Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study
Abstract
:1. Introduction
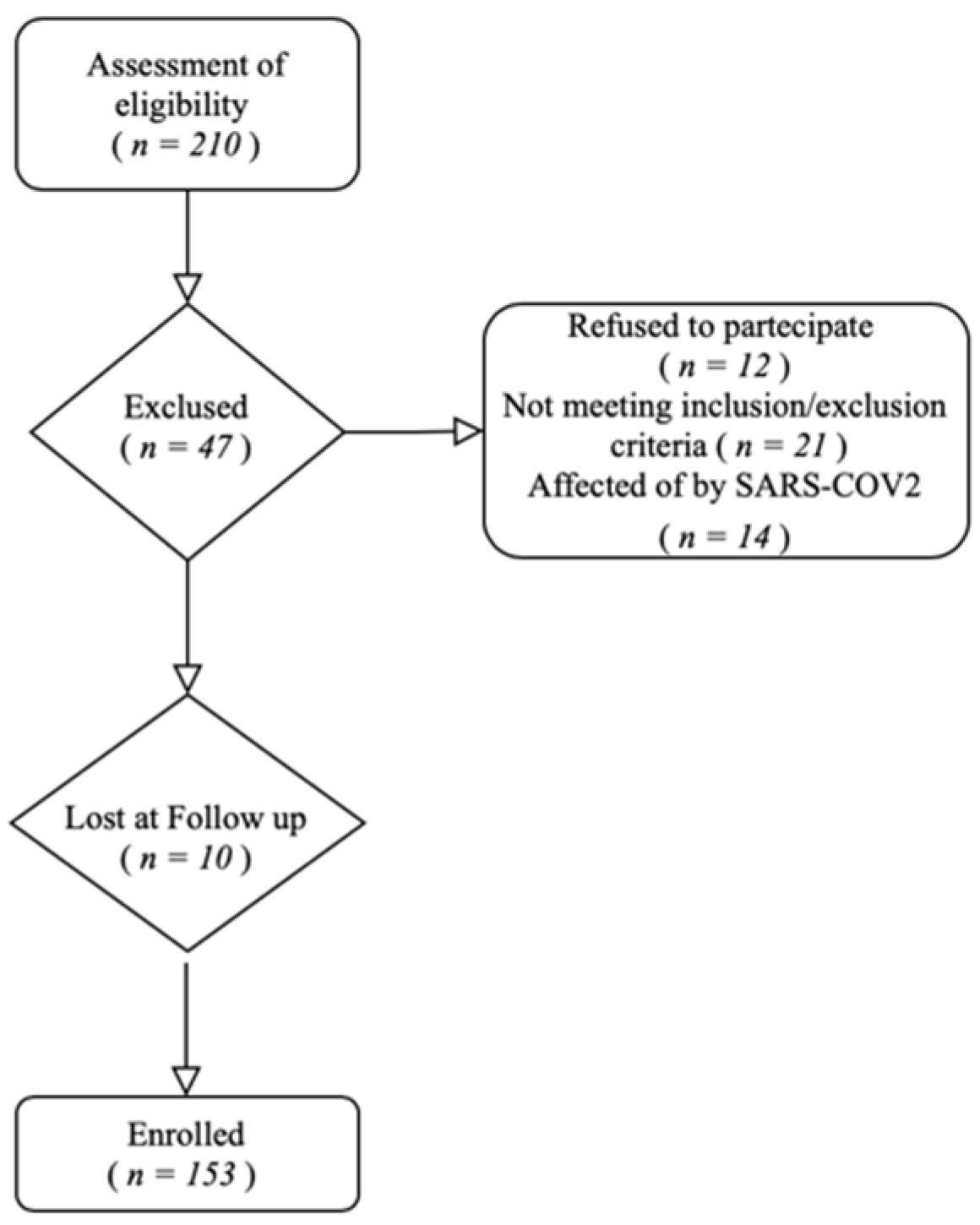
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. WOMAC
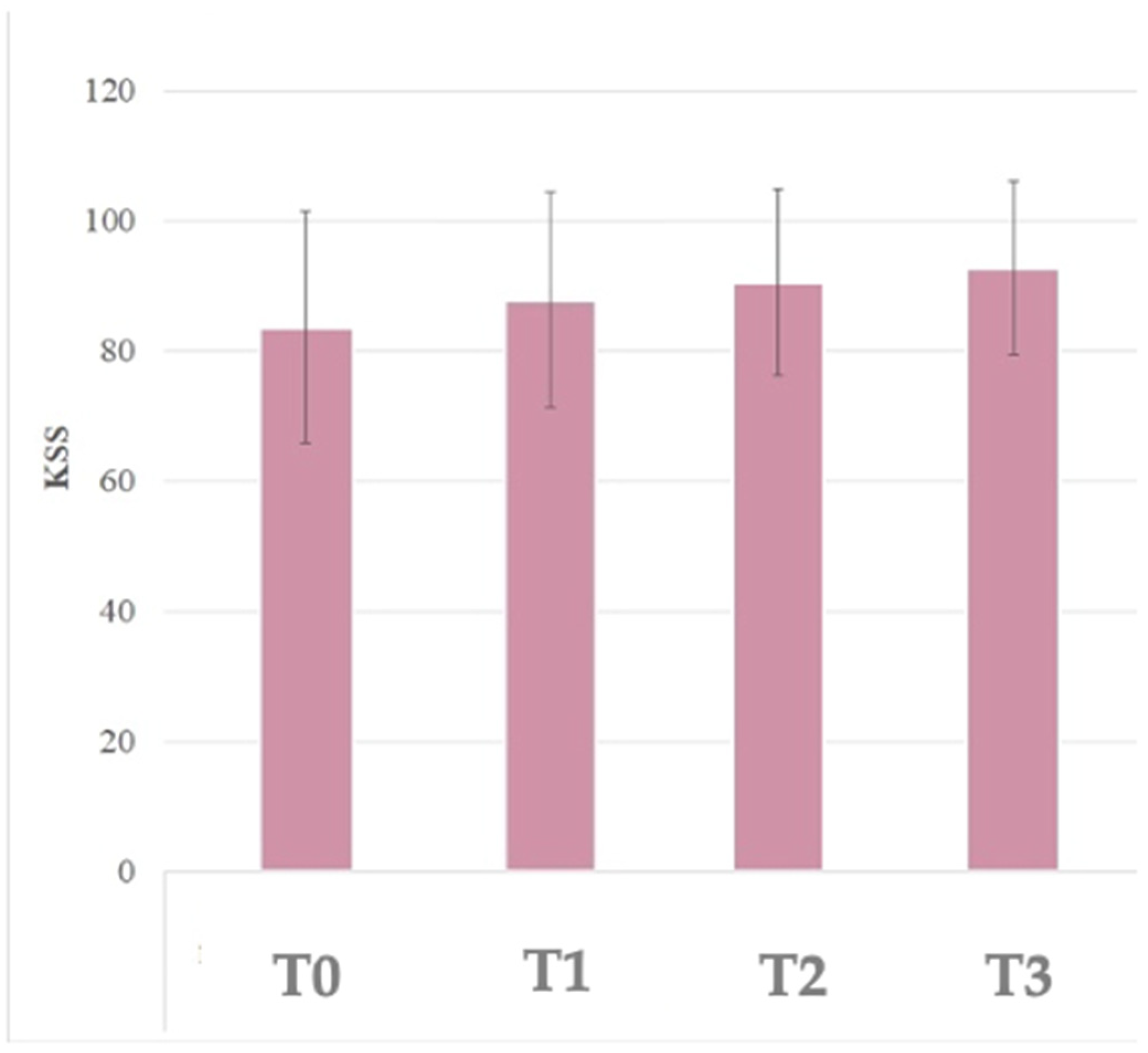
3.3. KSS
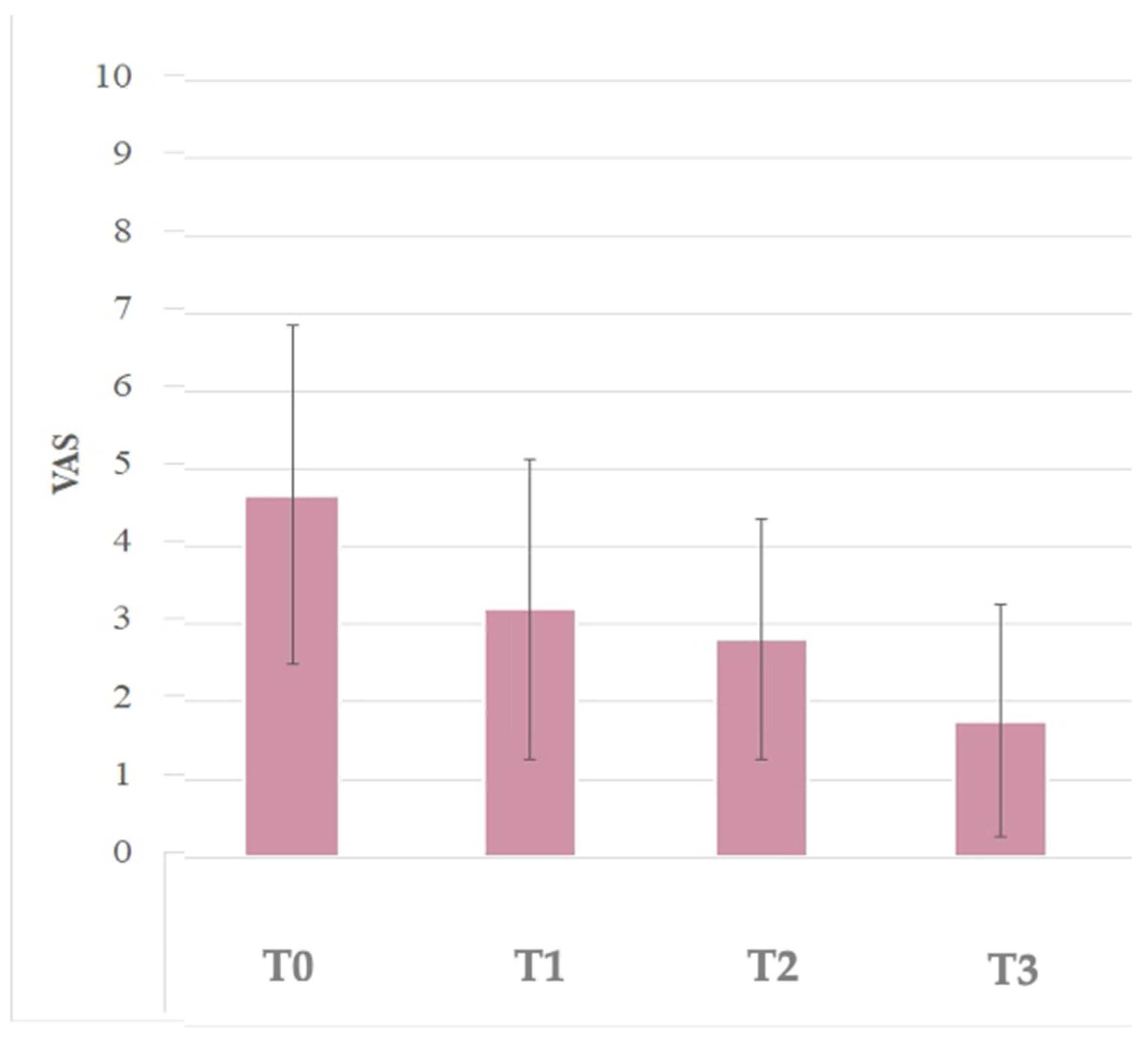
3.4. VAS
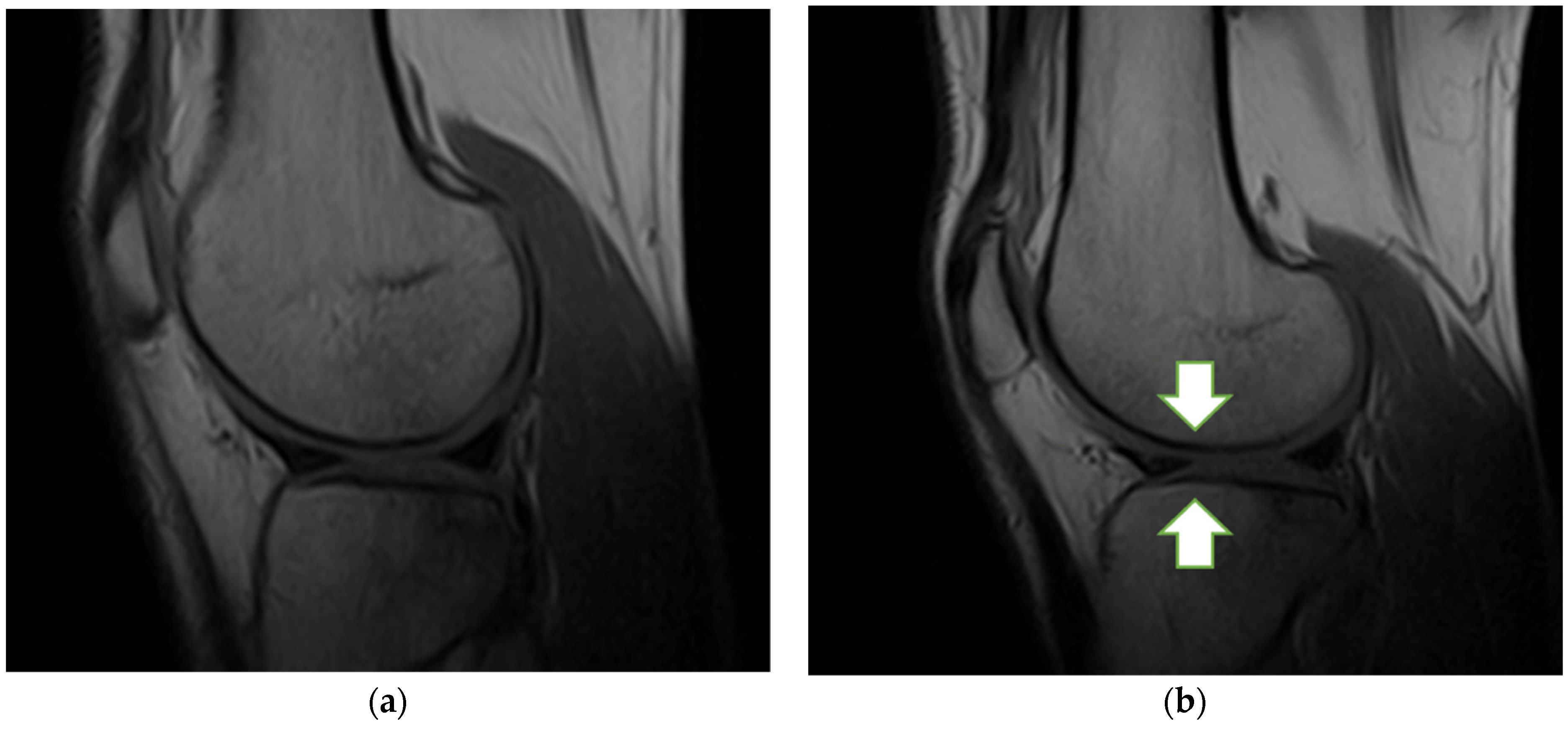
3.5. MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, W.-L.; Zhou, A.-G.; Zhang, H.; Zhang, J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy 2017, 33, 659–670.e1. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, S.; Wang, X.; Li, Z.; Guo, W. Role of platelet-rich plasma in the treatment of osteoarthritis: A meta-analysis. J. Int. Med. Res. 2020, 48, 1–10. [Google Scholar] [CrossRef]
- Paterson, K.L.; Nicholls, M.; Bennell, K.L.; Bates, D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet. Disord. 2016, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Ip, H.L.; Nath, D.K.; Sawleh, S.H.; Kabir, H.; Jahan, N. Regenerative Medicine for Knee Osteoarthritis—The Efficacy and Safety of Intra-Articular Platelet-Rich Plasma and Mesenchymal Stem Cells Injections: A Literature Review. Cureus 2020, 12, e10575. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg.—Ser. A 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Matada, M.S.; Holi, M.S.; Raman, R.; Jayaramu Suvarna, S.T. Visualization of Cartilage from Knee Joint Magnetic Resonance Images and Quantitative Assessment to Study the Effect of Age, Gender and Body Mass Index (BMI) in Progressive Osteoarthritis (OA). Curr. Med. Imaging 2019, 15, 565–572. [Google Scholar] [CrossRef]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Cattaneo, G.; De Caro, A.; Napoli, F.; Chiapale, D.; Trada, P.; Camera, A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 176. [Google Scholar] [CrossRef]
- Guillibert, C.; Charpin, C.; Raffray, M.; Benmenni, A.; Dehaut, F.X.; El Ghobeira, G.; Giorgi, R.; Magalon, J.; Arniaud, D. Single injection of high volume of autologous pure PRP provides a significant improvement in knee osteoarthritis: A prospective routine care study. Int. J. Mol. Sci. 2019, 20, 1327. [Google Scholar] [CrossRef] [Green Version]
- Raeissadat, S.A.; Hosseini, P.G.; Bahrami, M.H.; Roghani, R.S.; Fathi, M.; Ahangar, A.G.; Darvish, M. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet. Disord. 2021, 22, 134. [Google Scholar] [CrossRef]
- Antonelli, A.; Giudice, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef]
- Albilia, J.; Herrera-Vizcaíno, C.; Weisleder, H.; Choukroun, J.; Ghanaati, S. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: Preliminary results. Cranio 2020, 38, 292–304. [Google Scholar] [CrossRef]
- Samara, O.; Al-Ajlouni, J.; Al-Najar, M.; Saleh, M.; Al-Ryalat, N.; Gharaibeh, A.; Al Khayat, O.W.; Awidi, M.; Dweik, M.; Awidi, A. Intra-Articular Autologous Platelet Lysates Produce Positive Mri Structural Changes in Early and Intermediate Knee Osteoarthrosis. Pak. J. Radiol. 2017, 27, 14–18. [Google Scholar]
- Rahimzadeh, P.; Imani, F.; Faiz, S.-H.; Alebouyeh, M.-R.; Azad-Ehyaei, D.; Bahari, L.; Memarian, A.; Kim, K.-H. Adding Intra-Articular Growth Hormone to Platelet Rich Plasma under Ultrasound Guidance in Knee Osteoarthritis: A Comparative Double-Blind Clinical Trial. Anesthesiol. Pain Med. 2016, 6, e41719. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Chang, S.; Qian, L.; Wang, Y.; Yang, M. Extracorporeal shock wave therapy for isokinetic muscle strength around the knee joint in athletes with patellar tendinopathy. J. Sports Med. Phys. Fit. 2019, 59, 822–827. [Google Scholar] [CrossRef]
- Gobbi, A.; Lad, D.; Karnatzikos, G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2170–2177. [Google Scholar] [CrossRef]
- Asfaha, S.; Cenac, N.; Houle, S.; Altier, C.; Papez, M.D.; Nguyen, C.; Steinhoff, M.; Chapman, K.; Zamponi, G.W.; Vergnolle, N. Protease-activated receptor-4: A novel mechanism of inflammatory pain modulation. J. Cereb. Blood Flow Metab. 2007, 150, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Moretti, L.; Notarnicola, A.; Ostuni, A.; Pesce, V.; Maccagnano, G.; Coviello, M.; Covelli, I.; Franchini, A.; Bianchi, F.P.; Moretti, B. Umbilical cord blood platelet-rich plasma injections for epicondylitis treatment: A prospective clinical study. J. Biol. Regul. Homeost. Agents 2021, 35, 1337–1342. [Google Scholar]
- Notarnicola, A.; Tamma, R.; Moretti, L.; Panella, A.; Dell’Endice, S.; Zallone, A.; Moretti, B. Effect of shock wave treatment on platelet-rich plasma added to osteoblast cultures. Ultrasound Med. Biol. 2011, 37, 160–168. [Google Scholar] [CrossRef]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef]
- Migliorini, F.; Driessen, A.; Quack, V.; Sippel, N.; Cooper, B.; El Mansy, Y.; Tingart, M.; Eschweiler, J. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: A Bayesian network meta-analysis. Arch. Orthop. Trauma. Surg. 2020, 141, 1473–1490. [Google Scholar] [CrossRef]
- Vilchez-Cavazos, F.; Millan-Alanis, J.M.; Blázquez-Saldaña, J.; Álvarez-Villalobos, N.; Peña-Martínez, V.M.; Acosta-Olivo, C.A.; Simental-Mendía, M. Comparison of the Clinical Effectiveness of Single Versus Multiple Injections of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2020, 25, 1159–1167. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2017, 29, 48–55. [Google Scholar] [CrossRef]
- Huang, P.H.; Wang, C.J.; Chou, W.Y.; Wang, J.W.; Ko, J.Y. Short-term clinical results of intra-articular PRP injections for early osteoarthritis of the knee. Int. J. Surg. 2017, 42, 117–122. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Buendía-López, D.; Medina-Quirós, M.; Fernández-Villacañas Marín, M.Á. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: A randomized controlled trial. J. Orthop. Traumatol. 2018, 19, 3. [Google Scholar] [CrossRef]

| Participant’s Variables | |
|---|---|
| Age (year) | |
| Mean ± SD | 59.06 ± 8.78 |
| Range | 40–81 |
| Gender | |
| Male. n (%) | 72 (47%) |
| Female. n (%) | 81 (53%) |
| BMI (kg/m2) | |
| Mean ± SD | 25.4 ± 3.9 |
| Range | 21.5–29.3 |
| Side | |
| Left. n (%) | 67 (43.8%) |
| Right. n (%) | 86 (56.2%) |
| Kellgren-Lawrence Grade | |
| Grade 1. n (%) | 27 (17.7%) |
| Grade 2. n (%) | 58 (37.9%) |
| Grade 3. n (%) | 68 (44.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, L.; Maccagnano, G.; Coviello, M.; Cassano, G.D.; Franchini, A.; Laneve, A.; Moretti, B. Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study. J. Clin. Med. 2022, 11, 2640. https://doi.org/10.3390/jcm11092640
Moretti L, Maccagnano G, Coviello M, Cassano GD, Franchini A, Laneve A, Moretti B. Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study. Journal of Clinical Medicine. 2022; 11(9):2640. https://doi.org/10.3390/jcm11092640
Chicago/Turabian StyleMoretti, Lorenzo, Giuseppe Maccagnano, Michele Coviello, Giuseppe D. Cassano, Andrea Franchini, Andrea Laneve, and Biagio Moretti. 2022. "Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study" Journal of Clinical Medicine 11, no. 9: 2640. https://doi.org/10.3390/jcm11092640
APA StyleMoretti, L., Maccagnano, G., Coviello, M., Cassano, G. D., Franchini, A., Laneve, A., & Moretti, B. (2022). Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study. Journal of Clinical Medicine, 11(9), 2640. https://doi.org/10.3390/jcm11092640








