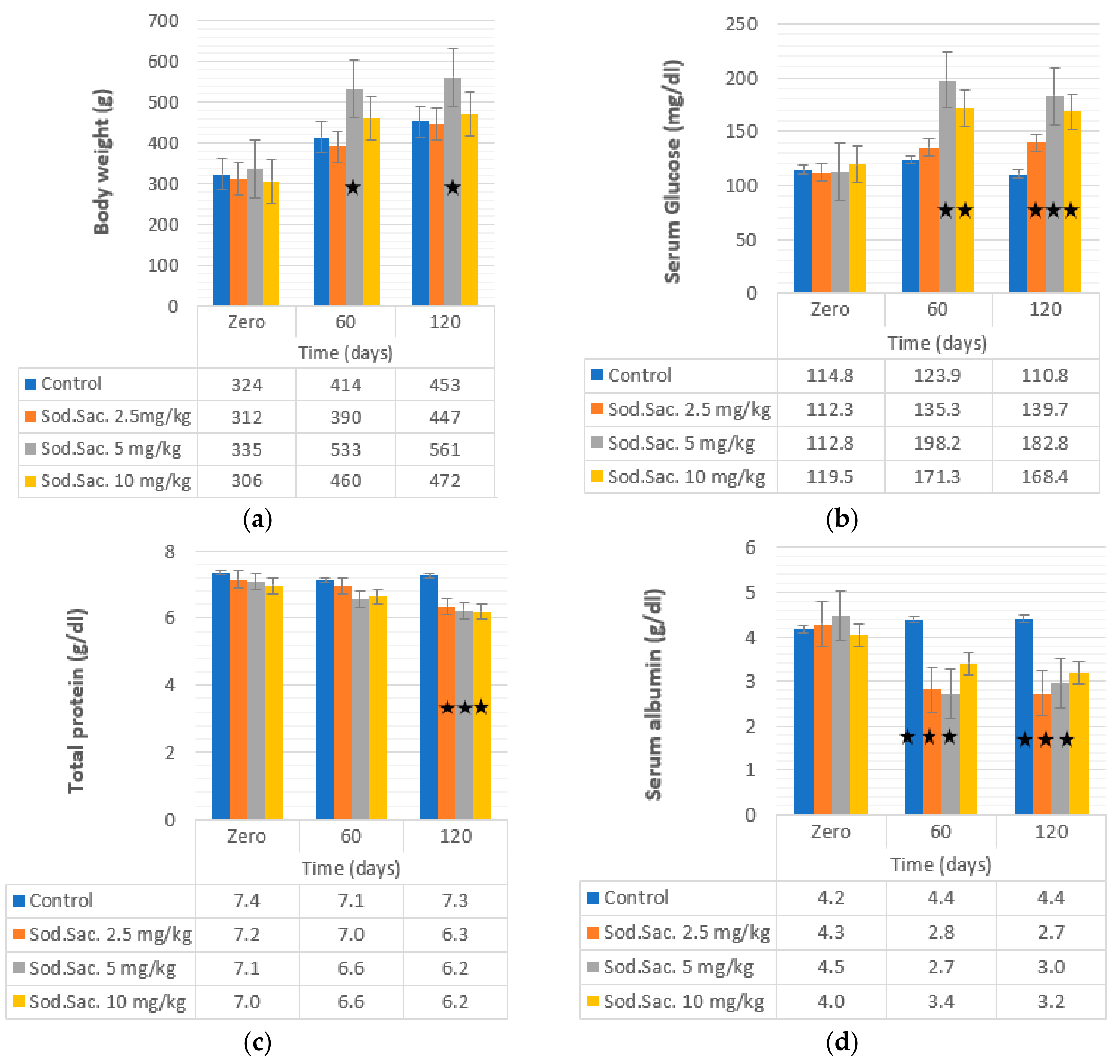
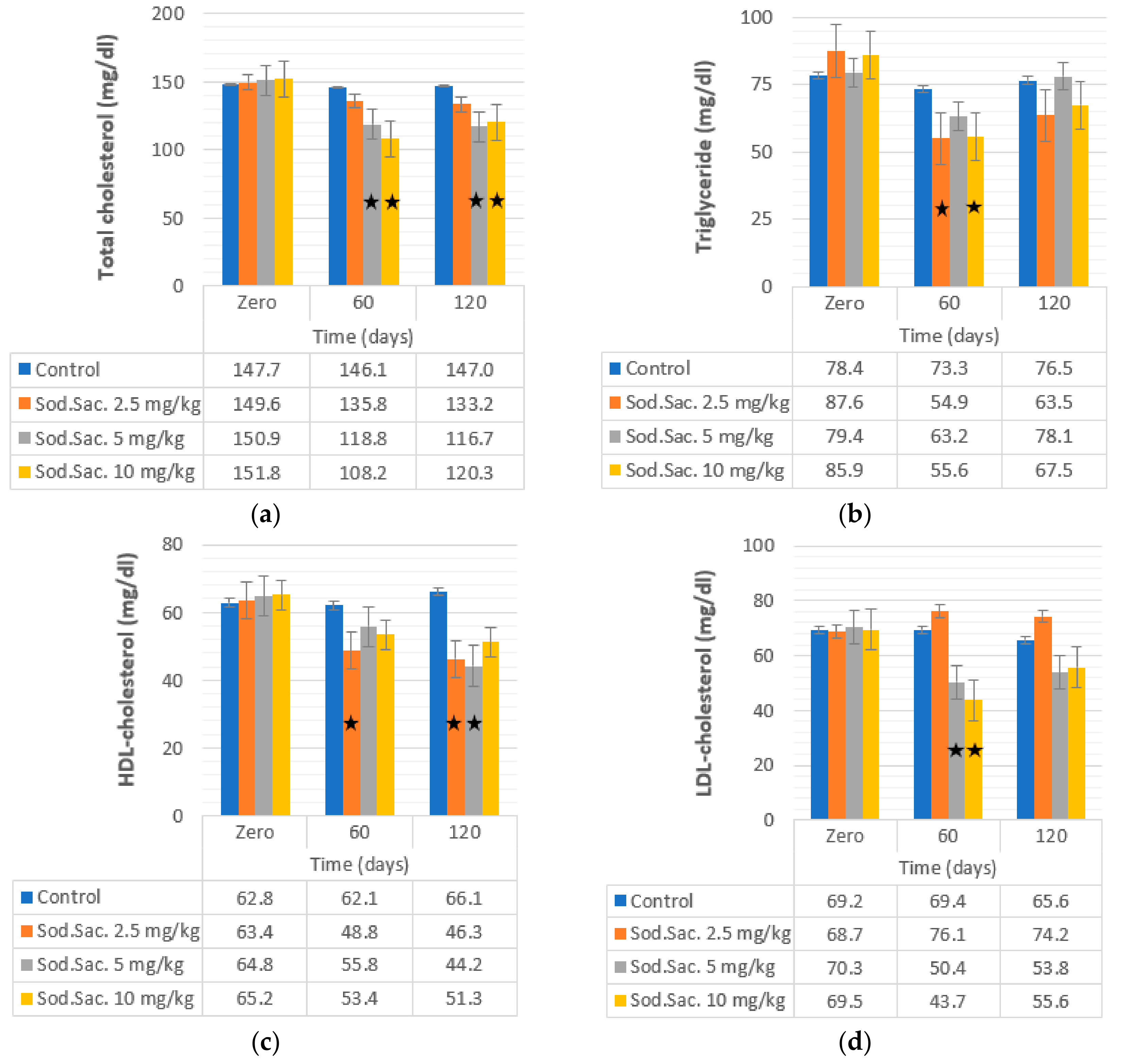
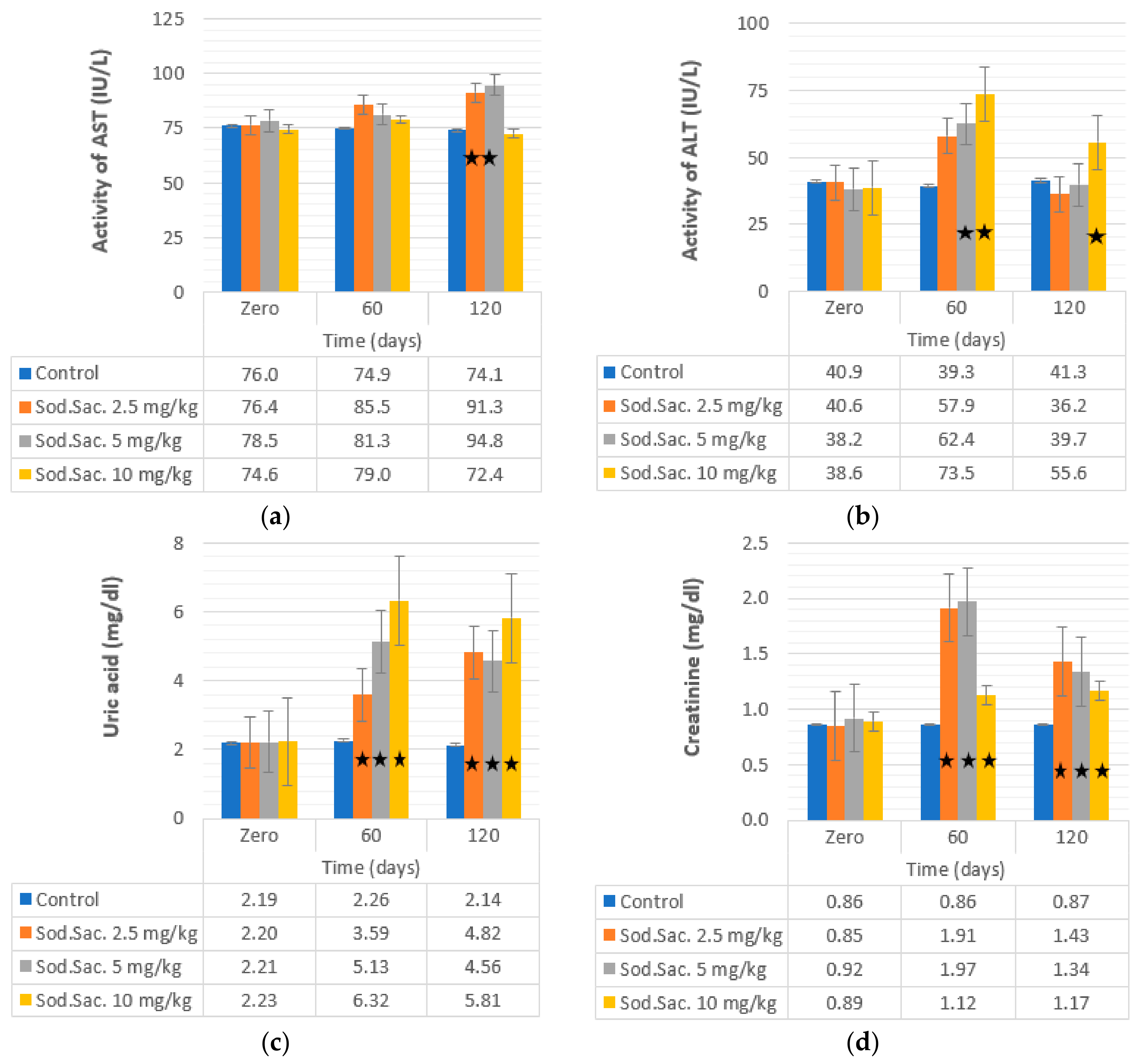
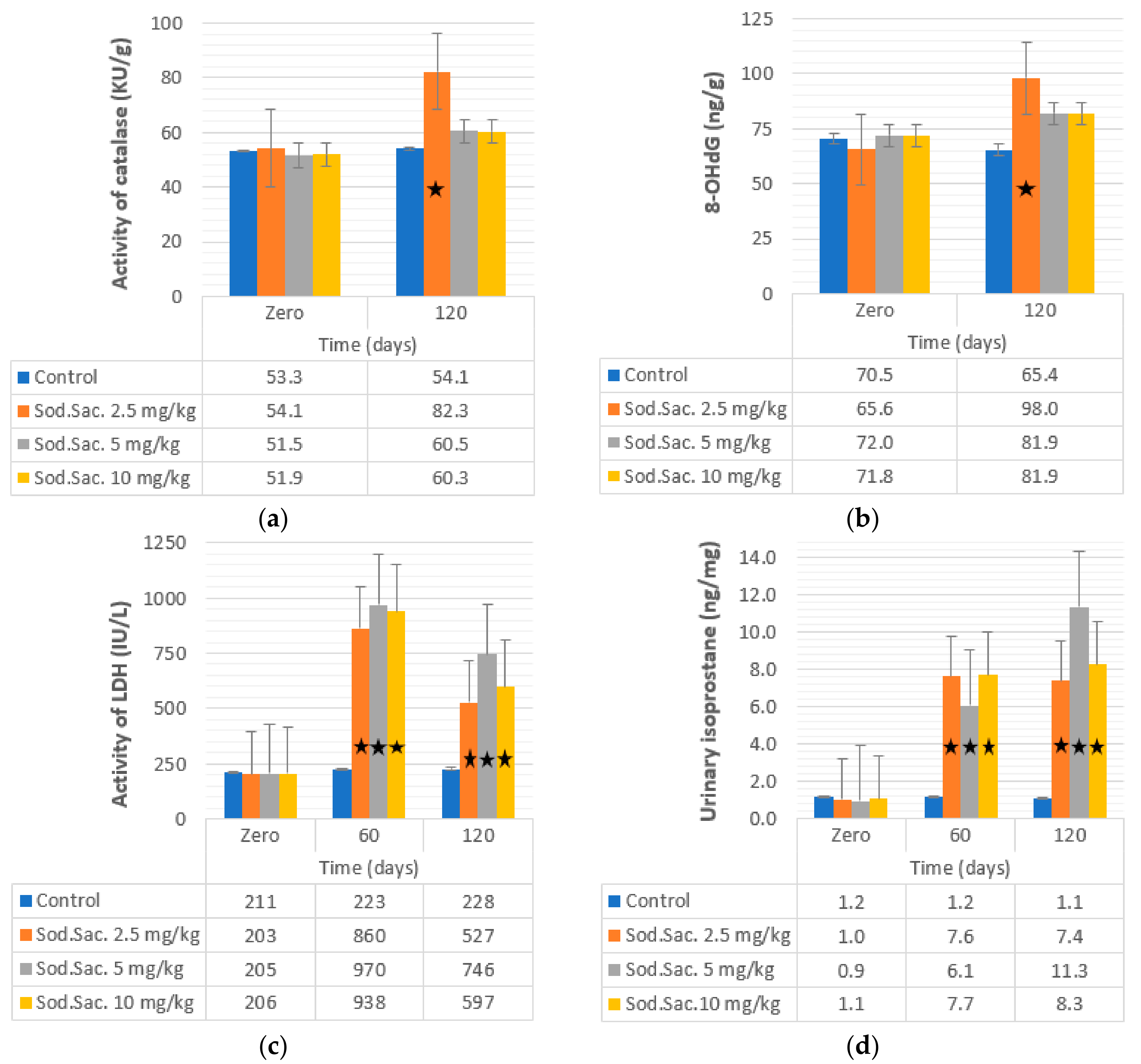
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garden, L.; Paterson, K. Artificial sweeteners and glucose intolerance: A dietitians’ perspective. Pract. Diabetes 2015, 32, 73–75. [Google Scholar] [CrossRef]
- Diabetes, U.K. Sugar, Sweeteners and Diabetes. 2018. Available online: https://www.diabetes.org.uk/guide-to-diabetes/enjoy-food/carbohydrates-and-diabetes/sugar-sweeteners-and-diabetes (accessed on 29 December 2018).
- Okoduwa, S.I.R.; Ebiloma, G.U.; Baba, J.; Ajide, S. The metabolism and toxicology of saccharin. Infohealth Aware. Artic. 2013, 1, 14–19. [Google Scholar]
- Abdelaziz, I.; Ashour, A.E.R.A. Effect of saccharin on albino rats’ blood indices and the therapeutic action of vitamins C and E. Hum. Exp. Toxicol. 2011, 30, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Grenby, T.H. Advances in Sweeteners, 1st ed.; Blackie Academic & Professional, Springer: Glasgow, UK; Springer: Boston, MA, USA, 1996; pp. 1–288. [Google Scholar]
- Renwick, A.G. The disposition of saccharin in animals and man, a review. Food Chem. Toxicol. 1985, 23, 429–435. [Google Scholar] [CrossRef]
- Ken-Ichiro, M.; Masato, A.T. The metabolism of saccharin and the related compounds in rats and guniea pigs. Chem. Pharm. Bull. 1972, 20, 1351–1356. [Google Scholar]
- Williamson, D.S.; Nagel, D.L.; Markin, R.S.; Cohen, S.M. Effect of PH and ions on the electronic structure of saccharin. Food Chem. Toxic. 1987, 25, 211–218. [Google Scholar] [CrossRef]
- Amin, K.A.; Almuzafar, H.M. Alterations in lipid profile, oxidative stress and hepatic function in rat fed with saccharin and methyl-salicylates. Int. J. Clin. Exp. Med. 2015, 8, 6133–6144. [Google Scholar]
- Mukherjee, M.; Sarkar, A. Sugar content in artificial sweetener. Adv. Appl. Sci. Res. 2011, 2, 407–409. [Google Scholar]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Uçar, A.; Yilmaz, S. Saccharin genotoxicity and carcinogenicity: A review. Adv. Food Sci. 2015, 37, 138–142. [Google Scholar]
- Gibaldi, M.; Perrier, D. Pharmacokinetics; Dekker: New York, NY, USA, 1975; p. 85. [Google Scholar]
- Ionescu, E.; Rohner-Jeanrenaud, F.; Proietto, J.; Rivest, R.W. Taste-induced changes in plasma insulin and glucose turnover in lean and genetically obese rats. Diabetes 1988, 37, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.R.; Boullata, J.; McCauley, L.A. The potential toxicity of artificial sweeteners. AAOHN J. 2008, 56, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Just, T.; Pau, H.W.; Engel, U.; Hummel, T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008, 51, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization; Food and Agriculture Organization of the United Nations & International Programme on Chemical Safety. Toxicological evaluation of certain food additives and naturally occurring toxicants/prepared by the thirty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), Rome, Italy, 1992; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Hoover, R.N.; Strasser, H.P. Artificial sweeteners and human bladder cancer: Preliminary results. Lancet 1980, 8173, 837–841. [Google Scholar] [CrossRef]
- Weihrauch, M.R.; Diehl, V. Artificial Sweeteners—Do they bear a carcinogenic risk? Ann. Oncol. 2004, 15, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.J. Saccharin, cyclamate, and human bladder cancer: No evidence of an association. JAMA 1978, 240, 249–255. [Google Scholar] [CrossRef]
- Campbell, T.C. Saccharin, cancer, and calories. Science 1978, 202, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.L. Relative risks of saccharin and calorie ingestion. Science 1978, 199, 983. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoff, R.K.; Levin, M.E. The saccharin controversy. Diabetes Care 1978, 1, 211–222. [Google Scholar] [CrossRef]
- Ellwein, L.B.; Cohen, S.M. The health risks of saccharin revisited. Crit. Rev. Toxicol. 1990, 20, 311–326. [Google Scholar] [CrossRef]
- Andrejić, B.M.; Mijatović, V.M.; Samojlik, I.N.; Horvat, O.J.; Ćalasan, J.D.; Dolai, M.A. The influence of chronic intake of saccharin on rat hepatic and pancreatic function and morphology: Gender differences. Bosn. J. Basic Med. Sci. 2013, 13, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.L.; McLane, M.; Kobe, P. Response to single dose of aspartame or saccharin by NIDDM patients. Diabetes Care 1988, 11, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C.; Knapper, J.M.E.; Turner, S.L.; Flatt, P.R. Antihyperglycaemic effect of saccharin in diabetic ob/ob mice. Br. J. Pharmacol. 1997, 468, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Alkafafy, M.E.S.; Ibrahim, Z.S.; Ahmed, M.M.; El-Shazly, S.A. Impact of aspartame and saccharin on the rat liver: Biochemical, molecular, and histological approach. Int. J. Immunopathol. Pharmacol. 2015, 28, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Al-muzafar, H.M.; Abd Elsttar, A.H. Effect of sweetener and flavoring agent on oxidative indices, liver and kidney function levels in rats. Indian J. Exp. Biol. 2016, 54, 56–63. [Google Scholar] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants. The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Unique bioactive products of lipid peroxidation free radicals, principally derived from oxygen, have been implicated in the pathophysiology of a wide variety of human diseases including cancer, atheroscler. Prog. Lipid Res. 1997, 36, 1–21. [Google Scholar] [CrossRef]
- Montusch, I.P.; Barnes, P.J.; Roberts, L.J. Insights into oxidative stress: The isoprostanes. Curr. Med. Chem. 2007, 14, 703–717. [Google Scholar] [CrossRef]
- Gnadt, B.J. Ethical and Legal Perspectives. In The Laboratory Rat, 2nd ed.; Suckow, M.A., Weisbroth, S.H., Franklin, C.L., Eds.; Elsevier, Academic Press: Cambridge, MA, USA, 2006; pp. 53–71. [Google Scholar]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Cohen, B.J.; Loew, F.M. Laboratory Animal Medicine; Academic Press: London, UK, 1984; pp. 19–120. [Google Scholar]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Allain, C.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [PubMed]
- Burtis, C.A.; Ashwood, E.R.; Saunders, W.B. Tietz Textbook of Clinical Chemistry, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1999; p. 283. [Google Scholar]
- William, T.F.; Robert, I.L.; Donald, S.F. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the Biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1997, 258, 21–30. [Google Scholar] [CrossRef]
- Labbé, D.; Vassault, A.; Cherruau, B.; Baltassat, P.; Bonète, R.; Carroger, G.; Costantini, A.; Guérin, S.; Houot, O.; Lacou, B.; et al. Method selected for the determination of creatinine in plasma or serum. Choice of optimal conditions of measurement. Ann. Biol. Clin. 1996, 54, 285–298. [Google Scholar]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3, 5-dichloro- 2-hydroxybenzenesulfonic acid/4-ami nophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980, 26, 227–231. [Google Scholar]
- Stanley, R.M.D.; Sam Frankel, P.D. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar]
- Young, D.S. Effects of Drugs on Clinical Laboratory Tests, 4th ed.; AACC Press: Washington, DC, USA, 1995; pp. 9383–9387. [Google Scholar]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Pinto, N.C.; Eide, L.; Hogue, B.A.; Thybo, T.; Stevnsner, T.; Seeberg, E.; Klungland, A.; Bohr, V.A. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res. 2001, 61, 5378–5381. [Google Scholar] [PubMed]
- Bruning, J.L.; Kintz, B.L. Computational Handbook of Statistics, 2nd ed.; Scott Foresman and Co.: Glenveiw, IL, USA, 1977; Volume 75–80, pp. 102–138. [Google Scholar]
- Saad, A.; Khan, F.A.; Hayee, A.; Nazir, M.S. A review on potential toxicity of artificial sweeteners vs safety of stevia: A natural bio-sweeteners. J. Biol. Agric. Health 2014, 4, 137–147. [Google Scholar]
- Zygler, A.; Wasik, A.; Namieśnik, J. Analytical methodologies for determination of artificial sweeteners in foodstuffs. Trends Anal. Chem. 2009, 28, 1082–1102. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Rogers, P.J.; Hill, A.J. Uncoupling sweetness and calories: Methodological aspects of laboratory studies on appetite control. Appetite 1988, 11, 54–61. [Google Scholar] [CrossRef]
- Davidson, T.L.; Swithers, S.E. A Pavlovian approach to the problem of obesity. Int. J. Obes. 2004, 28, 933–935. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Dlamini, S.N. Effects of Commercially Available Sugar Substitutes in an Experimentally Induced Rat Model of Type 2 Diabetes. Master’s Thesis, College of Agriculture, Engineering and Science School of Life Sciences Discipline of Biochemistry, University of KwaZulu-Natal, KwaZulu-Natal, South Africa, 2014. [Google Scholar]
- Abbott, A. Sugar substitutes linked to obesity. Nature 2014, 513, 290. [Google Scholar] [CrossRef]
- Swithers, S.E.; Davidson, T.L. A role for sweet taste: Calorie predictive relations in energy regulation. Behav. Neurosci. 2008, 122, 161. [Google Scholar] [CrossRef]
- Prokić, M.D.; Paunović, M.G.; Matić, M.M.; Djordjević, N.Z.; Ognjanović, B.I.; Štajn, A.; Saičić Zorica, S. Effect of aspartame on biochemical and oxidative stress parameters in rat blood. Arch. Biol. Sci. 2015, 67, 535–545. [Google Scholar] [CrossRef]
- Rosenman, K. Benefits of saccharin: A review. Environ. Res. 1978, 15, 70–81. [Google Scholar] [CrossRef]
- Smith, S.A.; Pogson, C.I. The hypoglycaemic action of tryptophan in the rat. Biochem. Soc. Trans. 1976, 4, 1049–1050. [Google Scholar] [CrossRef]
- Härtel, B.; Graubaum, J.-H.; Schneider, B. The influence of sweetener solutions on the secretion of insulin and the blood glucose level. Ernährungsumschau 1993, 40, 152–155. [Google Scholar]
- Swithers, S.E.; Laboy, A.F.; Clark, K.; Cooper, S.; Davidson, T.L. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res. 2013, 233, 1–14. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Saccharin (accessed on 22 April 2016).
- Katip, I.; Universitesi, C.; Khalilnezhad, A.; Solmaz, V. Evaluation of long-term effects of artificial sweeteners on rat brain: An Evaluation of long-term effects of artificial sweeteners on rat brain: A biochemical, behavioral, and histological study. J. Biochem. Mol. Toxicol. 2018, 32, e22053. [Google Scholar]
- Wong, O.T.; Williams, W.L.; Oswald, B.S.; Hall, I.H. The effects of cyclic imides on lipoprotein receptor binding and degradation of rat and human cells and effects on regulatory enzymes of lipid metabolism. Res. Commun. Chem. Pathol. Pharmacol. 1992, 76, 3–32. [Google Scholar]
- Hall, I.H.; Voorstad, P.J.; Chapman, J.M.; Cocolas, G.H. Antihyperlipidemic activity of saccharin analogues in rodents. J. Pharm. Sci. 1983, 72, 1192–1198. [Google Scholar] [CrossRef]
- Jamems, M.C.; Walter, J.S.; Ghassan, A.; Iris, H.H.; Oi, T.W. Hypolipicllemic activity of cyclic lmido alkyl ethers, thioethers, sulfoxides, and sulfones. J. Pharm. Sci. 2006, 78, 903–909. [Google Scholar]
- Jang, W.; Jeoung, N.H.; Cho, K.H. Modified apolipoprotein (apo) A-I by artificial sweetener causes severe premature cellular senescence and atherosclerosis with impairment of functional and structural properties of apoA-I in lipid-free and lipid-bound state. Mol. Cells 2011, 31, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P. Role of free radicals in liver diseases. Hepatol. Int. 2009, 3, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Azeez, O.H.; Alkass, S.Y. Effect of long-term consumption of aspartame on body weight. Int. J. Curr. Adv. Res. 2018, 7, 14464–14474. [Google Scholar]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical biochemistry of hepatotoxicity. J. Clin. Toxicol. 2014, 4, 1–19. [Google Scholar]
- Shakoori, A.R.; van Wijnen, A.J.; Bortell, R.; Owen, T.A.; Stein, J.L.; Lian, J.B.; Stein, G.S. Variations in vitamin D receptor transcription factor complexes associated with the osteocalcin gene vitamin D responsive element in osteoblasts and osteosarcoma cells. J. Cell. Biochem. 1994, 55, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Tetley, T.D. Proteinase inhibitors Secretory Leukoprotease Inhibitor and Elafin. In Encyclopedia of Respiratory Medicine; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; pp. 517–522. [Google Scholar]
- Turley, S.D.; Dietschy, J.M. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 2003, 14, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol. Ther. 1992, 53, 127–166. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Gimeno, C.J.; Ames, B.N. Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc. Natl. Acad. Sci. USA 1989, 86, 9697–9701. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Park, J.W.; Ames, B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 1988, 85, 6465–6467. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S.; Kurokawa, Y.; Hayashi, Y. Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis 1987, 8, 1959–1961. [Google Scholar] [CrossRef]
- Janssen, L.J. Isoprostanes: Generation, pharmacology, and roles in free-radical-mediated effects in the lung. Pulm. Pharmacol. Ther. 2000, 13, 149–155. [Google Scholar] [CrossRef]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Their role as an index of oxidant stress status in human pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [PubMed]
- Parks, D.A.; Williams, T.K.; Beckman, J.S. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: A reevaluation. Am. J. Physiol. 1988, 254, G768–G774. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.; Tsimoyiannis, E.; Kappas, A.; Galaris, D. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Esen, A.M.; Akcakoyun, M.; Esen, O.; Acar, G.; Yunus, E.; Pala, S.; Kargin, R.; Karapinar, H.; Ozcan, O.; Barutcu, I. Uric acid as a marker of oxidative stress in dilatation of the ascending aorta. Am. J. Hypertens. 2011, 24, 149–154. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeez, O.H.; Alkass, S.Y.; Persike, D.S. Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats. Medicina 2019, 55, 681. https://doi.org/10.3390/medicina55100681
Azeez OH, Alkass SY, Persike DS. Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats. Medicina. 2019; 55(10):681. https://doi.org/10.3390/medicina55100681
Chicago/Turabian StyleAzeez, Omar Hasan, Suad Yousif Alkass, and Daniele Suzete Persike. 2019. "Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats" Medicina 55, no. 10: 681. https://doi.org/10.3390/medicina55100681
APA StyleAzeez, O. H., Alkass, S. Y., & Persike, D. S. (2019). Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats. Medicina, 55(10), 681. https://doi.org/10.3390/medicina55100681




