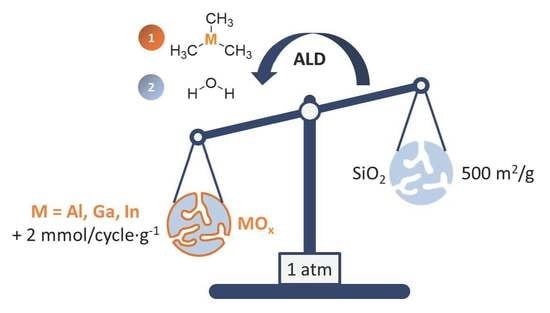
Synthesis of High Surface Area—Group 13—Metal Oxides via Atomic Layer Deposition on Mesoporous Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Atomic Layer Deposition of GaOx and InOx on SiO2
2.3. Characterization of the Materials
3. Results
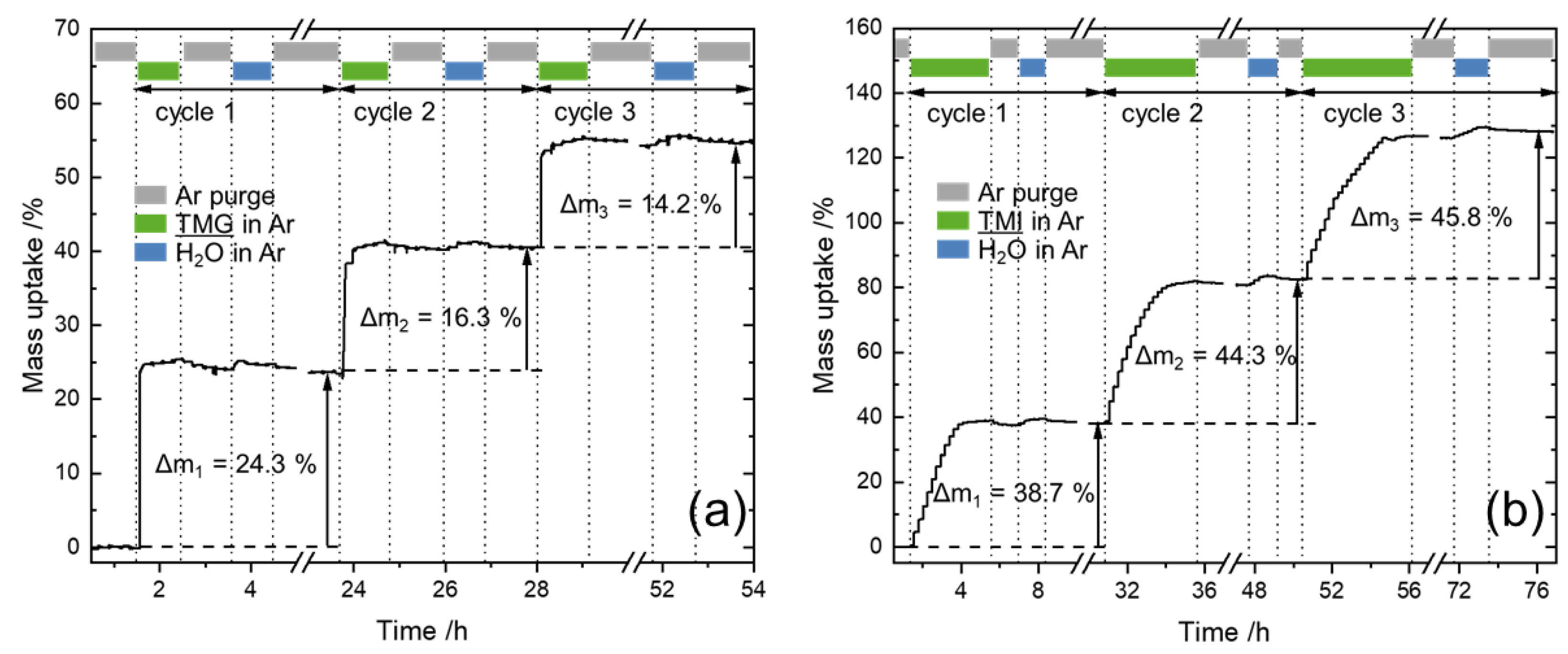
3.1. In-Situ Thermogravimetric Analysis
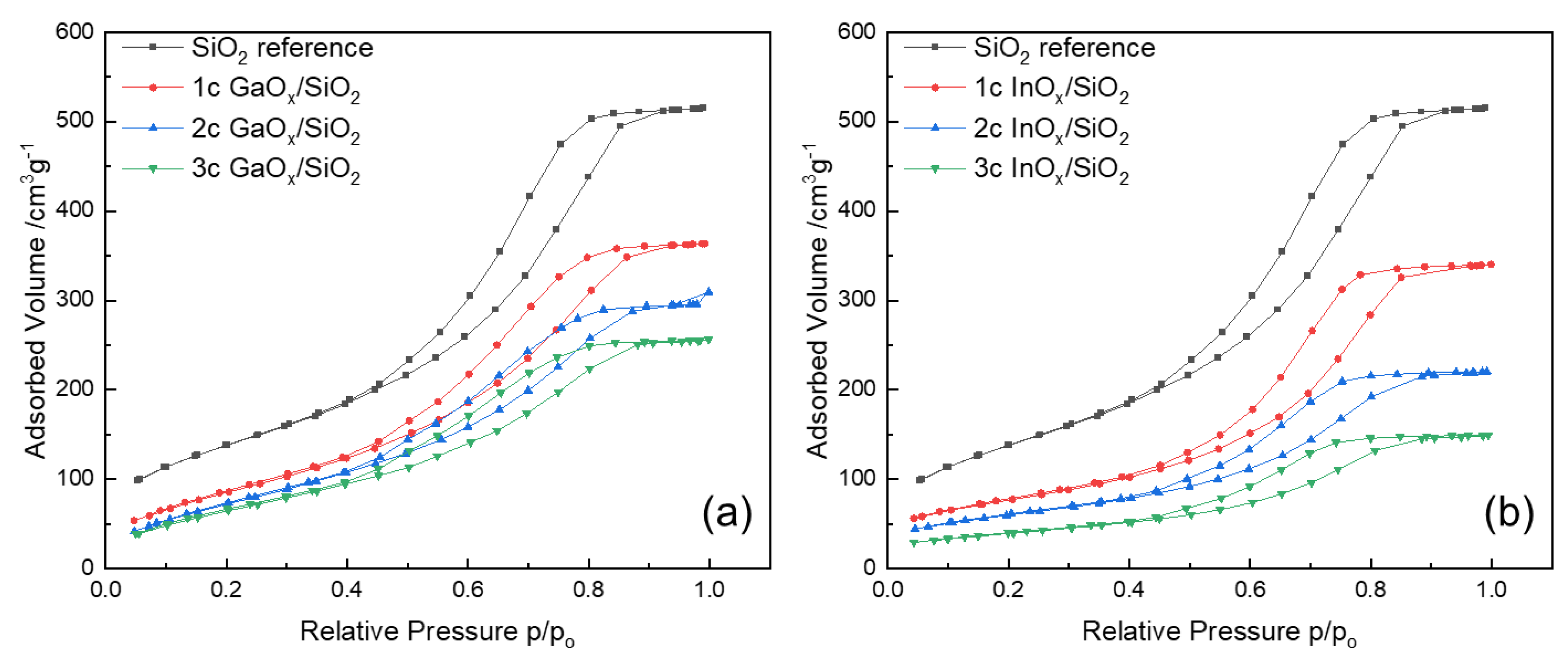
3.2. Effect of ALD on Surface Area and Pore Size
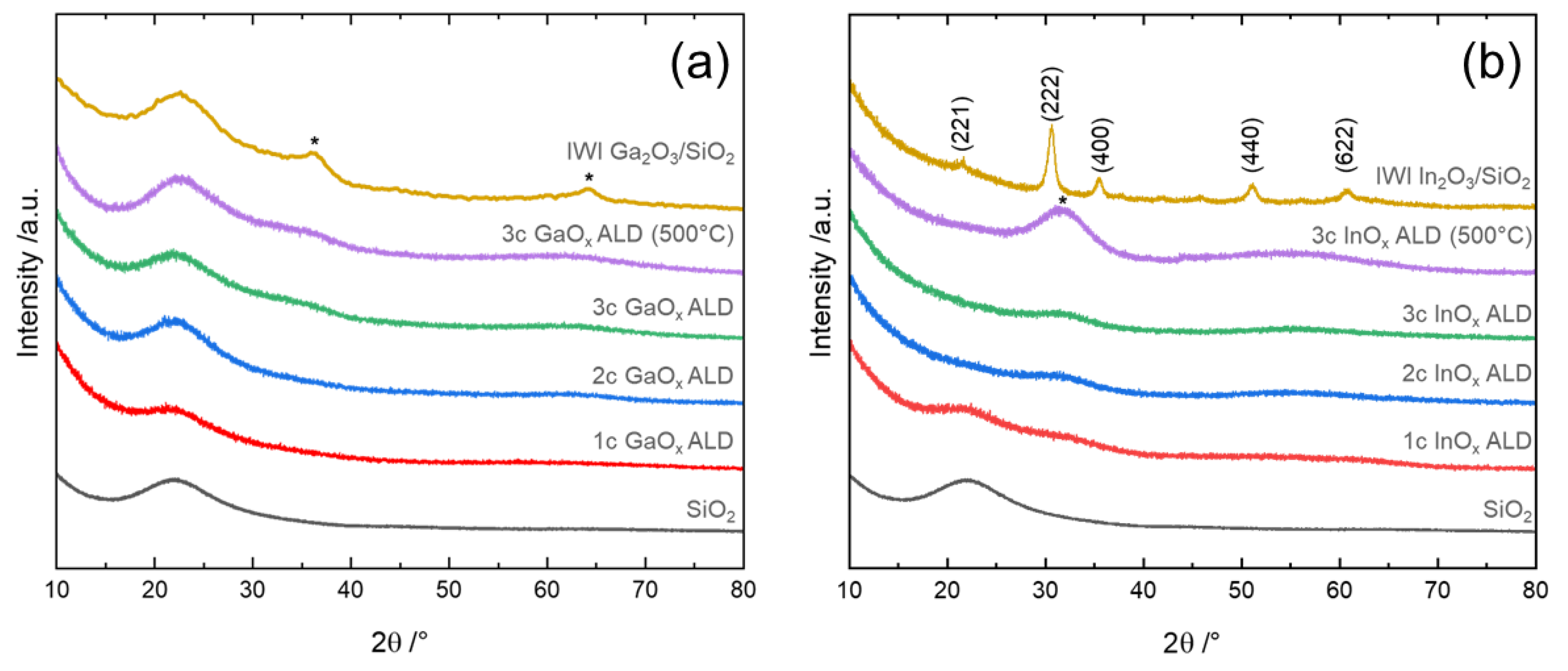
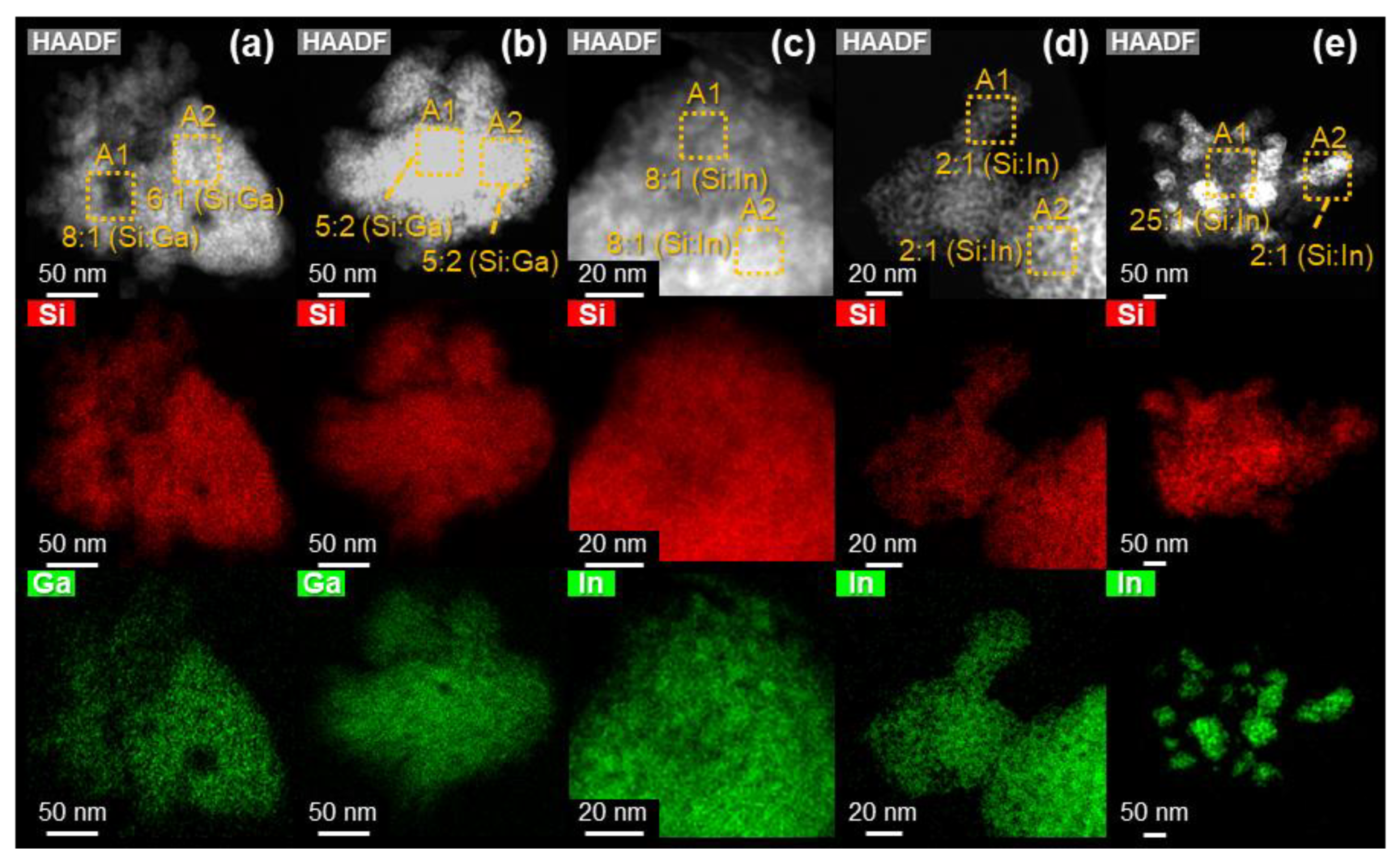
3.3. Investigation of the Formed Phase and Its Dispersion
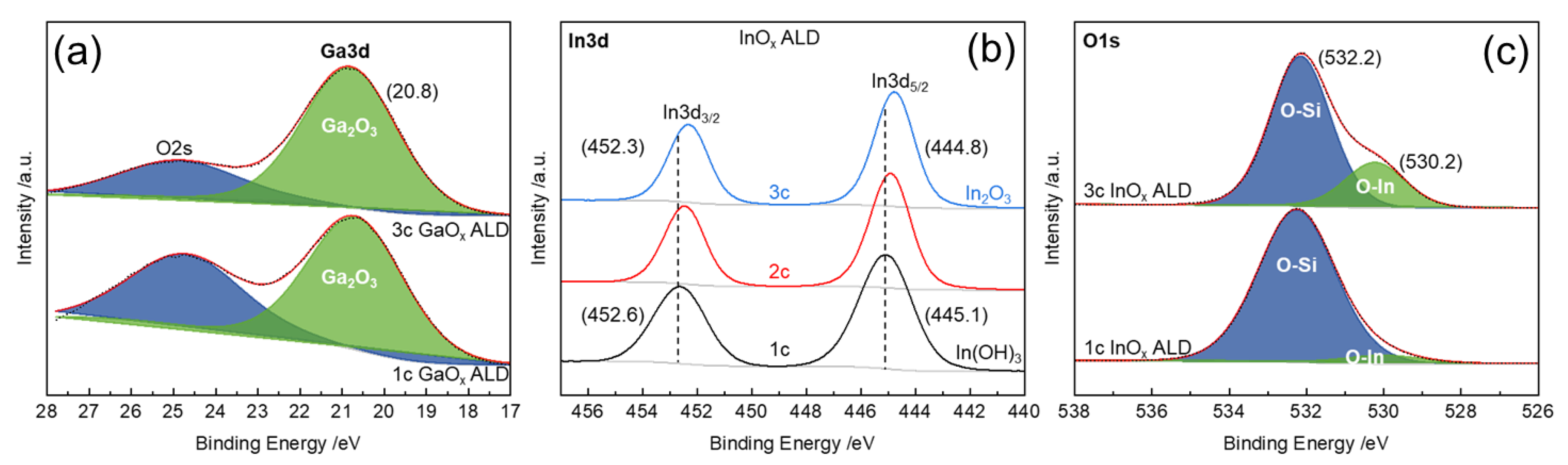
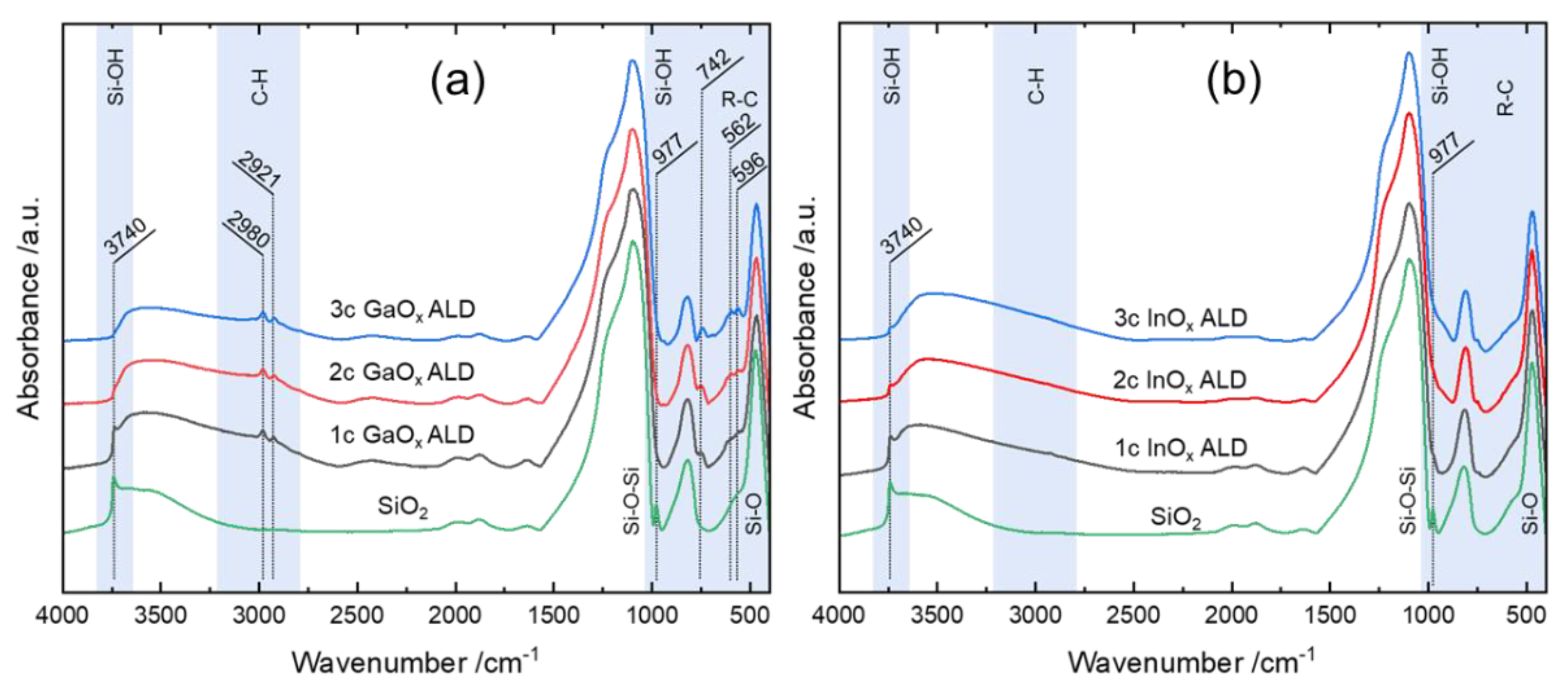
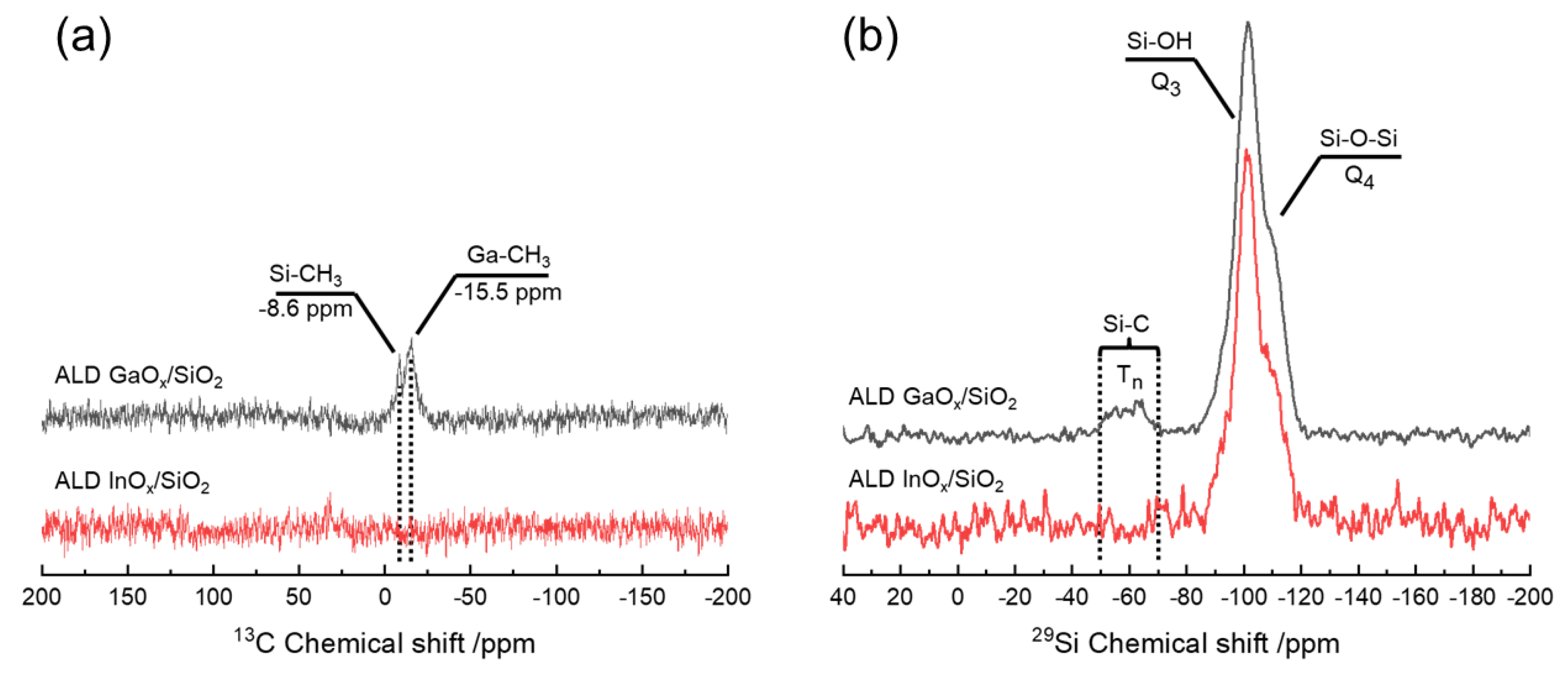
3.4. Determination of Ligand Implementation
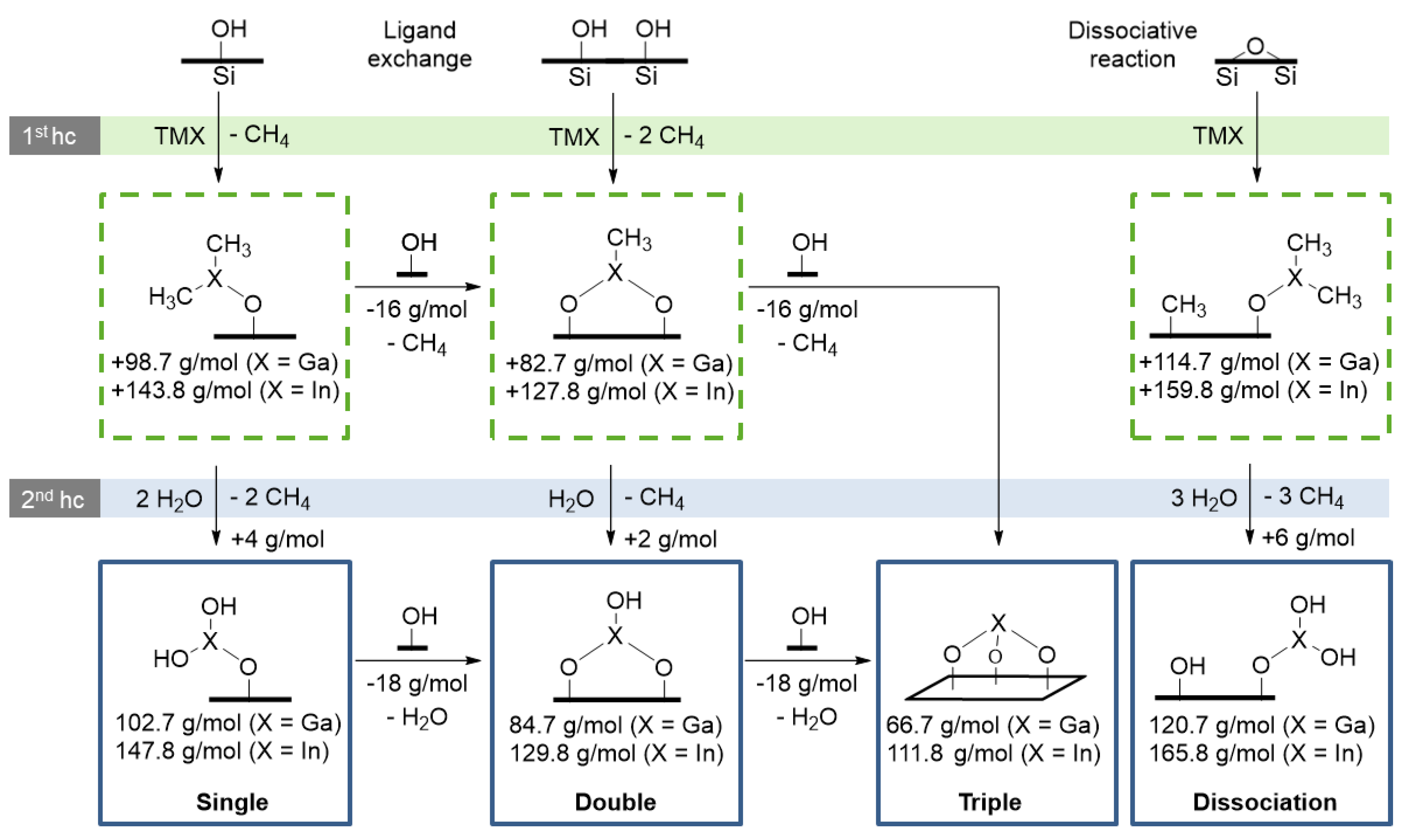
3.5. Decryption of the Growth Mechanism
3.6. Estimated ALD Oxide Layer Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katiyar, P.; Jin, C.; Narayan, R.J. Electrical Properties of Amorphous Aluminum Oxide Thin Films. Acta Mater. 2005, 53, 2617–2622. [Google Scholar] [CrossRef]
- Lin, D.G.; Vorobyeva, E.V.; Shapovalov, V.M. Influence of Chemically Inert Fillers on the Efficiency of Polyethylene Inhibition by Antioxidants. Russ. J. Appl. Chem. 2014, 87, 966–971. [Google Scholar] [CrossRef]
- Sinkó, K. Absorbability of Highly Porous Aluminum Oxide Ceramics. JMSE-A 2017, 7, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Almaev, A.V.; Chernikov, E.V.; Davletkildeev, N.A.; Sokolov, D.V. Oxygen Sensors Based on Gallium Oxide Thin Films with Addition of Chromium. Superlattices Microstruct. 2020, 139, 106392. [Google Scholar] [CrossRef]
- Garud, S.; Gampa, N.; Allen, T.G.; Kotipalli, R.; Flandre, D.; Batuk, M.; Hadermann, J.; Meuris, M.; Poortmans, J.; Smets, A.; et al. Surface Passivation of CIGS Solar Cells Using Gallium Oxide. Phys. Status Solidi A 2018, 215, 1700826. [Google Scholar] [CrossRef]
- Wellenius, P.; Suresh, A.; Foreman, J.V.; Everitt, H.O.; Muth, J.F. A Visible Transparent Electroluminescent Europium Doped Gallium Oxide Device. Mater. Sci. Eng. B 2008, 146, 252–255. [Google Scholar] [CrossRef]
- Qin, M.; Ma, J.; Ke, W.; Qin, P.; Lei, H.; Tao, H.; Zheng, X.; Xiong, L.; Liu, Q.; Chen, Z.; et al. Perovskite Solar Cells Based on Low-Temperature Processed Indium Oxide Electron Selective Layers. ACS Appl. Mater. Interfaces 2016, 8, 8460–8466. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Shen, G.; Chen, H.; Ha, Y.; Wu, C.; Sukcharoenchoke, S.; Fu, Y.; Liu, J.; Facchetti, A.; Marks, T.J.; et al. High-Performance Single-Crystalline Arsenic-Doped Indium Oxide Nanowires for Transparent Thin-Film Transistors and Active Matrix Organic Light-Emitting Diode Displays. ACS Nano 2009, 3, 3383–3390. [Google Scholar] [CrossRef]
- Charlet, E.; Grelet, E.; Brettes, P.; Bock, H.; Saadaoui, H.; Cisse, L.; Destruel, P.; Gherardi, N.; Seguy, I. Ultrathin Films of Homeotropically Aligned Columnar Liquid Crystals on Indium Tin Oxide Electrodes. Appl. Phys. Lett. 2008, 92, 24107. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Castro-Fernández, P.; Mance, D.; Liu, C.; Moroz, I.B.; Abdala, P.M.; Pidko, E.A.; Copéret, C.; Fedorov, A.; Müller, C.R. Propane Dehydrogenation on Ga2O3 -Based Catalysts: Contrasting Performance with Coordination Environment and Acidity of Surface Sites. ACS Catal. 2021, 11, 907–924. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. Engl. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Somorjai, G.A.; Li, Y. Introduction to Surface Chemistry and Catalysis, 2nd ed.; Wiley: Oxford, UK, 2010; ISBN 978-0-470-50823-7. [Google Scholar]
- Segawa, M.; Sato, S.; Kobune, M.; Sodesawa, T.; Kojima, T.; Nishiyama, S.; Ishizawa, N. Vapor-Phase Catalytic Reactions of Alcohols over Bixbyite Indium Oxide. J. Mol. Catal. A Chem. 2009, 310, 166–173. [Google Scholar] [CrossRef]
- Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of Propane to Propene over Different Polymorphs of Gallium Oxide. J. Catal. 2005, 232, 143–151. [Google Scholar] [CrossRef]
- Pan, B.; Yuan, G.; Zhao, X.; Han, N.; Huang, Y.; Feng, K.; Cheng, C.; Zhong, J.; Zhang, L.; Wang, Y.; et al. Highly Dispersed Indium Oxide Nanoparticles Supported on Carbon Nanorods Enabling Efficient Electrochemical CO2 Reduction. Small Sci. 2021, 1, 2100029. [Google Scholar] [CrossRef]
- Shao, C.-T.; Lang, W.-Z.; Yan, X.; Guo, Y.-J. Catalytic Performance of Gallium Oxide Based-Catalysts for the Propane Dehydrogenation Reaction: Effects of Support and Loading Amount. RSC Adv. 2017, 7, 4710–4723. [Google Scholar] [CrossRef] [Green Version]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface Chemistry of Atomic Layer Deposition: A Case Study for the Trimethylaluminum/Water Process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Dingemans, G.; Seguin, R.; Engelhart, P.; de van Sanden, M.C.M.; Kessels, W.M.M. Silicon Surface Passivation by Ultrathin Al2O3 Films Synthesized by Thermal and Plasma Atomic Layer Deposition. Phys. Status Solidi RRL 2010, 4, 10–12. [Google Scholar] [CrossRef]
- Cheng, H.-M.; Wang, F.-M.; Chu, J.P.; Santhanam, R.; Rick, J.; Lo, S.-C. Enhanced Cycleabity in Lithium Ion Batteries: Resulting from Atomic Layer Depostion of Al2O3 or TiO2 on LiCoO2 Electrodes. J. Phys. Chem. C 2012, 116, 7629–7637. [Google Scholar] [CrossRef]
- Wilson, C.A.; Grubbs, R.K.; George, S.M. Nucleation and Growth during Al2O3 Atomic Layer Deposition on Polymers. Chem. Mater. 2005, 17, 5625–5634. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef] [Green Version]
- Sels, B.; Van de Voorde, M. Atomic Layer Deposition for Catalysis. In Nanotechnology in Catalysis; Wiley: Weinheim, Germany, 2017. [Google Scholar]
- Lee, W.-J.; Bera, S.; Shin, H.-C.; Hong, W.-P.; Oh, S.-J.; Wan, Z.; Kwon, S.-H. Atomic Layer Deposition: Uniform and Size-Controlled Synthesis of Pt Nanoparticle Catalyst by Fluidized Bed Reactor Atomic Layer Deposition for PEMFCs. Adv. Mater. Interfaces 2019, 6, 1970133. [Google Scholar] [CrossRef] [Green Version]
- Mackus, A.J.M.; Weber, M.J.; Thissen, N.F.W.; Garcia-Alonso, D.; Vervuurt, R.H.J.; Assali, S.; Bol, A.A.; Verheijen, M.A.; Kessels, W.M.M. Atomic Layer Deposition of Pd and Pt Nanoparticles for Catalysis: On the Mechanisms of Nanoparticle Formation. Nanotechnology 2016, 27, 34001. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Qin, L.; Li, J.; Zhao, F.; Feng, H. Atomic Layer Deposition of Iron Oxide on Reduced Graphene Oxide and Its Catalytic Activity in the Thermal Decomposition of Ammonium Perchlorate. Appl. Surf. Sci. 2018, 451, 155–161. [Google Scholar] [CrossRef]
- Geerts, L.; Ramachandran, R.K.; Dendooven, J.; Radhakrishnan, S.; Seo, J.W.; Detavernier, C.; Martens, J.; Sree, S.P. Creation of Gallium Acid and Platinum Metal Sites in Bifunctional Zeolite Hydroisomerization and Hydrocracking Catalysts by Atomic Layer Deposition. Catal. Sci. Technol. 2020, 10, 1778–1788. [Google Scholar] [CrossRef]
- Yan, H.; He, K.; Samek, I.A.; Jing, D.; Nanda, M.G.; Stair, P.C.; Notestein, J.M. Tandem In2O3-Pt/Al2O3 Catalyst for Coupling of Propane Dehydrogenation to Selective H2 Combustion. Science 2021, 371, 1257–1260. [Google Scholar] [CrossRef]
- Lu, Z.; Tracy, R.W.; Abrams, M.L.; Nicholls, N.L.; Barger, P.T.; Li, T.; Stair, P.C.; Dameron, A.A.; Nicholas, C.P.; Marshall, C.L. Atomic Layer Deposition Overcoating Improves Catalyst Selectivity and Longevity in Propane Dehydrogenation. ACS Catal. 2020, 10, 13957–13967. [Google Scholar] [CrossRef]
- Ingale, P.; Guan, C.; Kraehnert, R.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Design of an Active and Stable Catalyst for Dry Reforming of Methane via Molecular Layer Deposition. Catal. Today 2021, 362, 47–54. [Google Scholar] [CrossRef]
- Ingale, P.; Knemeyer, K.; Preikschas, P.; Ye, M.; Geske, M.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Design of PtZn Nanoalloy Catalysts for Propane Dehydrogenation through Interface Tailoring via Atomic Layer Deposition. Catal. Sci. Technol. 2021, 11, 484–493. [Google Scholar] [CrossRef]
- Pallister, P.J.; Buttera, S.C.; Barry, S.T. Quantitative Surface Coverage Calculations via Solid-State NMR for Thin Film Depositions: A Case Study for Silica and a Gallium Amidinate. J. Phys. Chem. C 2014, 118, 1618–1627. [Google Scholar] [CrossRef]
- Allen, T.G.; Cuevas, A. Plasma Enhanced Atomic Layer Deposition of Gallium Oxide on Crystalline Silicon: Demonstration of Surface Passivation and Negative Interfacial Charge. Phys. Status Solidi RRL 2015, 9, 220–224. [Google Scholar] [CrossRef]
- Comstock, D.J.; Elam, J.W. Atomic Layer Deposition of Ga2O3 Films Using Trimethylgallium and Ozone. Chem. Mater. 2012, 24, 4011–4018. [Google Scholar] [CrossRef]
- Donmez, I.; Ozgit-Akgun, C.; Biyikli, N. Low Temperature Deposition of Ga2O3 Thin Films Using Trimethylgallium and Oxygen Plasma. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 01A110. [Google Scholar] [CrossRef]
- Kröncke, H.; Maudet, F.; Banerjee, S.; Albert, J.; Wiesner, S.; Deshpande, V.; Dubourdieu, C. Effect of O2 Plasma Exposure Time during Atomic Layer Deposition of Amorphous Gallium Oxide. J. Vac. Sci. Technol. A 2021, 39, 52408. [Google Scholar] [CrossRef]
- Rafie Borujeny, E.; Sendetskyi, O.; Fleischauer, M.D.; Cadien, K.C. Low Thermal Budget Heteroepitaxial Gallium Oxide Thin Films Enabled by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2020, 12, 44225–44237. [Google Scholar] [CrossRef]
- Shih, H.-Y.; Chu, F.-C.; Das, A.; Lee, C.-Y.; Chen, M.-J.; Lin, R.-M. Atomic Layer Deposition of Gallium Oxide Films as Gate Dielectrics in AlGaN/GaN Metal-Oxide-Semiconductor High-Electron-Mobility Transistors. Nanoscale Res. Lett. 2016, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Chung, K.-B.; Park, J.-S. Low Temperature Ga2O3 Atomic Layer Deposition Using Gallium Tri-Isopropoxide and Water. Thin Solid Film. 2013, 546, 31–34. [Google Scholar] [CrossRef]
- Mizutani, F.; Higashi, S.; Inoue, M.; Nabatame, T. Atomic Layer Deposition of High Purity Ga2O3 Films Using Liquid Pentamethylcyclopentadienyl Gallium and Combinations of H2O and O2 Plasma. J. Vac. Sci. Technol. A 2020, 38, 22412. [Google Scholar] [CrossRef]
- O’Donoghue, R.; Rechmann, J.; Aghaee, M.; Rogalla, D.; Becker, H.-W.; Creatore, M.; Wieck, A.D.; Devi, A. Low Temperature Growth of Gallium Oxide Thin Films via Plasma Enhanced Atomic Layer Deposition. Dalton Trans. 2017, 46, 16551–16561. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jung, E.A.; Mun, G.; Agbenyeke, R.E.; Park, B.K.; Park, J.-S.; Son, S.U.; Jeon, D.J.; Park, S.-H.K.; Chung, T.-M.; et al. Low-Temperature Growth of Indium Oxide Thin Film by Plasma-Enhanced Atomic Layer Deposition Using Liquid Dimethyl (N-ethoxy-2,2-dimethylpropanamido) indium for High-Mobility Thin Film Transistor Application. ACS Appl. Mater. Interfaces 2016, 8, 26924–26931. [Google Scholar] [CrossRef]
- Asikainen, T.; Ritala, M.; Leskelä, M. Atomic Layer Deposition Growth of Zirconium Doped In2O3 Films. Thin Solid Films 2003, 440, 152–154. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kwon, J.-Y.; Lee, J.I.; Kim, K.-B. Self-Limiting Film Growth of Transparent Conducting In2O3 by Atomic Layer Deposition using Trimethylindium and Water Vapor. J. Phys. Chem. C 2011, 115, 15384–15389. [Google Scholar] [CrossRef]
- Maeng, W.J.; Choi, D.; Park, J.; Park, J.-S. Indium Oxide Thin Film Prepared by Low Temperature Atomic Layer Deposition Using Liquid Precursors and Ozone Oxidant. J. Alloys Compd. 2015, 649, 216–221. [Google Scholar] [CrossRef]
- Mane, A.U.; Allen, A.J.; Kanjolia, R.K.; Elam, J.W. Indium Oxide Thin Films by Atomic Layer Deposition Using Trimethylindium and Ozone. J. Phys. Chem. C 2016, 120, 9874–9883. [Google Scholar] [CrossRef]
- Salami, H.; Uy, A.; Vadapalli, A.; Grob, C.; Dwivedi, V.; Adomaitis, R.A. Atomic Layer Deposition of Ultrathin Indium Oxide and Indium Tin Oxide Films Using a Trimethylindium, Tetrakis(dimethylamino)tin, and Ozone Precursor System. J. Vac. Sci. Technol. A 2019, 37, 10905. [Google Scholar] [CrossRef]
- Elam, J.W.; Libera, J.A.; Hryn, J.N. Indium Oxide ALD Using Cyclopentadienyl Indium and Mixtures of H2O and O2. ECS Trans. 2011, 41, 147–155. [Google Scholar] [CrossRef]
- Libera, J.A.; Hryn, J.N.; Elam, J.W. Indium Oxide Atomic Layer Deposition Facilitated by the Synergy between Oxygen and Water. Chem. Mater. 2011, 23, 2150–2158. [Google Scholar] [CrossRef]
- Han, J.H.; Jung, E.A.; Kim, H.Y.; Da Kim, H.; Park, B.K.; Park, J.-S.; Son, S.U.; Chung, T.-M. Atomic Layer Deposition of Indium Oxide Thin Film from a Liquid Indium Complex Containing 1-Dimethylamino-2-Methyl-2-Propoxy Ligands. Appl. Surf. Sci. 2016, 383, 1–8. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, H.-M.; Shao, Y.; Zhu, B.; Liu, W.-J.; Ding, S.-J.; Zhang, D.W. Atomic-Layer-Deposition of Indium Oxide Nano-films for Thin-Film Transistors. Nanoscale Res. Lett. 2018, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Elam, J.W.; Martinson, A.B.F.; Pellin, M.J.; Hupp, J.T. Atomic Layer Deposition of In2O3 Using Cyclopentadienyl Indium: A New Synthetic Route to Transparent Conducting Oxide Films. Chem. Mater. 2006, 18, 3571–3578. [Google Scholar] [CrossRef]
- Kim, D.; Nam, T.; Park, J.; Gatineau, J.; Kim, H. Growth Characteristics and Properties of Indium Oxide and Indium-Doped Zinc Oxide by Atomic Layer Deposition. Thin Solid Films 2015, 587, 83–87. [Google Scholar] [CrossRef]
- Nilsen, O.; Balasundaraprabhu, R.; Monakhov, E.V.; Muthukumarasamy, N.; Fjellvåg, H.; Svensson, B.G. Thin Films of In2O3 by Atomic Layer Deposition using In(acac)3. Thin Solid Films 2009, 517, 6320–6322. [Google Scholar] [CrossRef]
- Ramachandran, R.K.; Dendooven, J.; Poelman, H.; Detavernier, C. Low Temperature Atomic Layer Deposition of Crystalline In2O3 Films. J. Phys. Chem. C 2015, 119, 11786–11791. [Google Scholar] [CrossRef]
- Sheng, J.; Choi, D.; Lee, S.-H.; Park, J.; Park, J.-S. Performance Modulation of Transparent ALD Indium Oxide Films on Flexible Substrates: Transition between Metal-like Conductor and High Performance Semiconductor States. J. Mater. Chem. C 2016, 4, 7571–7576. [Google Scholar] [CrossRef]
- Yeom, H.-I.; Ko, J.B.; Mun, G.; Park, S.-H.K. High Mobility Polycrystalline Indium Oxide Thin-Film Transistors by Means of Plasma-Enhanced Atomic Layer Deposition. J. Mater. Chem. C 2016, 4, 6873–6880. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.S.; Raya, S.S.; Shong, B. Mechanistic Investigation on Thermal Atomic Layer Deposition of Group 13 Oxides. J. Phys. Chem. C 2020, 124, 17121–17134. [Google Scholar] [CrossRef]
- Singh, H.; Coburn, J.W.; Graves, D.B. Recombination Coefficients of O and N Radicals on Stainless Steel. J. Appl. Phys. 2000, 88, 3748–3755. [Google Scholar] [CrossRef]
- Schindler, P.; Logar, M.; Provine, J.; Prinz, F.B. Enhanced Step Coverage of TiO2 Deposited on High Aspect Ratio Surfaces by Plasma-Enhanced Atomic Layer Deposition. Langmuir 2015, 31, 5057–5062. [Google Scholar] [CrossRef]
- Lim, K.-B.; Lee, D.-C. Surface Modification of Glass and Glass Fibres by Plasma Surface Treatment. Surf. Interface Anal. 2004, 36, 254–258. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Giannazzo, F.; Nicotra, G.; Cora, I.; Gueorguiev, G.K.; Persson, P.O.; Pécz, B. Material Proposal for 2D Indium Oxide. Appl. Surf. Sci. 2021, 548, 149275. [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D Structures of Indium Oxide of Different Stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Knemeyer, K.; Baumgarten, R.; Ingale, P.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Toolbox for Atomic Layer Deposition Process Development on High Surface Area Powders. Rev. Sci. Instrum. 2021, 92, 25115. [Google Scholar] [CrossRef] [PubMed]
- Onn, T.; Küngas, R.; Fornasiero, P.; Huang, K.; Gorte, R. Atomic Layer Deposition on Porous Materials: Problems with Conventional Approaches to Catalyst and Fuel Cell Electrode Preparation. Inorganics 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Ingale, P.; Knemeyer, K.; Piernavieja Hermida, M.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis. Nanomaterials 2020, 10, 981. [Google Scholar] [CrossRef]
- Strempel, V.E.; Knemeyer, K.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Investigating the Trimethylaluminium/Water ALD Process on Mesoporous Silica by In Situ Gravimetric Monitoring. Nanomaterials 2018, 8, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knemeyer, K.; Piernavieja Hermida, M.; Ingale, P.; Schmidt, J.; Kröhnert, J.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Mechanistic Studies of Atomic Layer Deposition on Oxidation Catalysts—AlOx and POx Deposition. Phys. Chem. Chem. Phys. 2020, 22, 17999–18006. [Google Scholar] [CrossRef]
- Strempel, V.E.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Atomic Layer Deposition on Porous Powders with in Situ Gravimetric Monitoring in a Modular Fixed Bed Reactor Setup. Rev. Sci. Instrum. 2017, 88, 74102. [Google Scholar] [CrossRef]
- Lugmair, C.G.; Fujdala, K.L.; Tilley, T.D. New Tris(tert -butoxy)siloxy Complexes of Aluminum and Their Transformation to Homogeneous Aluminosilicate Materials via Low-Temperature Thermolytic Pathways. Chem. Mater. 2002, 14, 888–898. [Google Scholar] [CrossRef]
- Long, L.H.; Sackman, J.F. THe Heat of Formation and Physical Properties of Gallium Trimethyl. Trans. Faraday Soc. 1958, 54, 1797. [Google Scholar] [CrossRef]
- Shenai-Khatkhate, D.V.; DiCarlo, R.L.; Ware, R.A. Accurate Vapor Pressure Equation for Trimethylindium in OMVPE. J. Cryst. Growth 2008, 310, 2395–2398. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dendooven, J.; Goris, B.; Devloo-Casier, K.; Levrau, E.; Biermans, E.; Baklanov, M.R.; Ludwig, K.F.; van der Voort, P.; Bals, S.; Detavernier, C. Tuning the Pore Size of Ink-Bottle Mesopores by Atomic Layer Deposition. Chem. Mater. 2012, 24, 1992–1994. [Google Scholar] [CrossRef]
- Inoue, M.; Kimura, M.; Inui, T. Transparent Colloidal Solution of 2 nm Ceria Particles. Chem. Commun. 1999, 1999, 957–958. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Boulc’, F.; Knauth, P. The Big Problem of Small Particles: A Comparison of Methods for Determination of Particle Size in Nanocrystalline Anatase Powders. Chem. Mater. 2005, 17, 2378–2385. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Saraswat, H.; Chaudhary, S.; Parkash, J.; Shin, H.-J.; Chae, K.-H.; Won, S.-O. Nano-Structured Phases of Gallium Oxide (GaOOH, α-Ga2O3, β-Ga2O3, γ-Ga2O3, δ-Ga2O3, and ε-Ga2O3): Fabrication, Structural, and Electronic Structure Investigations. Int. Nano Lett. 2020, 10, 71–79. [Google Scholar] [CrossRef]
- Tan, S.; Gil, L.B.; Subramanian, N.; Sholl, D.S.; Nair, S.; Jones, C.W.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G. Catalytic Propane Dehydrogenation over In2O3–Ga2O3 Mixed Oxides. Appl. Catal. A Gen. 2015, 498, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K.; Lee, S.; Sohn, Y.; Pradhan, D. Biomolecule-assisted Synthesis of In(OH)3 Nanocubes and In2O3 Nanoparticles: Photocatalytic Degradation of Organic Contaminants and CO Oxidation. Nanotechnology 2015, 26, 485601. [Google Scholar] [CrossRef]
- Carli, R.; Bianchi, C.L. XPS Analysis of Gallium Oxides. Appl. Surf. Sci. 1994, 74, 99–102. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Alamé, S.; Esser, N.; Furthmüller, J.; Bechstedt, F.; Galazka, Z.; Skuridina, D.; Vogt, P. Near Valence-band Electronic Properties of Semiconducting β−Ga2O3 (100) Single Crystals. Phys. Rev. B 2015, 92, 195306. [Google Scholar] [CrossRef]
- Huang, C.; Mu, W.; Zhou, H.; Zhu, Y.; Xu, X.; Jia, Z.; Zheng, L.; Tao, X. Effect of OH− on Chemical Mechanical Polishing of β-Ga2O3 (100) Substrate using an Alkaline Slurry. RSC Adv. 2018, 8, 6544–6550. [Google Scholar] [CrossRef] [Green Version]
- Faur, M.; Faur, M.; Jayne, D.T.; Goradia, M.; Goradia, C. XPS Investigation of Anodic Oxides Grown on P-type InP. Surf. Interface Anal. 1990, 15, 641–650. [Google Scholar] [CrossRef]
- Miller, M.L.; Linton, W.R. X-ray Photoelectron Spectroscopy of Thermally Treated Silica (SiO2) Surfaces. Anal. Chem. 1985, 57, 2314–2319. [Google Scholar] [CrossRef]
- Hoch, L.B.; Wood, T.E.; O’Brien, P.G.; Liao, K.; Reyes, L.M.; Mims, C.A.; Ozin, G.A. The Rational Design of a Single-Component Photocatalyst for Gas-Phase CO2 Reduction Using Both UV and Visible Light. Adv. Sci. 2014, 1, 1400013. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, R.; Zhan, W.; Yuan, Y.; Wang, X.; Han, X.; Zhao, Y. Double-Shelled Hollow Rods Assembled from Nitrogen/Sulfur-Codoped Carbon Coated Indium Oxide Nanoparticles as Excellent Photocatalysts. Nat. Commun. 2019, 10, 2270. [Google Scholar] [CrossRef] [Green Version]
- El hadad, A.A.; Carbonell, D.; Barranco, V.; Jiménez-Morales, A.; Casal, B.; Galván, J.C. Preparation of SOL–Gel Hybrid Materials from γ-Methacryloxypropyltrimethoxysilane and Tetramethyl Orthosilicate: Study of the Hydrolysis and Condensation Reactions. Colloid. Polym. Sci. 2011, 289, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fu, S.; Fu, L.; Yang, H.; Chen, D. Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite. Minerals 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Chun, K.S.; Husseinsyah, S. Agrowaste-Based Composites from Cocoa Pod Husk and Polypropylene. J. Thermoplast. Compos. Mater. 2016, 29, 1332–1351. [Google Scholar] [CrossRef]
- Morrow, B.A.; McFarlane, R.A. Trimethylgallium Adsorbed on Silica and Its Reaction with Phosphine, Arsine, and Hydrogen Chloride: An Infrared and Raman Study. J. Phys. Chem. 1986, 90, 3192–3197. [Google Scholar] [CrossRef]
- Tubis, R.; Hamlett, B.; Lester, R.; Newman, C.G.; Ring, M.A. Formation of Dimethylgalliumoxy and Dichlorogalliumoxy Groups on Silica Surfaces and Catalysis by the Dichlorogalliumoxy Group. Inorg. Chem. 1979, 18, 3275–3276. [Google Scholar] [CrossRef]
- Lakomaa, E.-L.; Root, A.; Suntola, T. Surface Reactions in Al2O3 Growth from Trimethylaluminium and Water by Atomic Layer Epitaxy. Appl. Surf. Sci. 1996, 107, 107–115. [Google Scholar] [CrossRef]
- Maudoux, N.; Tan, E.; Hu, Y.; Roisnel, T.; Dorcet, V.; Carpentier, J.-F.; Sarazin, Y. Aluminium, Gallium and Indium Complexes Supported by a Chiral Phenolato-Prolinolato Dianionic Ligand. Main Group Met. Chem. 2016, 39, 131–143. [Google Scholar] [CrossRef]
- Metroke, T.; Wang, Y.; van Ooij, W.J.; Schaefer, D.W. Chemistry of Mixtures of Bis-[trimethoxysilylpropyl]amine and Vinyltriacetoxysilane: An NMR Analysis. J. Sol.-Gel. Sci. Technol. 2009, 51, 23–31. [Google Scholar] [CrossRef]
- Iliashevsky, O.; Rubinov, E.; Yagen, Y.; Gottlieb, M. Functionalization of Silica Surface with UV-Active Molecules by Multivalent Organosilicon Spacer. OJIC 2016, 06, 163–174. [Google Scholar] [CrossRef] [Green Version]
- López, T.D.-F.; González, A.F.; Del Reguero, Á.; Matos, M.; Díaz-García, M.E.; Badía-Laíño, R. Engineered Silica Nanoparticles as Additives in Lubricant Oils. Sci. Technol. Adv. Mater. 2015, 16, 55005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadzała-Kopciuch, R.; Kluska, M.; Wełniak, M.; Buszewski, B. Silicon Dioxide Surfaces with Aryl Interaction Sites for Chromatographic Applications. Mater. Chem. Phys. 2005, 89, 228–237. [Google Scholar] [CrossRef]
- Szwarc-Rzepka, K.; Ciesielczyk, F.; Jesionowski, T. Preparation and Physicochemical Properties of Functionalized Silica/Octamethacryl-Silsesquioxane Hybrid Systems. J. Nanomater. 2013, 2013, 674237. [Google Scholar] [CrossRef]
- Schütz, M.R.; Sattler, K.; Deeken, S.; Klein, O.; Adasch, V.; Liebscher, C.H.; Glatzel, U.; Senker, J.; Breu, J. Improvement of Thermal and Mechanical Properties of a Phenolic Resin Nanocomposite by in Situ Formation of Silsesquioxanes from a Molecular Precursor. J. Appl. Polym. Sci. 2010, 117, 2272–2277. [Google Scholar] [CrossRef]
- Kapusuz, D.; Durucan, C. Synthesis of DNA-encapsulated Silica Elaborated by Sol–Gel Routes. J. Mater. Res. 2013, 28, 175–184. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Han, J.H.; Park, B.K.; Chung, T.-M. Atomic Layer Deposition of Pure In2O3 Films for a Temperature Range of 200–300 °C using Heteroleptic Liquid In(DMAMP)2(OiPr) Precursor. Ceram. Int. 2020, 46, 3139–3143. [Google Scholar] [CrossRef]
- Hagleitner, D.R.; Menhart, M.; Jacobson, P.; Blomberg, S.; Schulte, K.; Lundgren, E.; Kubicek, M.; Fleig, J.; Kubel, F.; Puls, C.; et al. Bulk and Surface Characterization of In2O3 (001) Single Crystals. Phys. Rev. B 2012, 85, 115441. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.; Catlow, C.R.A. Structure, Stability and Work Functions of the Low Index Surfaces of Pure Indium Oxide and Sn-doped Indium Oxide (ITO) from Density Functional Theory. J. Mater. Chem. 2010, 20, 10438. [Google Scholar] [CrossRef]
- Geller, S. Crystal Structure of β-Ga2O3. J. Chem. Phys. 1960, 33, 676–684. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, C.; Zhang, Y.; Qi, C.; Li, H.; Wang, T.; Ma, G.; Liu, Y.; Dong, S.; Huo, M. Modulation of Electronic and Optical Properties by Surface Vacancies in Low-Dimensional β-Ga2O3. Phys. Chem. Chem. Phys. 2019, 21, 14745–14752. [Google Scholar] [CrossRef]
| AlOx/SiO2 [68] | GaOx/SiO2 | InOx/SiO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALD Cycles | Mass Up./% | Molar Up./mmol·g−1 | Mass Frac./% | Mass Up./% | Molar Up. /mmol·g−1 | Mass Frac./% | Mass Up./% | Molar Up. /mmol·g−1 | Mass Frac./% |
| 1 | +11.9 | +1.0 | 10.6 | +24.3 | +1.0 | 19.6 | +38.7 | +1.0 | 27.9 |
| 2 | +11.4 | +1.0 | 18.9 | +16.3 | +0.8 | 28.9 | +44.3 | +1.1 | 45.4 |
| 3 | +13.4 | +1.2 | 26.9 | +14.2 | +0.7 | 35.4 | +45.8 | +1.1 | 56.3 |
| Sum | +36.7 | +3.2 | 26.9 | +54.8 | +2.5 | 35.4 | +128.8 | +3.2 | 56.3 |
| Sample | Mass Frac./% | 1 M2O3 Frac./% | 2 M(OH)2 Frac./% | Mass Frac./% (ICP-OES) |
|---|---|---|---|---|
| 1c GaOx | 19.6 (GaOx) | 14.5 (Ga) | 13.1 (Ga) | 14.6 (Ga) |
| 3c GaOx | 35.4 (GaOx) | 26.3 (Ga) | 23.8 (Ga) | 26.0 (Ga) |
| 1c InOx | 27.9 (InOx) | 23.1 (In) | 21.5 (In) | 21.1 (In) |
| 3c InOx | 56.3 (InOx) | 46.6 (In) | 43.4 (In) | 48.5 (In) |
| AlOx/SiO2 [68] | GaOx/SiO2 | InOx/SiO2 | ||||
|---|---|---|---|---|---|---|
| ALD Cycles | SA/m2g−1 | PV/cm3g−1 | SA/m2g−1 | PV/cm3g−1 | SA/m2g−1 | PV/cm3g−1 |
| 0 | 505 | 0.79 | 505 | 0.79 | 505 | 0.79 |
| 1 | 435 | 0.66 | 336 | 0.57 | 277 | 0.55 |
| 2 | 383 | 0.55 | 296 | 0.46 | 216 | 0.34 |
| 3 | 337 | 0.47 | 259 | 0.39 | 142 | 0.23 |
| Sample [68] | Thickness/Å | Sample | Thickness/Å | Sample | Thickness/Å |
|---|---|---|---|---|---|
| 1c AlOx | 0.8 | 1c GaOx | 0.9 | 1c InOx | 1.1 |
| 2c AlOx | 1.6 | 2c GaOx | 1.6 | 2c InOx | 2.8 |
| 3c AlOx | 2.5 | 3c GaOx | 2.2 | 3c InOx | 4.6 |
| gpc | 0.8 | gpc | 0.7 | gpc | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgarten, R.; Ingale, P.; Knemeyer, K.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Synthesis of High Surface Area—Group 13—Metal Oxides via Atomic Layer Deposition on Mesoporous Silica. Nanomaterials 2022, 12, 1458. https://doi.org/10.3390/nano12091458
Baumgarten R, Ingale P, Knemeyer K, Naumann d’Alnoncourt R, Driess M, Rosowski F. Synthesis of High Surface Area—Group 13—Metal Oxides via Atomic Layer Deposition on Mesoporous Silica. Nanomaterials. 2022; 12(9):1458. https://doi.org/10.3390/nano12091458
Chicago/Turabian StyleBaumgarten, Robert, Piyush Ingale, Kristian Knemeyer, Raoul Naumann d’Alnoncourt, Matthias Driess, and Frank Rosowski. 2022. "Synthesis of High Surface Area—Group 13—Metal Oxides via Atomic Layer Deposition on Mesoporous Silica" Nanomaterials 12, no. 9: 1458. https://doi.org/10.3390/nano12091458
APA StyleBaumgarten, R., Ingale, P., Knemeyer, K., Naumann d’Alnoncourt, R., Driess, M., & Rosowski, F. (2022). Synthesis of High Surface Area—Group 13—Metal Oxides via Atomic Layer Deposition on Mesoporous Silica. Nanomaterials, 12(9), 1458. https://doi.org/10.3390/nano12091458