Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of MSU Crystals
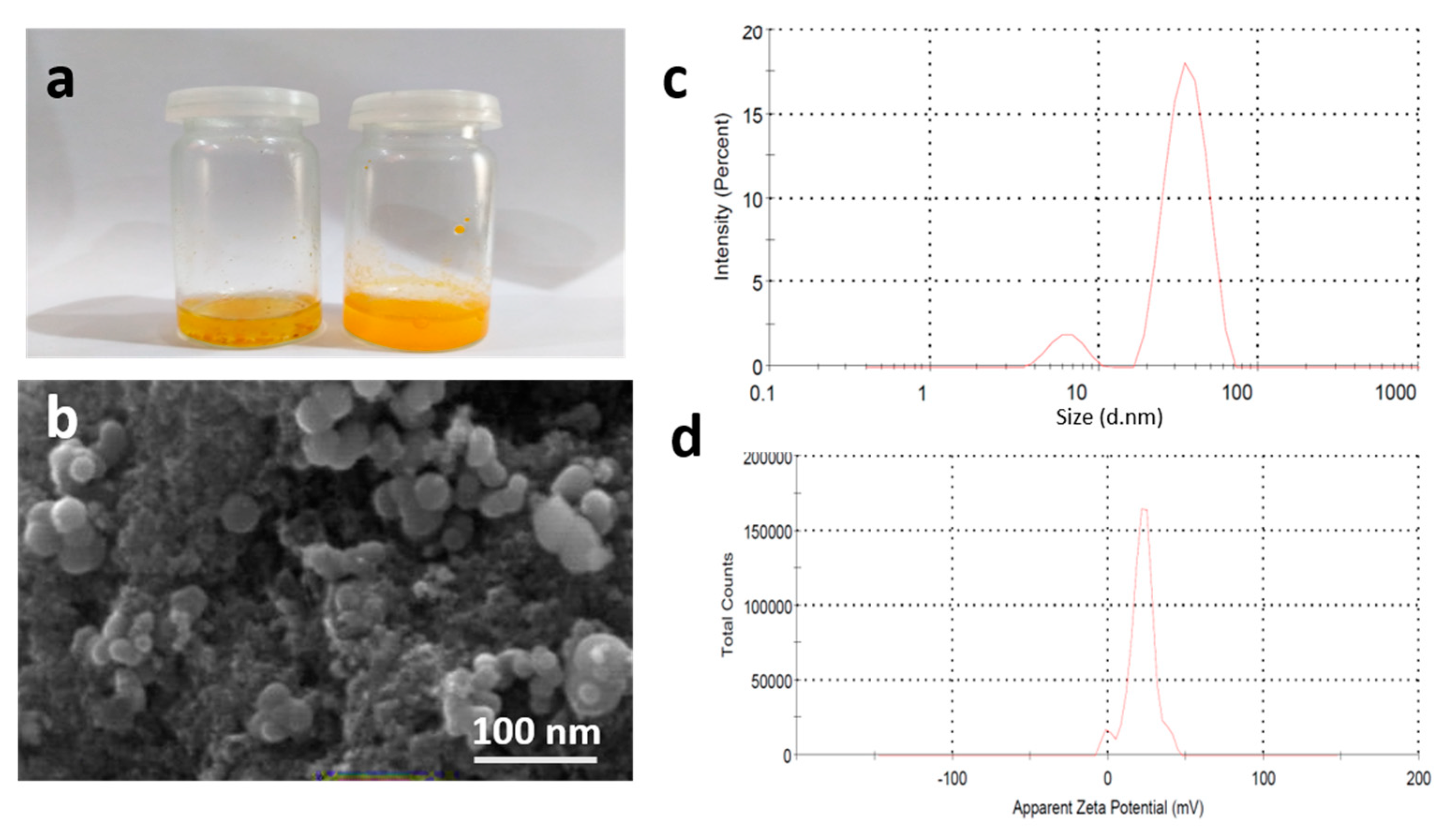
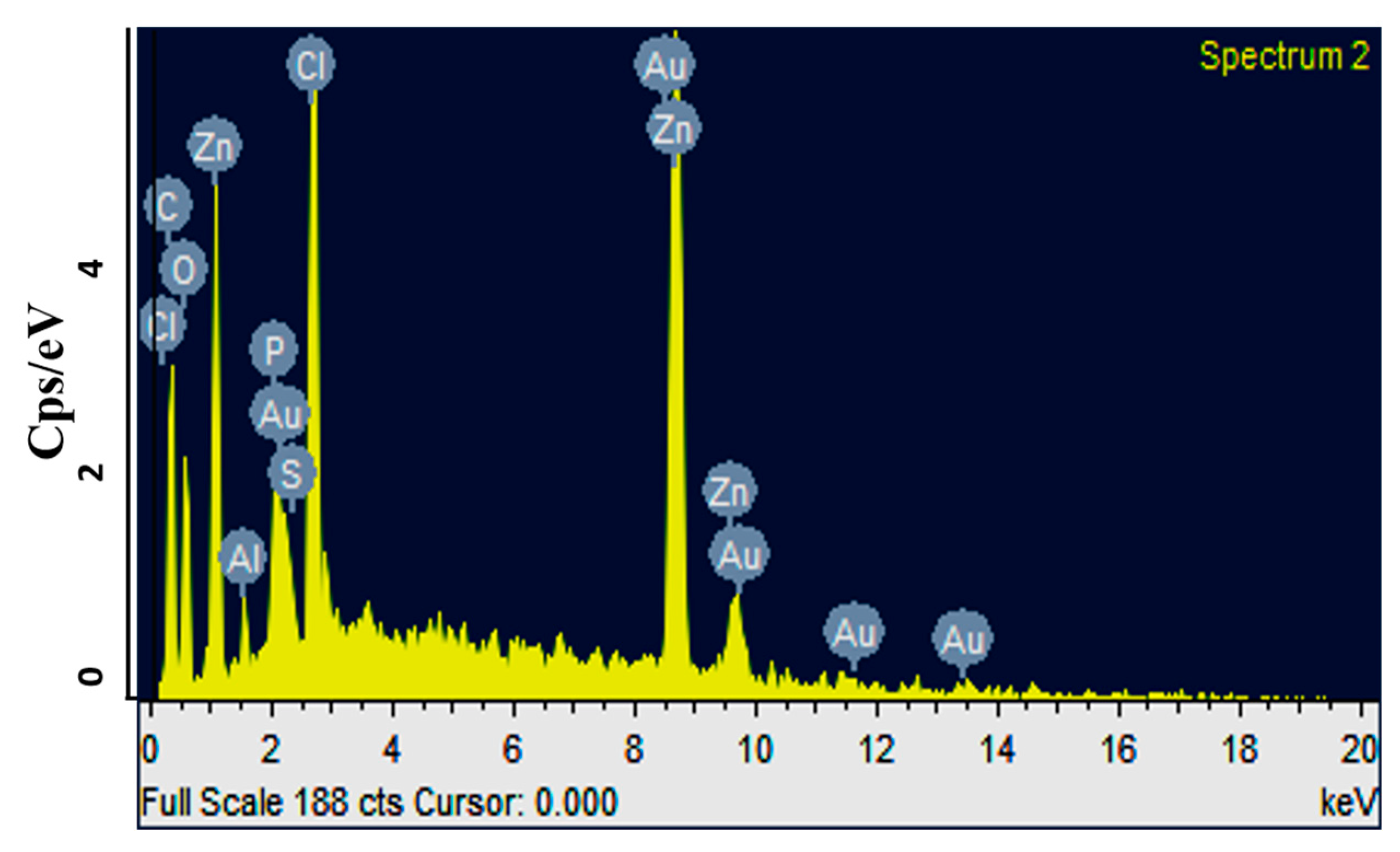
2.2. Characterization of Turmeric Nanoparticles
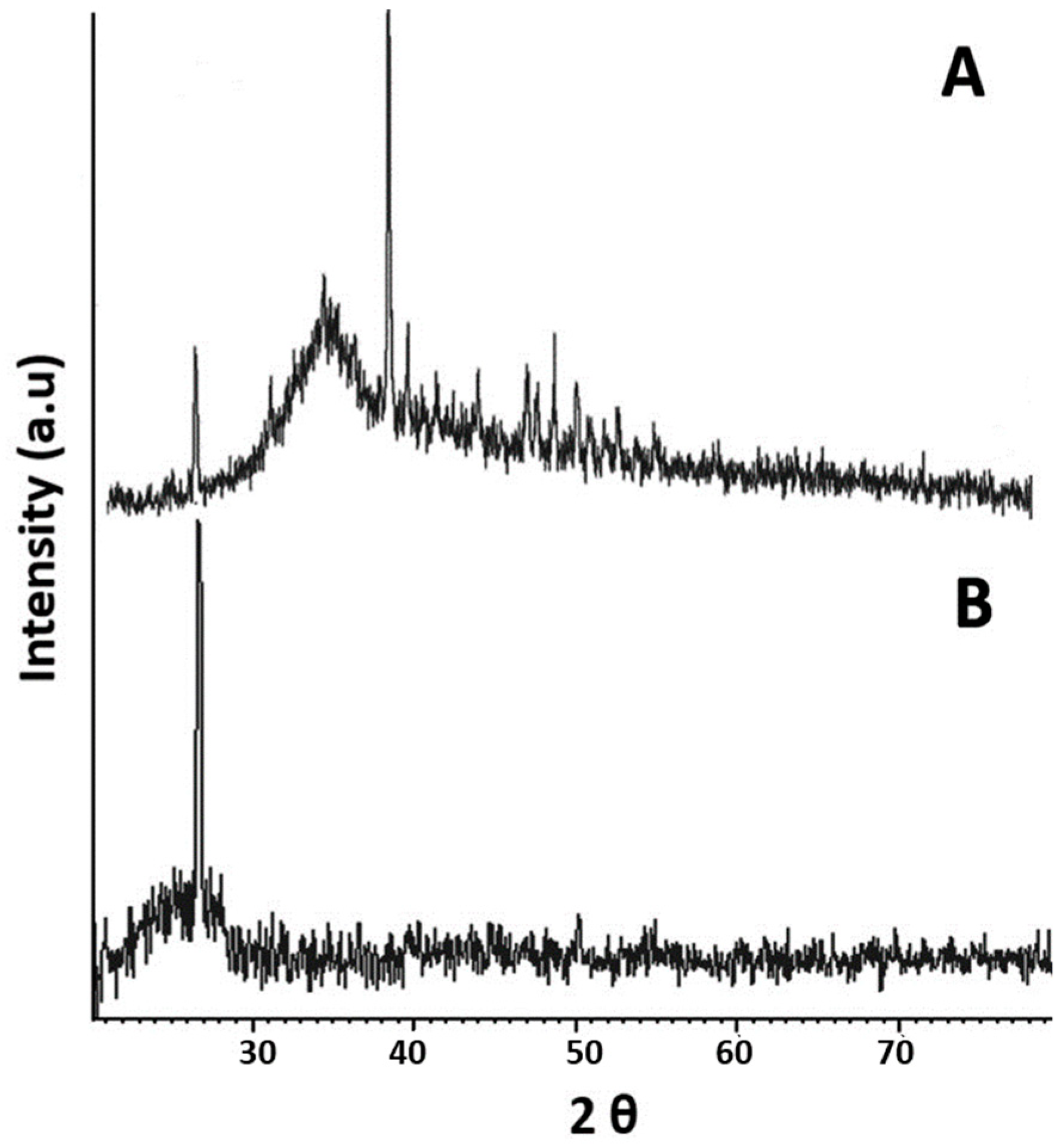
2.3. XRD Analysis
2.4. Evaluation of Anti-Gout Potential
2.4.1. Induction of Gouty Arthritis in Mice
2.4.2. In Vivo Studies
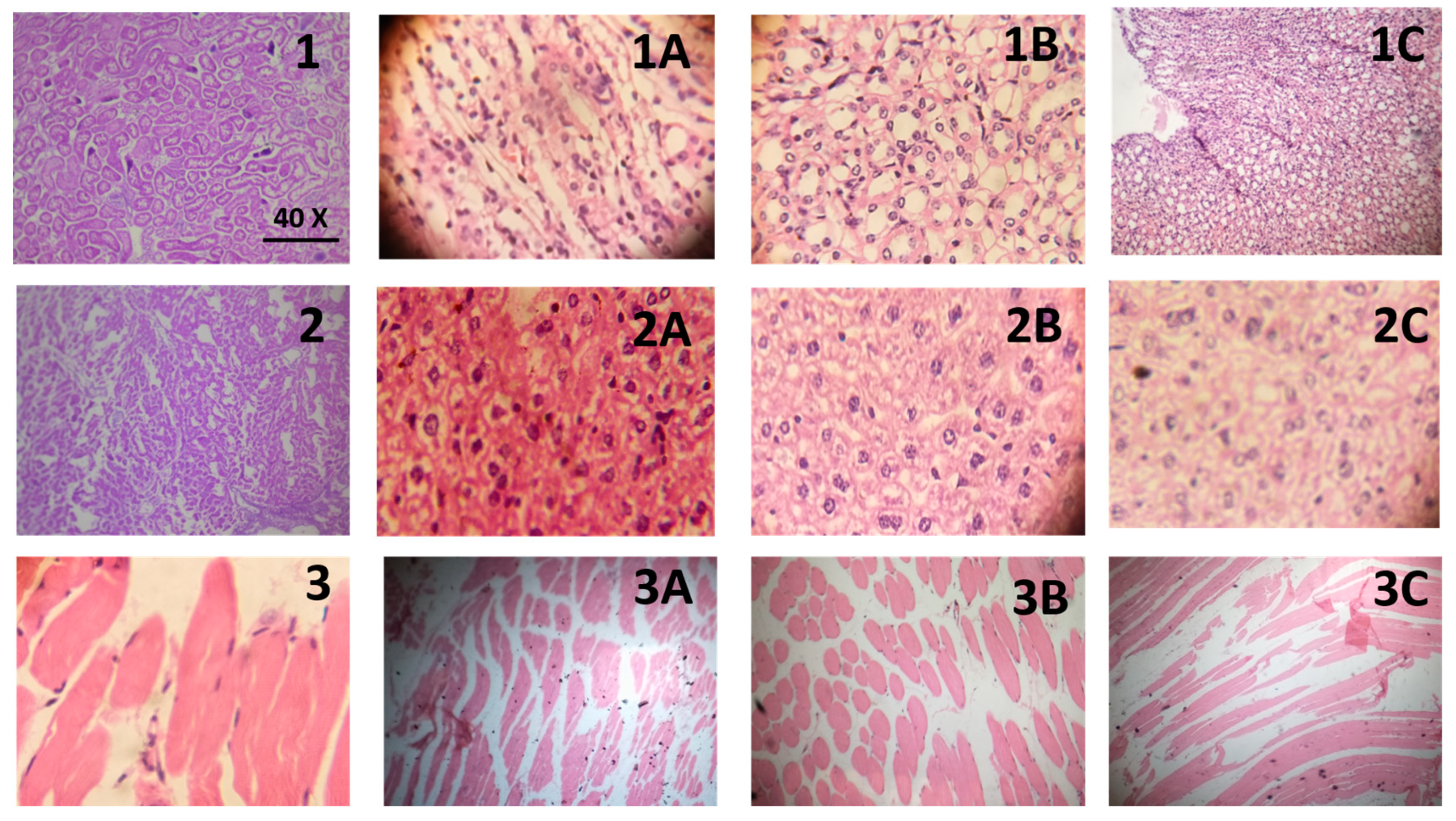
2.4.3. Histopathological Evaluation
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of MSU Crystals
3.2.2. Synthesis of Turmeric Nanoparticles
3.2.3. Particle Size, PDI, and Zeta Potential of NPs
3.2.4. Scanning Electron Microscopy and EDX Analysis of NPs
3.2.5. XRD Analysis of NPs
3.2.6. Evaluation of Anti-Gout Potential
Induction of Gouty Arthritis in Mice
In Vivo Studies
Biochemical Analysis
Histopathological Evaluation
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, L.; Saseen, J.J. Gouty arthritis: A review of acute management and prevention. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Roddy, E.; Doherty, M. Gout-A guide for the general and acute physicians. Clin. Med. 2017, 17, 54–59. [Google Scholar] [CrossRef]
- Burns, C.M.; Wortmann, R.L. Gout therapeutics: New drugs for an old disease. Lancet 2011, 377, 165–177. [Google Scholar] [CrossRef]
- Alsultanee, I.R.; Ewadh, M.J.; Mohammed, M.F. Novel natural anti gout medication extract from Momdica Charantia. J. Nat. Sci Res. 2014, 4, 16–23. [Google Scholar]
- Pant, M.K.; Panthari, P.; Kharkwal, A.; Kharkwal, H.; Kharkwal, H. Curcumin: A wonder therapeutical drug. World J. Pharm. Pharm. Sci. 2014, 3, 374–396. [Google Scholar]
- Ranade, S.Y.; Gaud, R.S. Current strategies in herbal drug delivery for arthritis: An overview. Int. J. Pharm. Sci. Res. 2013, 4, 3782–3794. [Google Scholar]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. Advances in experimental medicine and biology. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: Berlin, Germany, 2007; Volume 595. [Google Scholar]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Ansari, S.; Farha Islam, M. Influence of nanotechnology on herbal drugs: A Review. J. Adv. Pharm. Technol. Res. 2012, 3, 142–146. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Hassan, S.; Prakash, G.; Ozturk, A.B.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dokmeci, M.R.; Zhang, Y.S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef]
- Pandit, R.S.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. Springerplus 2016, 5, 1147. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: A systematic review and meta-analysis of randomized clinical trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Hasan, S.T.; Meydani, M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors 2013, 39, 101–121. [Google Scholar] [CrossRef]
- Jang, E.-M.; Choi, M.-S.; Jung, U.J.; Kim, M.-J.; Kim, H.-J.; Jeon, S.-M.; Shin, S.-K.; Seong, C.-N.; Lee, M.-K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat–fed hamsters. Metabolism 2008, 57, 1576–1583. [Google Scholar] [CrossRef]
- Iracheta-Vellve, A.; Petrasek, J.; Satishchandran, A.; Gyongyosi, B.; Saha, B.; Kodys, K.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Szabo, G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 2015, 63, 1147–1155. [Google Scholar] [CrossRef]
- Jhang, J.-J.; Lu, C.-C.; Ho, C.-Y.; Cheng, Y.-T.; Yen, G.-C. Protective effects of catechin against monosodium urate-induced inflammation through the modulation of NLRP3 inflammasome activation. J. Agric. Food Chem. 2015, 63, 7343–7352. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bravo, E.; Schumacher, J.H. Components generated locally as well as serum alter the phlogistic effect of monosodium urate crystals in vivo. J. Rheumatol. 1993, 20, 1162–1166. [Google Scholar] [PubMed]
- Sarwar, H.S.; Sohail, M.F.; Saljoughian, N.; Rehman, A.U.; Akhtar, S.; Nadhman, A.; Yasinzai, M.; Gendelman, H.E.; Satoskar, A.R.; Shahnaz, G. Design of mannosylated oral amphotericin B nanoformulation: Efficacy and safety in visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 1–11. [Google Scholar] [CrossRef]
- Lu, P.-J.; Fu, W.-E.; Huang, S.-C.; Lin, C.-Y.; Ho, M.-L.; Chen, Y.-P.; Cheng, H.-F. Methodology for sample preparation and size measurement of commercial ZnO nanoparticles. J. Food Drug Anal. 2018, 26, 628–636. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Rehman, M.; Jahan, S.; Hussain, I.; Shahnaz, G. Polymeric nanocapsules embedded with ultra-small silver nanoclusters for synergistic pharmacology and improved oral delivery of Docetaxel. Sci. Rep. 2018, 8, 13304. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Javed, I.; Hussain, S.Z.; Sarwar, S.; Akhtar, S.; Nadhman, A.; Batool, S.; Bukhari, N.I.; Saleem, R.S.Z.; Hussain, I. Folate grafted thiolated chitosan enveloped nanoliposomes with enhanced oral bioavailability and anticancer activity of docetaxel. J. Mater. Chem. B 2016, 4, 6240–6248. [Google Scholar] [CrossRef]
- Sohail, M.F.; Sarwar, H.S.; Javed, I.; Nadhman, A.; Hussain, S.Z.; Saeed, H.; Raza, A.; Bukhari, N.I.; Hussain, I.; Shahnaz, G. Cell to rodent: Toxicological profiling of folate grafted thiomer enveloped nanoliposomes. Toxicol. Res. 2017, 6, 814–821. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ashraf, M.; Javeed, A.; Anjum, A.A.; Sharif, A.; Saleem, M.; Mustafa, G.; Ashraf, M.; Saleem, A.; Akhtar, B. Association of textile industry effluent with mutagenicity and its toxic health implications upon acute and sub-chronic exposure. Environ. Monit. Assess. 2018, 190, 179. [Google Scholar] [CrossRef]
| Parameter | Unit | Normal Range | Control Group | Diseased Group | Allp-Treated Group (50 mg) | T-NPs Treated Group (5 ppm) | T-NPs Treated Group (10 ppm) | T-NPs Treated Group (20 ppm) |
|---|---|---|---|---|---|---|---|---|
| ALT | U/L | 10–40 | 34 ± 6.48 a | 61.16 ± 9.40 b | 51 ± 5.01 b | 48 ± 9.16 | 63 ± 5.35 b | 42 ± 11.3 |
| AST | U/L | 10–40 | 36 ± 3.55 a | 55.83 ± 7.31 b | 45.16 ± 2.13 | 49 ± 5.53 | 68 ± 2.58 b | 50 ± 3.21 b |
| Total bilirubin | mg/dL | 0.2–1.0 | 0.46 ± 0.16 | 0.41 ± 0.10 | 0.41 ± 0.09 | 0.5 ± 0.16 | 0.5 ± 0.12 | 0.6 ± 0.08 |
| ALP | U/L | 65–306 | 189 ± 22.99 | 183.33 ± 53.60 | 171 ± 18.97 | 69 ± 3.78 ab | 328 ± 35.59 ab | 142 ± 31.28 |
| Parameter | Unit | Normal Range | Control Group | Diseased Group | Allp-Treated Group (50 mg) | T-NPs Treated Group (5 ppm) | T-NPs Treated Group (10 ppm) | T-NPs Treated Group (20 ppm) |
|---|---|---|---|---|---|---|---|---|
| Cholesterol | mg/dL | <200 | 146.66 ± 35.51 | 207.33 ± 32.92 | 135.5 ± 29.78 | 142 ± 23.49 | 87 ± 13.06 a | 85 ± 4.51 ab |
| HDL | mg/dL | 35–65 | 34.66 ± 14.42 | 24.33 ± 2.74 | 23 ± 0.89 | 28 ±4.32 | 20 ± 2.58 | 20 ± 1.63 |
| LDL | mg/dL | <150 | 68 ± 8.6 | 108.16 ± 50.32 | 61 ± 11.67 | 62 ± 12.94 | 14 ± 1.29 ab | 15.16 ± 1.95 ab |
| Triglycerides | mg/dL | <150 | 122 ± 22.75 a | 298.66 ± 47.43 b | 306 ± 61.69 a | 331.66 ± 44.13 a | 215 ± 38.11 a | 331 ± 12.93 a |
| Parameter | Unit | Normal Range | Control Group | Diseased Group | Allp-Treated Group (50 mg) | T-NPs Treated Group (5 ppm) | T-NPs Treated Group (10 ppm) | T-NPs Treated Group (20 ppm) |
|---|---|---|---|---|---|---|---|---|
| Urea | mg/dL | 10–50 | 33 ± 7.25a | 61.5 ± 7.04 b | 38.16 ± 3.60 a | 14.5 ± 2.21 ab | 35 ± 5.94 a | 10 ± 2.51 ab |
| Creatinine | mg/dL | 0.5–1.3 | 0.63 ± 0.12 | 0.98 ± 0.22 | 0.41 ± 0.14 a | 0.4 ± 0.21 a | 0.32 ± 0.26 a | 0.3 ± 0.15 a |
| Uric Acid | mg/dL | 4–7 | 4.63 ± 0.69 a | 9.8 ± 1.29 | 2.11 ± 0.37 ab | 1.5 ± 0.41 ab | 2.16 ± 0.47 ab | 1.55 ± 0.13 ab |
| Parameter | Unit | Normal Range | Control Group | Diseased Group | Allp-Treated Group (50 mg) | T-NPs Treated Group (5 ppm) | T-NPs Treated Group (10 ppm) | T-NPs Treated Group (20 ppm) |
|---|---|---|---|---|---|---|---|---|
| WBCs Count | 103/mm3 | 4–10 | 5.33 ± 0.91 a | 34.33 ± 4.86 b | 3.66 ± 0.56 a | 6.0 ± 0.74 a | 7.0 ± 0.86 a | 6.6 ± 0.61 a |
| RBCs Count | mL/mm3 | 4.50–6.00 | 6.193 ± 0.22 | 7.12 ± 1.20 | 6.34 ± 0.02 | 8.74 ± 0.17 | 7.795 ± 0.31 | 7.82 ± 0.18 |
| Hemoglobin | g/dL | 11.0–15.0 | 11.5 ± 0.96 | 8.83 ± 1.99 | 8.76 ± 2.12 | 14.78 ± 0.42 ab | 14.28 ± 0.46 ab | 12.4 ± 0.62 |
| Hematocrit | % | 40–50 | 36 ± 4.32 | 34 ± 8.46 | 30.83 ± 5.67 | 47.16 ± 4.05 | 46 ± 1.29 | 44 ± 4.54 |
| MCV | Fl | 76–92 | 80 ± 2.43 a | 47.83 ± 4.66 b | 47.66 ± 3.50 b | 54 ± 5.85 b | 59 ± 1.82 b | 56 ± 5.62 b |
| MCH | Pg | 23–31 | 27.33 ± 2.49 a | 14.83 ± 2.03 b | 14.16 ± 2.04 b | 17 ± 1.41 b | 18 ± 1.0 b | 16 ± 2.08 b |
| MCHC | g/dL | 32–36 | 30.66 ± 1.24 | 32.66 ± 5.05 | 31 ± 2.75 | 31 ± 2.38 | 31 ± 0.81 | 28 ± 2.31 |
| RDW CV | % | 11.5–16.0 | 18.66 ± 3.29 | 21.83 ± 2.79 | 21 ± 3.84 | 16 ± 1.29 | 19 ± 4.47 | 17 ± 1.00 |
| Platelet Count | 103/mm3 | 150–450 | 231.7 ± 77.15 | 182.7 ± 42.71 | 1040 ± 44.3 ab | 554± 247.3 | 240 ± 37.02 | 771.66 ± 80.06 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa Kiyani, M.; Sohail, M.F.; Shahnaz, G.; Rehman, H.; Akhtar, M.F.; Nawaz, I.; Mahmood, T.; Manzoor, M.; Imran Bokhari, S.A. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina 2019, 55, 10. https://doi.org/10.3390/medicina55010010
Mustafa Kiyani M, Sohail MF, Shahnaz G, Rehman H, Akhtar MF, Nawaz I, Mahmood T, Manzoor M, Imran Bokhari SA. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina. 2019; 55(1):10. https://doi.org/10.3390/medicina55010010
Chicago/Turabian StyleMustafa Kiyani, Mubin, Muhammad Farhan Sohail, Gul Shahnaz, Hamza Rehman, Muhammad Furqan Akhtar, Irum Nawaz, Tariq Mahmood, Mobina Manzoor, and Syed Ali Imran Bokhari. 2019. "Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug" Medicina 55, no. 1: 10. https://doi.org/10.3390/medicina55010010
APA StyleMustafa Kiyani, M., Sohail, M. F., Shahnaz, G., Rehman, H., Akhtar, M. F., Nawaz, I., Mahmood, T., Manzoor, M., & Imran Bokhari, S. A. (2019). Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina, 55(1), 10. https://doi.org/10.3390/medicina55010010






