Abstract
Programming is triggered through events during critical developmental phases that alter offspring health outcomes. High fat programming is defined as the maintenance on a high fat diet during fetal and/or early postnatal life that induces metabolic and physiological alterations that compromise health. The maternal nutritional status, including the dietary fatty acid composition, during gestation and/or lactation, are key determinants of fetal and postnatal development. A maternal high fat diet and obesity during gestation compromises the maternal metabolic state and, through high fat programming, presents an unfavorable intrauterine milieu for fetal growth and development thereby conferring adverse cardiac outcomes to offspring. Stressors on the heart, such as a maternal high fat diet and obesity, alter the expression of cardiac-specific factors that alter cardiac structure and function. The proper nutritional balance, including the fatty acid balance, particularly during developmental windows, are critical for maintaining cardiac structure, preserving cardiac function and enhancing the cardiac response to metabolic challenges.
1. Introduction
1.1. The Programming Concept and Cardiovascular Disease
Programming is triggered through events during critical developmental phases that alter offspring health outcomes. Fetal programming refers to exposure to a stimulus or insult during fetal (in utero) life. Lactational programming refers to exposure to a stimulus or insult during early postnatal life i.e., from birth up to weaning. Developmental programming spans both fetal and lactational programming i.e., from fetal life up to weaning. Most animal studies focus on either fetal or lactational programming, the latter can be extended to include childhood. The preconception phase and paternal contribution to offspring health outcomes are also recognized as forms of programming.
Table 1 summarizes the consequences of fetal programming. There are diverse programming effects that induce specific metabolic derangements that have been demonstrated across species. Some programming effects include altered expression profiles of growth, proliferation and circadian rhythm factors, altered antioxidant enzyme actions, and the induction of increased atherogenesis, blood pressure, vascular activity and heart rate.

Table 1.
Programming of metabolic disease.
1.2. Overview of High Fat Programming
Nutrition is a programming stimulus or insult that can typically be low/high protein, carbohydrate or fat diets, or combinations, administered during specific life stages (i.e., developmental windows) [24]. Maternal nutrition and dietary fatty acid (FA) composition during gestation and/or lactation are key determinants for normal fetal and postnatal development and contribute to fetal programming effects that typically influence the offspring’s susceptibility to metabolic diseases over the life course [25]. High fat programming is defined as the maintenance on a high fat diet (HFD) during fetal and/or early postnatal life that induces metabolic and physiological alterations that compromise health. Specifically, high fat programming refers to the exposure of offspring to a high saturated fat diet during fetal and/or lactational life via maternal nutrition [24]. The HFD is typically high in saturated fat content, usually ≥40% saturated fat (as energy), that is often derived from animal fat sources. However, some programming effects can be observed from ≥30% fat (as energy) diet depending on the dietary constituents. The proportions of the other macronutrients viz. carbohydrates and protein are adjusted due to the increase in fat content in the diets. However, protein content is usually maintained at high enough levels i.e., ≥15% protein as energy to avoid the adverse programming effects of protein deficiency. Glucolipotoxicity refers to the simultaneous elevation of glucose and lipids that results in intracellular accumulation of lipids and lipid metabolites, with adverse effects on pancreatic [26] and cardiac structure, function and survival. The exposure of the fetus to hyperglycemia and hyperlipidemia implicates glucolipotoxicity [27] in the onset of metabolic diseases such as cardiovascular disease (CVD).
1.3. High Fat Programming of Cardiovascular Disease
Cardiac insulin signaling can be impaired due to a shift in FA metabolism resulting in insulin resistance. A maternal HFD diminishes cardiac function in offspring exposed to diabetic pregnancy through metabolic abnormalities, oxidative stress and mitochondrial dysfunction [28]. Further, a maternal HFD compromises organ development and renders the offspring prone to metabolic diseases later in life including CVD [29]. Maintenance on an HFD during gestation altered the placenta resulting in fetuses that were either smaller or larger [29]. In rodent models, it was revealed that maternal obesity adversely impacted the offspring, evident by hypertension, adiposity, hyperphagia, dyslipidemia, insulin resistance and hepatic steatosis [30,31,32]. Primates closely mimic human obesity, diabetes and CVD in pathology, altered glycemia and complications [16]. In primates, HFD maintenance during pregnancy led to broad developmental health issues in the offspring [3,33,34,35,36,37]. The high fat programming effects in primate offspring were restricted fetal growth, placental insufficiency with reduced placental blood flow volume, increased cytokine release, dyslipidemia and increased hepatic fat deposition [3,33,34,35,36,37]. Postnatally, the offspring maintained on an HFD displayed catch-up growth, increased fat mass and persistent fatty liver reflecting an increased risk for CVD. In primates, maintenance on an HFD (through maternal feeding and lactation) impaired offspring vascular function evident by diminished endothelium-dependent vasodilatation, thickened intima walls, and the onset of inflammation and prothrombosis which predisposes them to an increased risk of early-onset atherogenesis [16]. Importantly, the vascular function impairments observed in HFD offspring presented prior to development of obesity [16].
Prenatal insults that adversely affect fetal growth increase the incidence of hypertension in adulthood [38,39,40]. This demonstrates the early initiation of CVD and how it manifests later in life. The unfavorable intrauterine milieu can induce cardiac derangements that prompt the onset of CVD in adulthood. Maternal and early postnatal nutrition can alter the trajectories of metabolic and neural pathways involved in energy homeostasis, linked to the programming of obesity, thereby leading to disease in adulthood [41].
1.4. Maternal Obesity
Maternal obesity adversely impacts the heart and is associated with CVD in both human and animal offspring [42]. Offspring of overweight and obese mothers are more susceptible to cardiovascular anomalies [43], with obesity during pregnancy associated with a higher risk of CVD in adult human offspring [44]. Further, the programming of hypertension may initiate during intrauterine life; however, it may also emerge over the offspring’s life course [38]. Low birth weights followed by altered subsequent growth trajectories (such as catch up growth) contributes to cardiovascular morbidities [38]. Atherosclerosis has a lengthy asymptomatic phase [45] as pathological manifestations emerge in the arteries of children and young adults, decades prior to overt clinical atherosclerosis [45]. Early life (in utero, infancy and childhood) nutritional factors determine the progression of atherosclerosis [45]. Maternal hypercholesterolemia, obesity and diabetes particularly increase CVD risk [39,46] over the life course of offspring. There are also global changes in fetal lipid and mitochondrial metabolic pathways that predict later onset metabolic disease, obesity [47] and CVD. The maternal programming influences, viz. maternal HFD consumption and obesity, are discussed in the context of offspring cardiovascular outcomes.
2. High Fat Programming: Sex-Specificity, Altered Cardiac Gene and microRNA Expression, and Modified Cardiac Structure and Physiology
2.1. Sex-Specific Influences
Differential sex mechanisms of fetal cardiac programming are caused by an adverse intrauterine milieu [48] such as high fat programming. In offspring, sex-specific metabolic derangements are triggered by high fat programming [49], and although conflicting, males are more susceptible to the programming of CVD [50]. Maternal hyperglycemia in pregnancy is independently associated with the offspring’s risk for glucose intolerance, obesity and an increase in blood pressure in seven-year-old children, with only girls being obese [51]. In female sheep, after prenatal bisphenol A (BPA, commonly found in polycarbonate plastic and epoxy resins) exposure, postnatal overfeeding/adiposity, and the combination, led to myocardial transcriptional changes, and despite different gene profiles, still influenced similar signaling pathways [21]. Genes altered by the programming insult were implicated in obesity, hypertension or heart disease [21]. In rats, a maternal HFD causes cardiac hypertrophy; increases cardiac susceptibility to ischemic-reperfusion injury only in adult male offspring, and differentially regulates cardiac angiotensin II (AngII) receptor type 1 (AGTR1) and type 2 (AGTR2) expression [48]. Multiple mechanisms are involved in the sex-specific effects of maternal HFD [48].
2.2. Altered Cardiac Gene and miRNA Expression
AngII, a hormone that regulates blood pressure, and its receptors, viz. AGTR1 and AGTR2, play key roles in the regulation of cardiovascular homeostasis and are implicated in the fetal programming of cardio-cerebrovascular diseases [48,52,53,54,55]. Further, upregulation of AGTR2 played a causal role in maternal HFD-induced higher cardiac susceptibility to ischemia-reperfusion injury in male offspring; and decreased glucocorticoid receptors (GR) binding to glucocorticoid response elements (GREs) at the Agtr2 promoter through HFD-mediated Agtr2 gene upregulation [48]. Therefore, a maternal HFD influences the expression of key blood pressure regulatory factors that alter cardiac structure and susceptibility to ischemia-reperfusion injury.
There is an intricate cardiac miRNA network, modulated by HFD administration, and implicated in the pathogenesis of CVD. Poor cardiac outcomes are linked to miRNAs modulated after HFD exposure in mice [56]. In young adult mouse offspring (12 weeks of age), exposure to maternal obesity altered some cardiac-relevant microRNA (miRNA, small, non-coding endogenous RNAs of 21–25 nucleotides) expression, overlaid on a cardiac miRNAome that was markedly changed after 9 weeks of exposure to a HFD (the HFD mimicked a Western fast-food diet) [56]. After 9 weeks of the HFD administration, 33 cardiac miRNA expression profiles were altered [56]. miR-30 family members were prominent as their dysregulation is associated with cardiac abnormalities and ischemia-reperfusion injury [57], cardiomyocyte endoplasmic reticulum (ER) stress [58] and pathological hypertrophy [59,60]. Diet directly impacts cardiac miR-126 (a negative regulator of insulin signaling) expression [56] evident by its 2.5-fold upregulation induced by diet-induced maternal obesity [61]. miR-499 overexpression (>2 fold after HFD exposure) augmented heart mass and hypertrophy and exacerbated contractile dysfunction [62]. Both miR-24 and miR199a were overexpressed in failing human hearts, murine cardiac hypertrophy, and induced hypertrophy in vitro [63]. About two-thirds of miRNAs were diet-upregulated >2 fold in failing hearts [64], therefore the miRNAs altered by poor nutrition, such as exposure to a HFD in utero, are likely implicated in poor cardiac health outcomes [56]. miR-208a-3p was upregulated >3 fold by the HFD [56]. The key cardiac-specific miR-208a is required for electrical conduction but its transgenic overexpression induces hypertrophy [63,65]. Cardiac-specific miR-208a knockdown improves whole-body energy homeostasis, yielding leaner mice resistant to diet-induced obesity [66]. The alterations in cardiac miRNA expression profiles by a HFD demonstrates the importance of proper nutrition particularly during critical developmental periods. Maternal nutrition during gestation and lactation sustains and shapes offspring growth and therefore the correct healthy balance of nutrients is necessary for positive offspring development, growth and health outcomes.
The intrauterine milieu can considerably alter fetal genome expression thereby stimulating or inhibiting fetal growth and adiposity [67]. Downregulation in the miR-17-92 cluster resulted in septal defects in mice [68] whereas miR-181a plays a role in cardiac neural crest migration [69]. The dysregulation of developmentally key and relevant miRNAs may explain why the offspring of obese women are more susceptible to several congenital heart defects [70,71]. Differential miRNA expression, through developmental programming such as maternal high fat feeding and obesity, therefore influences cardiac development and health outcomes in offspring.
Some miRNA expression altered by a maternal HFD reflected expression profiles in adult cardiac diseases, such cardiac hypertrophy (miR-21, miR-143 and miR-499) [72], heart failure (miR-21 and miR-223) [73] and myocardial infarction (miR-30c, miR-139 and miR-451) [72]. Overexpressed miRNAs were implicated in increasing fibrosis (miR-21, miR-499, miRs-30 family and miRs-133 family) [74], intracellular trafficking and cell adhesion (miR-30 family) [59]. Altered cardiac miRNA expression likely diminishes cardiac function in offspring maintained on a HFD [67]. A maternal HFD, administered preconception and during gestation, programmed fetal cardiac fibrosis concomitant with differential cardiac miRNA expression likely implicated in the programming of impaired cardiac development [67]. Altered miRNA expression, through HFD modulation, therefore alters cardiac structure with implications on cardiac function and health.
2.3. Modified Cardiac Structure and Physiology
A maternal HFD had no effect on body weight but increased the heart weight and heart weight-to-body weight in the adult male offspring, which was supported by the echocardiographic (ECG) analysis showing increased wall thickness and decreased left ventricle internal diameters [48]. Thus, heart weight or its ratio to body weight (independent of body weight), is important as body weight solely may not alter despite structural and potentially functional cardiac alterations. Further, a maternal HFD caused cardiomyocyte hypertrophy in adult male offspring rats [48] consistent with another study reporting that maternal overnutrition during gestation and lactation in mice induced cardiac hypertrophy in male offspring [75]. Alterations in the myocardium have also been reported in a primate model of fetal programming [16]. In rodents, early life overnutrition programmed cardiac hypertrophy and decreased myocardial vessel density during cardiac development [76]. In addition, ECG analysis showed that a maternal HFD maintained normal systolic and diastolic function, indicating that the concentric geometric remodeling with a reduction in left ventricle chamber size relative to wall thickness is an adaptation to preserve left ventricle pump function [48]. Thus, after maintenance on a maternal HFD, the heart adapts by remodeling as a compensatory mechanism to maintain function. However, this may only be temporary as cardiac dysfunction may ensue with a persistent HFD or from the later effects of programming that present over the life course i.e., adulthood and ageing.
Clinical and experimental studies observed that the perinatal period and early development milieu regulate metabolic predispositions in adulthood [77] and ageing. Several epidemiological studies reported that early nutrition may permanently influence body weight trajectories and cardiometabolic risks viz. hypertension, dyslipidemia and insulin resistance, that render individuals susceptible to develop CVD [78,79,80,81,82]. Genomic plasticity during neonatal life is necessary for orientating adult phenotypic traits, since epigenetic modifications may alter gene expression and prompt the pathogenesis of chronic diseases [77]. In rodents, early overnutrition by decreasing litter size [83,84] limits competition for milk during lactation thereby resulting in lactational overnutrition [85] which reflects lactational programming. A reduction in rodent litter size led to a ~30% increase in body weight at weaning that was maintained as the rodents matured, albeit to a lesser extent [86,87,88]. Several metabolic syndrome traits such as overweight, insulin resistance and hypertension were reported in postnatally overfed rats [83,89]. In adult postnatally overfed rats, impaired cardiac insulin signaling was demonstrated [90,91] and lactational overnutrition programmed cardiac gene expression, that may permanently alter cardiac structure and metabolism, impair cardiac contraction and predispose offspring to myocardial ischemia-reperfusion injury [87,92,93]. Therefore, overnutrition, that often prompts overweight and obesity, modulates the expression of key cardiac factors, which alters cardiac structure and function. Neonatal cardiomyocytes exposed to a HFD had lipid droplet accumulation and mitochondrial hyperplasia with increased oxidative stress (lipid peroxidation) [28]. Neonatal cardiomyocytes exposed to both a HFD and diabetes presented impaired metabolic fuel flexibility, mitochondrial dysfunction, and diastolic and systolic dysfunction [28]. The maternal metabolic milieu therefore shapes and predicts offspring cardiovascular outcomes.
3. Maternal Obesity Triggers Cardiovascular Disease
Obesity encompasses the dysregulation of several molecular pathways and organ systems, including adipose tissue, pancreas, liver, the central nervous system, gastrointestinal tract and microbiome [94], and the cardiovascular system. Maternal hyperglycemia increased offspring susceptibility to obesity, hypertension and glucose intolerance during early childhood; this was independent of maternal obesity, macrosomia and childhood obesity [51]. Although irregular glucose tolerance levels in children may be low, the cardiometabolic risk perpetually increases over the life course [51]. A high omega-6/omega-3 ratio is prothrombotic and proinflammatory which fuels the high prevalence of atherosclerosis, diabetes and obesity [95,96,97,98,99,100]. HFDs rich in omega-6 FAs increase susceptibility to obesity, leptin resistance and diabetes in humans and rodents [101,102]. In humans and animals, a high omega-6 FA intake and a high omega-6/omega-3 ratio stimulate weight gain, whereas a high omega-3 FA intake limits weight gain [94]. The increasing incidence in obesity is most pronounced during the reproductive ages [103] and, thus, more children are conceived from overweight/obese parents [56] which contributes to the global increased incidence and prevalence of obesity. However, early exposure to omega-3 FAs appears favorable for lipid homeostasis and catalase protein production in offspring during childhood [104]. The maternal intake of omega-3 FAs in normocaloric and normolipidic diets during gestation and lactation decreased serum concentrations of triacylglycerol (TAG) and total cholesterol [104]. Therefore, the quality of FAs influences offspring lipidemia with consequences for cardiometabolic health.
Both epidemiological and experimental evidence demonstrate that overnutrition during intrauterine life, often accompanied by obesity, can program susceptibility to metabolic dysfunction in adulthood [105] and that an adverse intrauterine environment increases the risk for CVD in adulthood [106,107]. These adverse metabolic effects are genome-independent suggesting that the intrauterine milieu exerts its influences on offspring susceptibility to metabolic disease [56]. Maternal overweight and obesity are also considered as important risk factors for adult diseases in offspring, including metabolic disorders, type 2 diabetes, hypertension and CVD [108,109,110,111]. Maternal obesity is associated with various adverse outcomes for the mother, such as preeclampsia and gestational diabetes [48]. Maternal obesity and HFD consumption during gestation increase the risk of offspring obesity through complex mechanisms, which involve metabolic dysregulation and alteration of food intake behavior [112]. Exposure to maternal adiposity during gestation is linked to offspring with heavier birth weights and greater adiposity through childhood and over their life course [113]. Developmental overnutrition enables excessive transplacental passage of nutrients that results in larger babies with greater fat mass [114]. Metabolic dysregulation predisposes to CVD, and in humans, offspring from obese mothers are predisposed to CVD [44,115]. Animal studies have identified the risk of hypertension [116,117], vascular dysfunction [118], cardiac hypertrophy and contractile dysfunction [75,119] to all be shaped by maternal obesity during gestation [56]. Further, maternal obesity during gestation is associated with the adverse long-term offspring cardiovascular health outcomes, that may be independent of the genome and adult lifestyle choices [105]. High omega-6 FA intake during the perinatal period contributes to higher offspring adiposity [94]. Maternal macronutrient (HFD) and micronutrient (FA) profiles shape offspring cardiovascular outcomes. Maintaining the optimal nutrition to support the pregnant and lactating mothers’ dynamic metabolism is critical to ensure proper development, growth and maturation of their offspring.
4. Maternal High Fat Feeding and Obesity Influences on Offspring Cardiac Outcomes
Figure 1 summarizes the influences of maternal high fat feeding on their offspring’s cardiac outcomes. Maternal factors, such as malnutrition during gestation, impair myocardial cardioprotection, resulting in cardiac vulnerability. Glucolipotoxicity, with excess circulating glucose and saturated FAs, induces adverse cardiac outcomes [120], therefore maintaining an optimal intrauterine milieu is important for the developing heart. High fat programming often presents a glucolipotoxic milieu, usually co-existing with maternal obesity, which translates into undesirable consequences for offspring health outcomes. Maternal hyperglycemia during gestation also independently contributes to the offspring’s risk of hypertension, glucose intolerance and obesity [51]. Thus, the heart is susceptible to structural and functional changes.
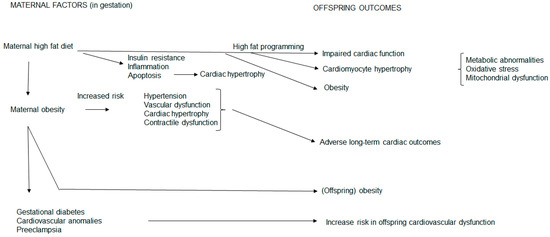
Figure 1.
Maternal high fat feeding and obesity influences on offspring cardiac outcomes. A maternal high fat diet and obesity compromises the maternal phenotype and exposes the fetal offspring to an unfavorable intrauterine milieu. This prompts structural and functional cardiac alterations in offspring through conferrance of undesirable metabolic sequalae thereby increasing their risk for cardiovascular disease.
In the mother, a HFD during gestation induces insulin resistance, inflammation, apoptosis and cardiac hypertrophy. These unfavorable maternal metabolic derangements, therefore, present an undesirable intrauterine milieu for fetal development and growth. Hence a maternal HFD confers undesirable metabolic sequelae to offspring which influences their cardiac outcomes. This occurs through high fat programming and reflects maternal metabolic conferrance to the fetal offspring. In these offspring, the mother confers impaired cardiomyocyte hypertrophy and cardiac function concomitant with obesity. In addition, metabolic abnormalities, oxidative stress and mitochondrial dysfunction [28] also contribute to offspring cardiac hypertrophy, dysfunction and obesity.
As a maternal HFD and maternal obesity are usually entwined, in the mother, maternal obesity increases the risk of gestational diabetes, increases cardiovascular anomalies and increases the risk of preeclampsia reflecting metabolic maternal derangements. Maternal obesity also increases the inherent risk of hypertension, vascular dysfunction, cardiac hypertrophy and contractile dysfunction that confers long-term adverse cardiac outcomes to offspring concomitant with obesity.
Thus, the compromised maternal metabolic state during gestation, through maternal HFD consumption and obesity, programs adverse cardiovascular outcomes in offspring. At birth, offspring may be equipped with varying abilities to compensate in response to cardiac demand. However, some offspring may be more susceptible to develop CVD. Hence, prenatal, and later postnatal, nutrition are also critical periods for shaping offspring health outcomes.
5. Conclusions
Nutrition in pregnancy requires a careful balance of both the quality and quantity of fat intake to optimize fetal growth and development, also reducing maternal morbidity [121]. The maternal nutritional status and dietary FA composition during gestation and/or lactation shape offspring development with high fat programming conferring cardiovascular risk to offspring that may present at any time over the life course. A well balanced maternal diet during gestation and lactation, with a favorable FA balance, is therefore critical for good offspring cardiovascular health.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Napoli, C.; De Nigris, F.; Welch, J.S.; Calara, F.B.; Stuart, R.O.; Glass, C.K.; Palinski, W. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation 2002, 105, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Merki, E.; Yamashita, T.; Johnson, S.; Armando, A.M.; Quehenberger, O.; Napoli, C.; Palinski, W. Maternal immunization affects in utero programming of insulin resistance and type 2 diabetes. PLoS ONE 2012, 7, e45361. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.; Bocock, P.; Showalter, L.; Hu, M.; Shope, C.; McKnight, R.; Grove, K.; Lane, R.; Aagaard-Tillery, K. Epigenomics: Maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011, 25, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Freigang, S.; Eberle, C.; Pattison, J.; Gupta, S.; Napoli, C.; Palinski, W. Maternal immunization programs postnatal immune responses and reduces atherosclerosis in offspring. Circ. Res. 2006, 99, e51–e64. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Yamashita, T.; Armando, A.M.; Dennis, E.A.; Palinski, W. Effect of gestational hypercholesterolemia and maternal immunization on offspring plasma eicosanoids. Am. J. Obstet. Gynecol. 2011, 205, 156.e15–156.e25. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, A.T.; Tooke, J.E. The fetal insulin hypothesis: An alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 1999, 353, 1789–1792. [Google Scholar] [CrossRef]
- Dabelea, D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007, 30, S169–S174. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Pettitt, D.J.; Hanson, R.L.; Imperatore, G.; Bennett, P.H.; Knowler, W.C. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care 1999, 22, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Lindsay, R.M.; Edwards, C.R.W.; Seckl, J.R. Inhibition of 11β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 1996, 27, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Goharkhay, N.; Tamayo, E.H.; Yin, H.; Hankins, G.D.V.; Saade, G.R.; Longo, M. Maternal hypercholesterolemia leads to activation of endogenous cholesterol synthesis in the offspring. Am. J. Obstet. Gynecol. 2008, 199, 273.e1–273.e6. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; D’Armiento, F.P.; Mancini, F.P.; Postiglione, A.; Witztum, J.L.; Palumbo, G.; Palinski, W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Investig. 1997, 100, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lindsley, S.R.; Comstock, S.M.; Takahashi, D.L.; Evans, A.E.; He, G.W.; Thornburg, K.L.; Grove, K.L. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int. J. Obes. 2013, 37, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Koukkou, E.; Ghosh, P.; Lowy, C.; Poston, L. Offspring of normal and diabetic rats fed saturated fat in pregnancy demonstrate vascular dysfunction. Circulation 1998, 98, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Dekou, V.; Hanson, M.; Poston, L.; Taylor, P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 2004, 110, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.D.; Khan, I.Y.; Hanson, M.A.; Poston, L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J. Physiol. 2004, 558, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Langenveld, J.; Lu, F.; Bytautiene, E.; Anderson, G.D.; Saade, G.R.; Longo, M. In utero programming of adult vascular function in transgenic mice lacking low-density lipoprotein receptor. Am. J. Obstet. Gynecol. 2008, 199, 165.e1–165.e5. [Google Scholar] [CrossRef] [PubMed]
- Koneva, L.A.; Vyas, A.K.; McEachin, R.C.; Puttabyatappa, M.; Wang, H.S.; Sartor, M.A.; Padmanabhan, V. Developmental programming: Interaction between prenatal BPA and postnatal overfeeding on cardiac tissue gene expression in female sheep. Environ. Mol. Mutagen. 2017, 58, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Sinclair, K.D. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. AJP Heart Circ. Physiol. 2014, 306, H1444–H1452. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.S.; Gardner, D.S.; Rhind, S.M.; Rae, M.T.; Kyle, C.E.; Brooks, A.N.; Walker, R.M.; Ramsay, M.M.; Keisler, D.H.; Stephenson, T.; et al. Programming of adult cardiovascular function after early maternal undernutrition in sheep. AJP Regul. Integr. Comp. Physiol. 2004, 287, R12–R20. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. High fat programming of beta cell compensation, exhaustion, death and dysfunction. Pediatr. Diabetes 2015, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E.; Louw, J. High fat-induced programming of beta-cell development and function in neonatal and weanling offspring. In Developmental Programming of Diabetes and Metabolic Syndrome; Cerf, M.E., Ed.; Transworld Research Network: Trivandrum, Kerala, India, 2008; pp. 133–158. [Google Scholar]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.R.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Cagampang, F.R.; Mukhtar, D.; Anthony, F.W.; Ohri, S.K.; Hanson, M.A. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 2009, 102, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.J.M.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Williams, S.; Grove, K.; Lane, R.H.; Aagaard-Tillery, K.M. A maternal high-fat diet is accompanied by alterations in the fetal primate metabolome. Am. J. Obstet. Gynecol. 2009, 201, 281.e1–281.e9. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.E.; Bishop, J.M.; Williams, S.M.; Grayson, B.E.; Smith, M.S.; Friedman, J.E.; Grove, K.L. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Investig. 2009, 119, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.E.; Levasseur, P.R.; Williams, S.M.; Smith, M.S.; Marks, D.L.; Grove, K.L. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010, 151, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Grayson, B.; Takahashi, D.; Robertson, N.; Maier, A.; Bethea, C.L.; Smith, M.S.; Coleman, K.; Grove, K.L. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 2010, 30, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Frias, A.E.; Morgan, T.K.; Evans, A.E.; Rasanen, J.; Oh, K.Y.; Thornburg, K.L.; Grove, K.L. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011, 152, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Nuyt, A.M.; Alexander, B.T. Developmental programming and hypertension. Curr. Opin. Nephrol. Hypertens. 2009, 18, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Nicolaides, E.; Liguori, A.; Napoli, C. Influence of maternal dysmetabolic conditions during pregnancy on cardiovascular disease. J. Cardiovasc. Transl. Res. 2009, 2, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Baum, M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am. J. Physiol. Renal Physiol. 2010, 298, F235–F247. [Google Scholar] [CrossRef] [PubMed]
- Velkoska, E.; Morris, M.J. Mechanisms behind early life nutrition and adult disease outcome. World J. Diabetes 2011, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zheng, Q.; Ford, S.P.; Nathanielsz, P.W.; Ren, J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J. Mol. Cell. Cardiol. 2013, 55, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Stothard, K.J.; Tennant, P.W.G.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular events in adult offspring: Follow-up of 1,323,275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef] [PubMed]
- Remacle, C.; Bieswal, F.; Bol, V.; Reusens, B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am. J. Clin. Nutr. 2011, 94, 1846S–1852S. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A. The early origins of atherosclerosis. Adv. Exp. Med. Biol. 2009, 646, 51–58. [Google Scholar] [PubMed]
- McCurdy, C.E.; Schenk, S.; Hetrick, B.; Houck, J.; Drew, B.G.; Kaye, S.; Lashbrook, M.; Bergman, B.C.; Takahashi, D.L.; Dean, T.A.; et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016, 1, e86612. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Dasgupta, C.; Chen, M.; Zhang, L. Foetal hypoxia increases cardiac AT2R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc. Res. 2011, 89, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dearden, L.; Balthasar, N. Sexual dimorphism in offspring glucose-sensitive hypothalamic gene expression and physiological responses to maternal high-fat diet feeding. Endocrinology 2014, 155, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo Pinho Franco, M.; Nigro, D.; Fortes, Z.B.; Tostes, R.C.; Carvalho, M.H.; Lucas, S.R.; Gomes, G.N.; Coimbra, T.M.; Gil, F.Z. Intrauterine undernutrition—Renal and vascular origin of hypertension. Cardiovasc. Res. 2003, 60, 228–234. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, D.; Dasgupta, C.; Xiong, F.; Tong, W.; Yang, S.; Zhang, L. Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: Role of angiotensin II receptors. Stroke 2012, 43, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J.L. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 1999, 33, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.D.; Lorell, B.H. AT2, judgment day: Which angiotensin receptor is the culprit in cardiac hypertrophy? Circulation 2001, 104, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Wing-Lun, E.; Eaton, S.A.; Hur, S.S.J.; Aiken, A.; Young, P.E.; Buckland, M.E.; Li, C.C.Y.; Cropley, J.E.; Suter, C.M. Nutrition has a pervasive impact on cardiac microRNA expression in isogenic mice. Epigenetics 2016, 11, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Forini, F.; Kusmic, C.; Nicolini, G.; Mariani, L.; Zucchi, R.; Matteucci, M.; Iervasi, G.; Pitto, L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014, 155, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ma, G.; Yue, Y.; Wei, Y.; Li, Q.; Tong, Z.; Zhang, L.; Miao, G.; Zhang, J. Downregulation of the miR-30 family microRNAs contributes to endoplasmic reticulum stress in cardiac muscle and vascular smooth muscle cells. Int. J. Cardiol. 2014, 173, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; Van Der Made, I.; Herias, V.; Van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhong, Y.; Cheng, C.; Liu, B.; Wang, L.; Li, A.; Xiong, L.; Liu, S. miR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS ONE 2013, 8, e53950. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Twinn, D.S.; Alfaradhi, M.Z.; Martin-Gronert, M.S.; Duque-Guimaraes, D.E.; Piekarz, A.; Ferland-McCollough, D.; Bushell, M.; Ozanne, S.E. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol. Metab. 2014, 3, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.T.C.; Huang, Y.; Gilmore, J.; Srivastava, D. Elevated miR-499 levels blunt the cardiac stress response. PLoS ONE 2011, 6, e19481. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Matkovich, S.J.; Van Booven, D.J.; Youker, K.A.; Torre-Amione, G.; Diwan, A.; Eschenbacher, W.H.; Dorn, L.E.; Watson, M.A.; Margulies, K.B.; Dorn, G.W. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 2009, 119, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Callis, T.E.; Pandya, K.; Hee, Y.S.; Tang, R.H.; Tatsuguchi, M.; Huang, Z.P.; Chen, J.F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef] [PubMed]
- Grueter, C.E.; Van Rooij, E.; Johnson, B.A.; Deleon, S.M.; Sutherland, L.B.; Qi, X.; Gautron, L.; Elmquist, J.K.; Bassel-Duby, R.; Olson, E.N. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 2012, 149, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Maloyan, A.; Muralimanoharan, S.; Huffman, S.; Cox, L.A.; Nathanielsz, P.W.; Myatt, L.; Nijland, M.J. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol. Genom. 2013, 45, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.Z. MicroRNAs in cardiovascular development. J. Mol. Cell. Cardiol. 2012, 52, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cedergren, M.I.; Källén, B.A.J. Maternal obesity and infant heart defects. Obes. Res. 2003, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Troendle, J.; Conley, M.R.; Carter, T.; Druschel, C.M. Maternal obesity and congenital heart defects: A population-based study. Am. J. Clin. Nutr. 2010, 91, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Martins, P.A.; De Windt, L.J. MicroRNAs in control of cardiac hypertrophy. Cardiovasc. Res. 2012, 91, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Toli, J.; Abdellatif, M. MicroRNAs in the cardiovascular system. Curr. Opin. Cardiol. 2011, 26, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J. Regulation of myocardial fibrosis by micrornas. J. Cardiovasc. Pharmacol. 2010, 56, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Twinn, D.S.; Blackmore, H.L.; Siggens, L.; Giussani, D.A.; Cross, C.M.; Foo, R.; Ozanne, S.E. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 2012, 153, 5961–5971. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Teixeira, M.; da Silveira Osso, F.; Pereira, R.O.; de Oliveira Silva-Junior, G.; Garcia de Souza, E.P.; Mandarim de Lacerda, C.A.; Moura, A.S. Left ventricular hypertrophy induced by overnutrition early in life. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guenancia, C.; Rigal, E.; Hachet, O.; Chollet, P.; Desmoulins, L.; Leloup, C.; Rochette, L.; Vergely, C. Short-term moderate diet restriction in adulthood can reverse oxidative, cardiovascular and metabolic alterations induced by postnatal overfeeding in mice. Sci. Rep. 2016, 6, 30817. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Kennedy, K.; Stephenson, T.; Elias-Jones, A.; Lucas, A. Promotion of faster weight gain in infants born small for gestational age: Is there an adverse effect on later blood pressure? Circulation 2007, 115, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Lucas, A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: Follow-up of a prospective randomised study. Lancet 2004, 115, 213–220. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. Br. Med. J. 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989, 298, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Boullu-Ciocca, S.; Achard, V.; Tassistro, V.; Dutour, A.; Grino, M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and proinflammatory adipokines gene expression in adulthood. Diabetes 2008, 57, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Dorner, G.; Plagemann, A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm. Metab. Res. 1994, 26, 213–221. [Google Scholar] [CrossRef] [PubMed]
- de Souza Rodrigues Cunha, A.C.; Pereira, R.O.; dos Santos Pereira, M.J.; de Melo Soares, V.; Martins, M.R.; Teixeira, M.; Souza, É.P.G.; Moura, A.S. Long-term effects of overfeeding during lactation on insulin secretion - the role of GLUT-2. J. Nutr. Biochem. 2009, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Rake, A.; Voits, M.; Fink, H.; Rohde, W.; Dörner, G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999, 836, 146–155. [Google Scholar] [CrossRef]
- Habbout, A.; Guenancia, C.; Lorin, J.; Rigal, E.; Fassot, C.; Rochette, L.; Vergely, C. Postnatal overfeeding causes early shifts in gene expression in the heart and long-term alterations in cardiometabolic and oxidative parameters. PLoS ONE 2013, 8, e56981. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Ziko, I.; Barwood, J.; Soch, A.; Sominsky, L.; Molero, J.C.; Spencer, S.J. Overfeeding during a critical postnatal period exacerbates hypothalamic-pituitary-adrenal axis responses to immune challenge: A role for adrenal melanocortin 2 receptors. Sci. Rep. 2016, 6, 21097. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Heidrich, I.; Götz, F.; Rohde, W.; Dörner, G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp. Clin. Endocrinol. Diabetes 1992, 99, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.O.; Moreira, A.S.B.; de Carvalho, L.; Moura, A.S. Overfeeding during lactation modulates insulin and leptin signaling cascade in rats’ hearts. Regul. Pept. 2006, 136, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.R.; Gonzalez Vieira, A.K.; Garcia de Souza, É.P.; Moura, A.S. Early overnutrition impairs insulin signaling in the heart of adult Swiss mice. J. Endocrinol. 2008, 198, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Habbout, A.; Delemasure, S.; Goirand, F.; Guilland, J.C.; Chabod, F.; Sediki, M.; Rochette, L.; Vergely, C. Postnatal overfeeding in rats leads to moderate overweight and to cardiometabolic and oxidative alterations in adulthood. Biochimie 2012, 94, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Habbout, A.; Li, N.; Rochette, L.; Vergely, C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J. Nutr. 2013, 143, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet and essential fatty acids. In Fatty Acids and Lipids—New Findings; Hamazaki, T., Okuyama, H., Eds.; Karger: Basel, Switzerland, 2001; pp. 18–27. [Google Scholar]
- Kang, J.X. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. In Omega-6/Omega-3 Essential Fatty Acid Ratio: The Scientific Evidence; Simopoulos, A.P., Cleland, L., Eds.; Karger: Basel, Switzerland, 2003; pp. 23–36. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome brain, metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients 2013, 5, 2901–2923. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; De Goede, J. Update on cardiometabolic health effects of ω-3 fatty acids. Curr. Opin. Lipidol. 2014, 25, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; Ordovas, J.M.; Cupples, L.A.; Defoort, C.; Lovegrove, J.A.; Drevon, C.A.; Blaak, E.E.; et al. Leptin receptor polymorphisms interact with polyunsaturated fatty acids to augment risk of insulin resistance and metabolic syndrome in adults. J. Nutr. 2010, 140, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, K.; Breier, B.H.; Jayasinghe, S.N.; Bergmann, H.; Thompson, N.; Nuernberg, G.; Dannenberger, D.; Schneider, F.; Renne, U.; Langhammer, M.; et al. Metabolic responses to high-fat diets rich in n-3 or n-6 long-chain polyunsaturated fatty acids in mice selected for either high body weight or leanness explain different health outcomes. Nutr. Metab. 2011, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013, A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oyama, L.M.; Santamarina, A.B.; do Nascimento, O.; Pisani, L.P. Influence of maternal consumption of different types of fatty acids during pregnancy and lactation on lipid and glucose metabolism of the 21-day-old male offspring in rats. Prostaglandins Leukot. Essent. Fatty Acids 2018, 135, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.M.; Morrison, J.L.; Rattanatray, L.; Zhang, S.; Ozanne, S.E.; McMillen, I.C. The early origins of obesity and insulin resistance: Timing, programming and mechanisms. Int. J. Obes. 2016, 40, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Eriksson, J.; Forsen, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.R.; Reynolds, R.M. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. 2013, 78, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.; Ozanne, S.E. Maternal over-nutrition and offspring obesity predisposition: Targets for preventative interventions. Int. J. Obes. 2011, 35, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Howie, G.J.; Sloboda, D.M.; Kamal, T.; Vickers, M.H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 2009, 587, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Matthews, P.; Poston, L. Maternal metabolism and obesity: Modifiable determinants of pregnancy outcome. Hum. Reprod. Update 2009, 16, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Smith, A.D.A.C.; Fraser, A.; Sattar, N.; Lindsay, R.S.; Ring, S.M.; Tilling, K.; Smith, G.D.; Lawlor, D.A.; Nelson, S.M. Programming of adiposity in childhood and adolescence: Associations with birth weight and cord blood adipokines. J. Clin. Endocrinol. Metab. 2017, 102, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Teramo, K.; Osmond, C.; Barker, D.J. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: Follow up study. BMJ 1997, 315, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Oest, M.E.; Prater, M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2009, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Guberman, C.; Jellyman, J.K.; Han, G.; Ross, M.G.; Desai, M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am. J. Obstet. Gynecol. 2013, 209, 262.e1–262.e8. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Bitsanis, D.; Ghebremeskel, K.; Crawford, M.A.; Poston, L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J. Physiol. 2001, 533, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, H.L.; Niu, Y.; Fernandez-Twinn, D.S.; Tarry-Adkins, J.L.; Giussani, D.A.; Ozanne, S.E. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 2014, 155, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Cardiac glucolipotoxicity and cardiovascular outcomes. Medicina 2018, 54, 70. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Flynn, A.C.; Pasupathy, D. Nutrition in pregnancy. Obstet. Gynaecol. Reprod. Med. 2016, 26, 259–264. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).