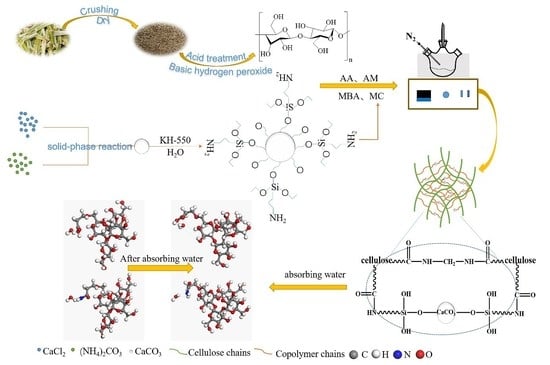
Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
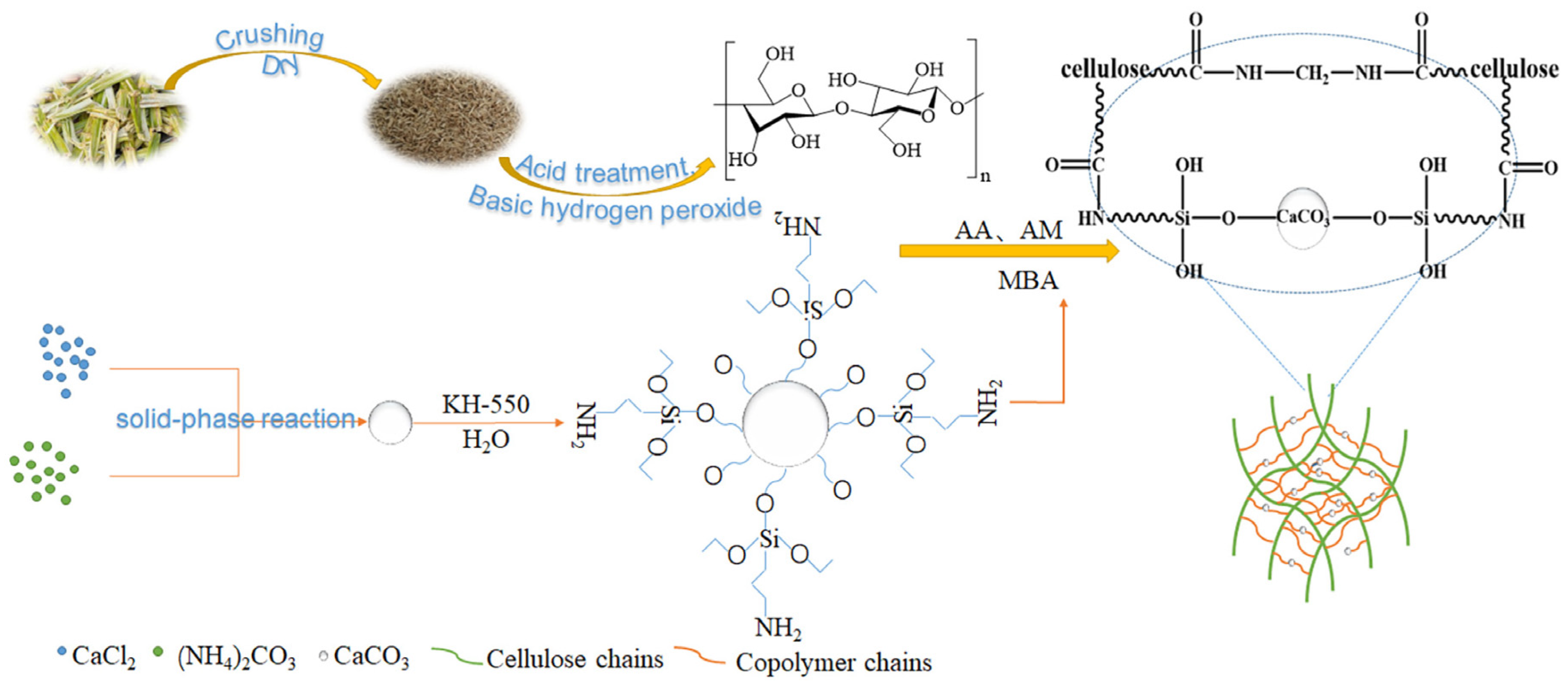
2.1.1. Preparation Method of CAAMC Graft Copolymer
2.1.2. Characterization Method
2.2. Measurement of Water Absorbency
2.3. Measurement of Water Retention in Soil
2.4. Computation Details
3. Results
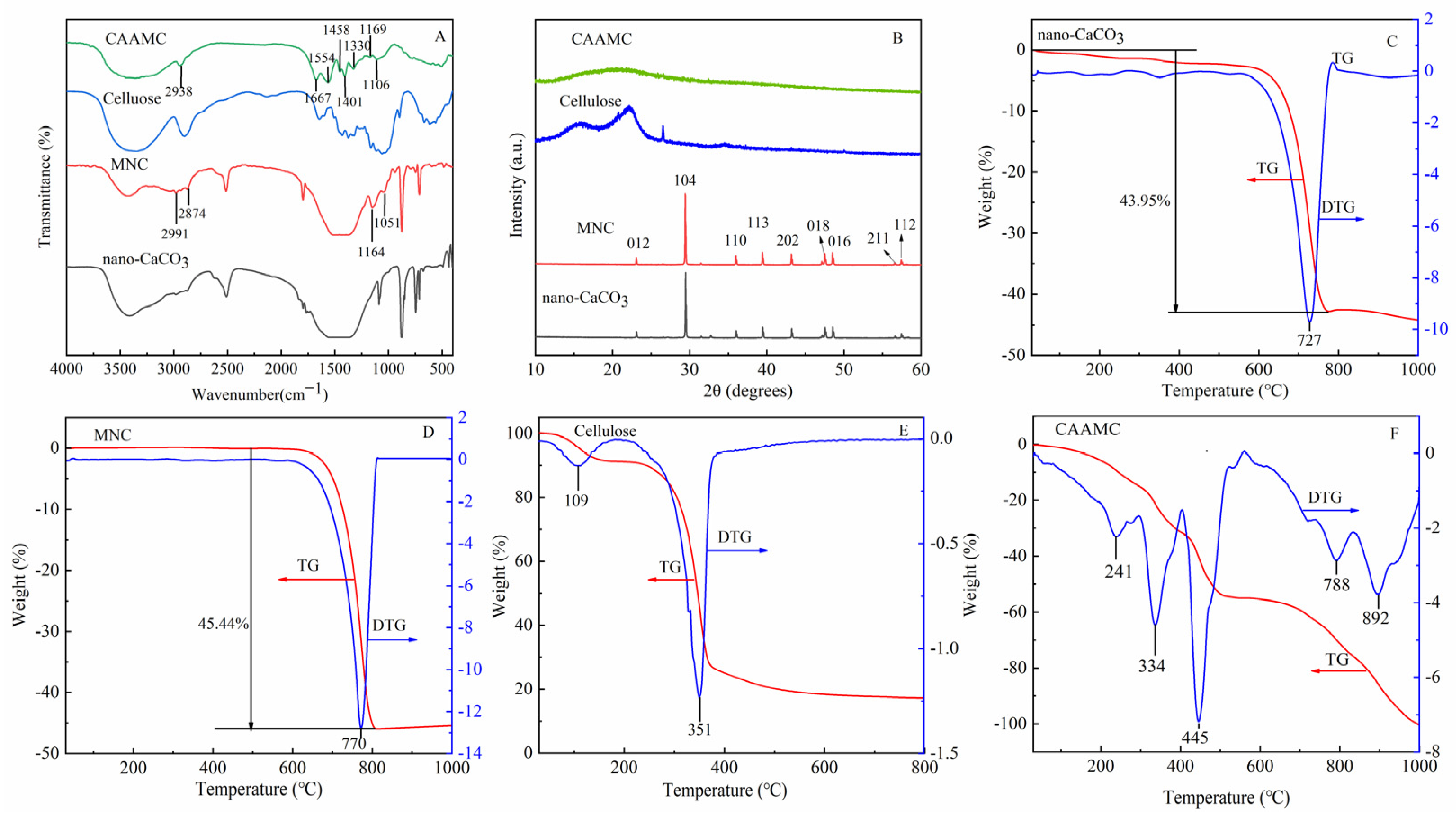
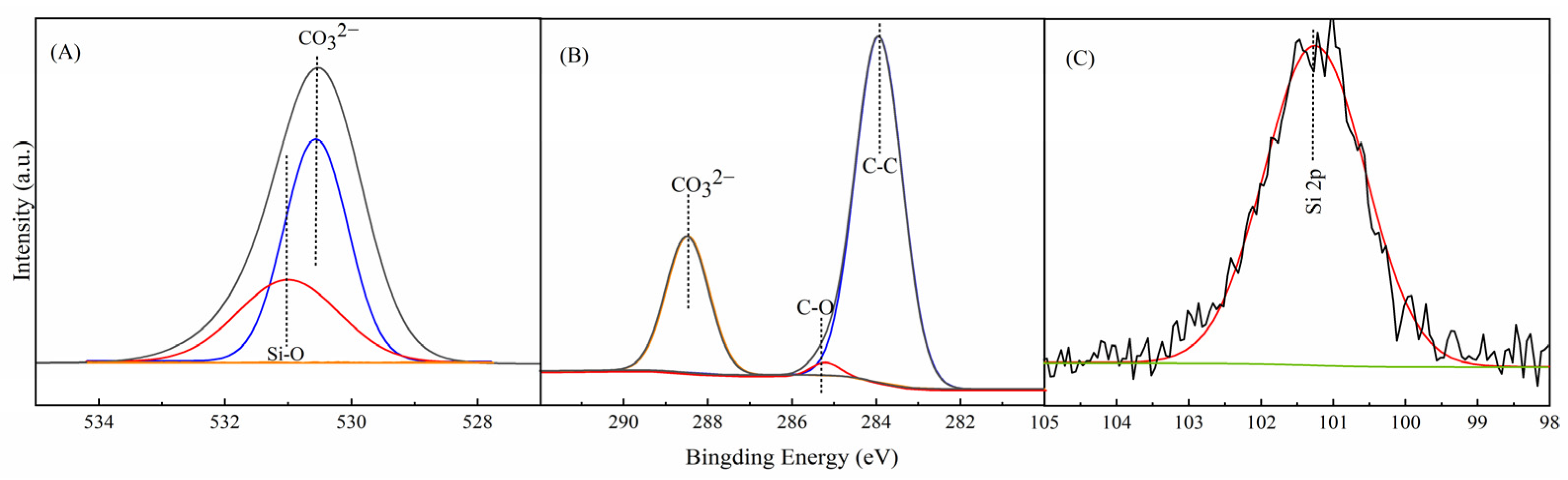
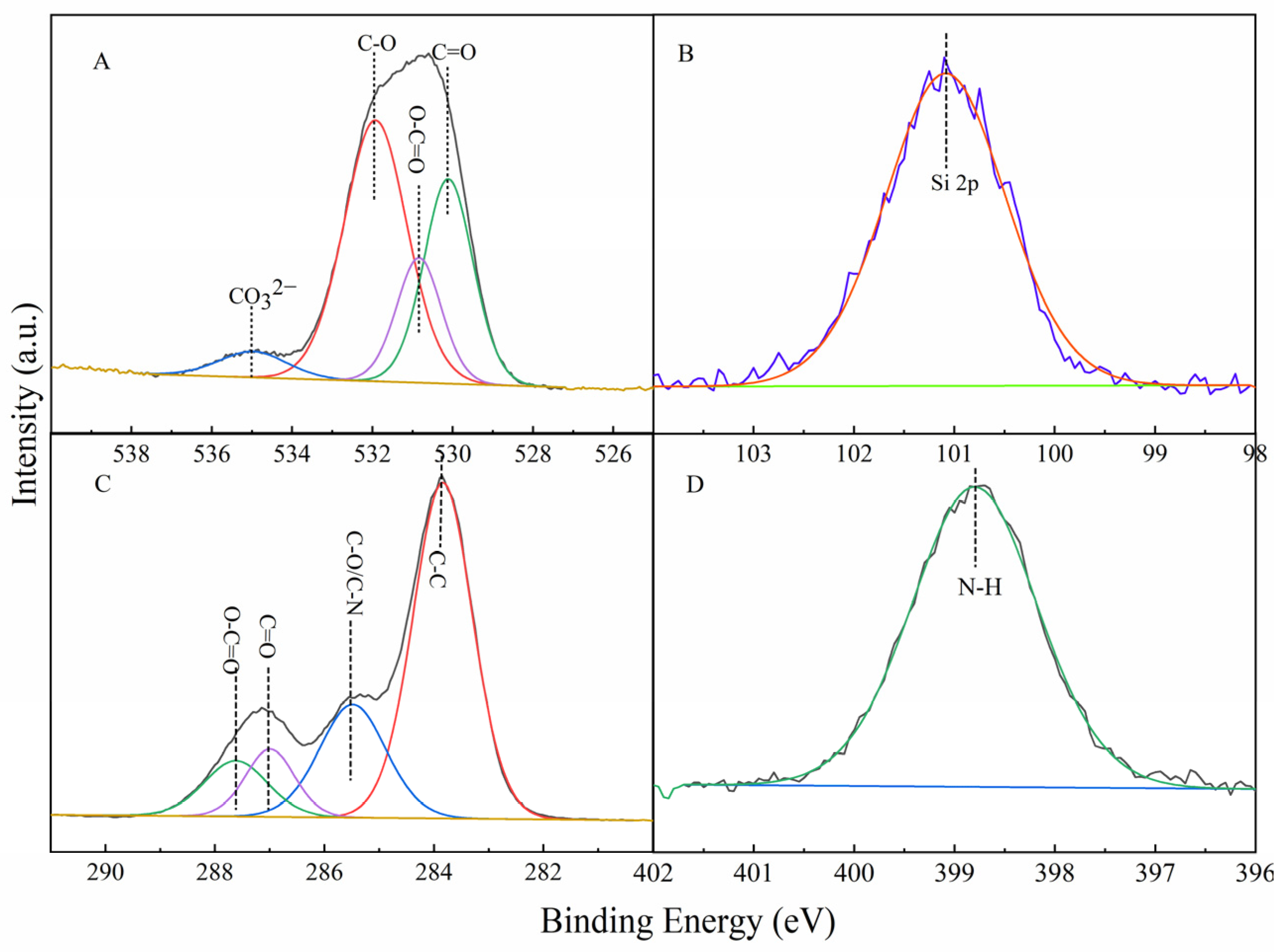
3.1. Characterization of CAAMC
3.2. Polymerization Mechanism of CAAMC
3.3. Effect of MNC Content on the Properties of CAAMC
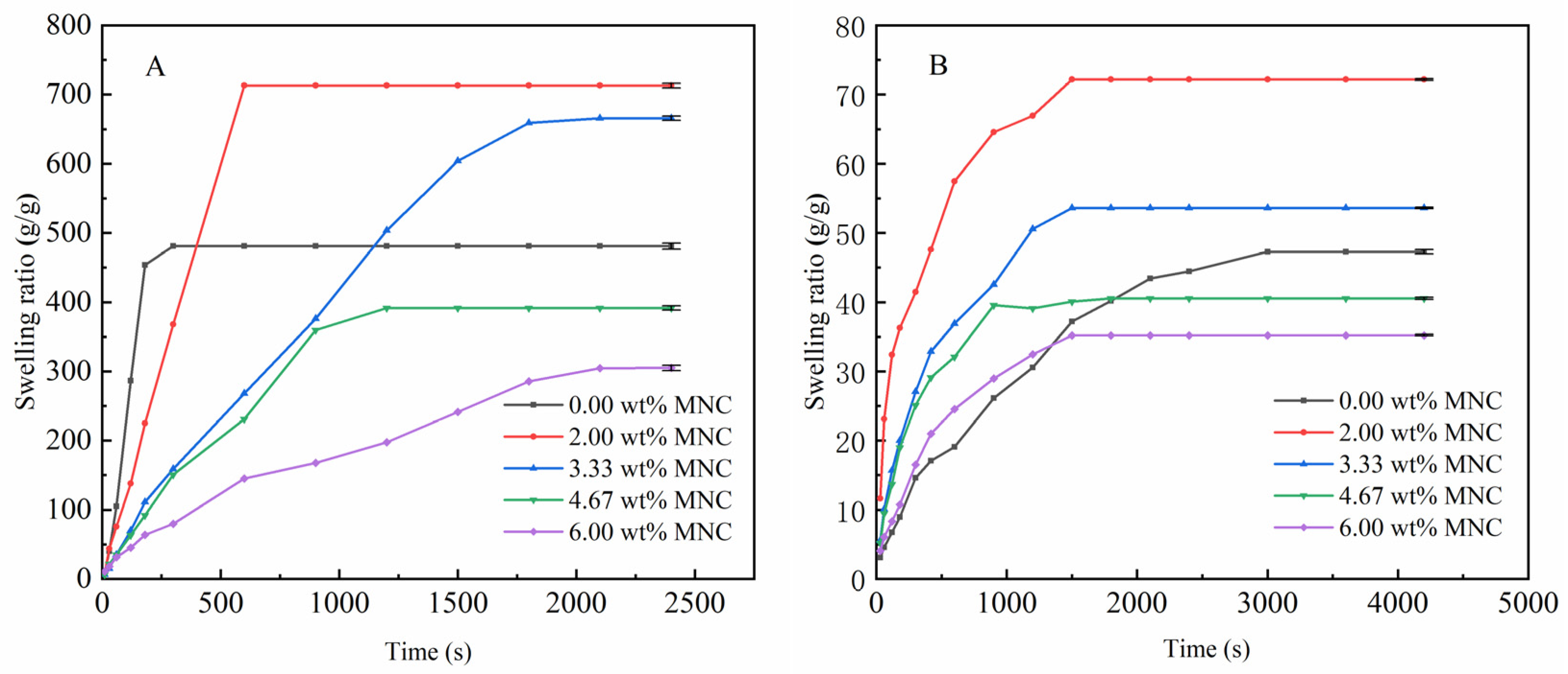
3.3.1. Effect of MNC Content on the Water Absorbency
3.3.2. Effect of MNC Content on the Reswelling Water Absorbency of CAAMC
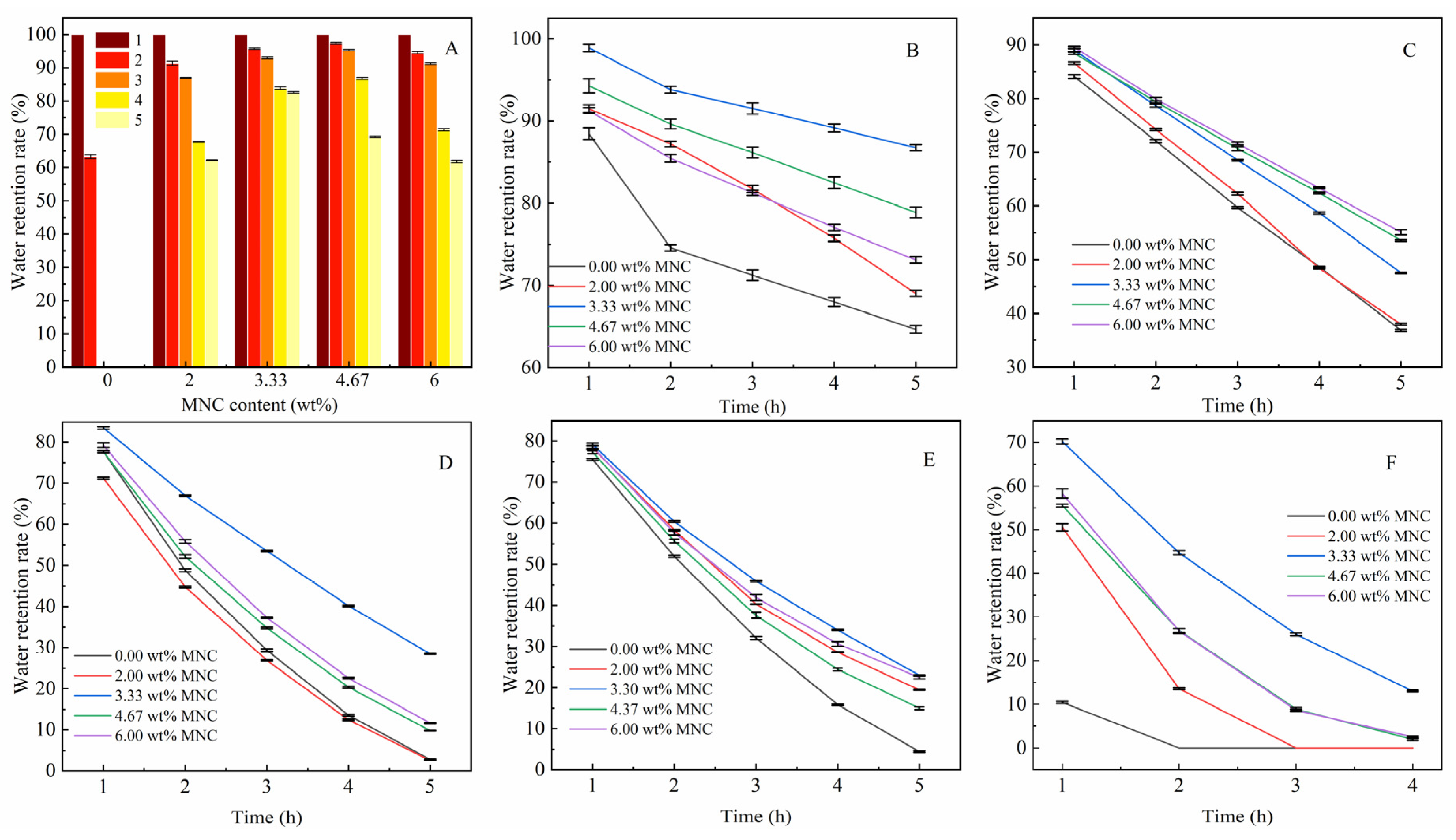
3.3.3. Effect of MNC Content on Water Retention of CAAMC at Different Temperatures
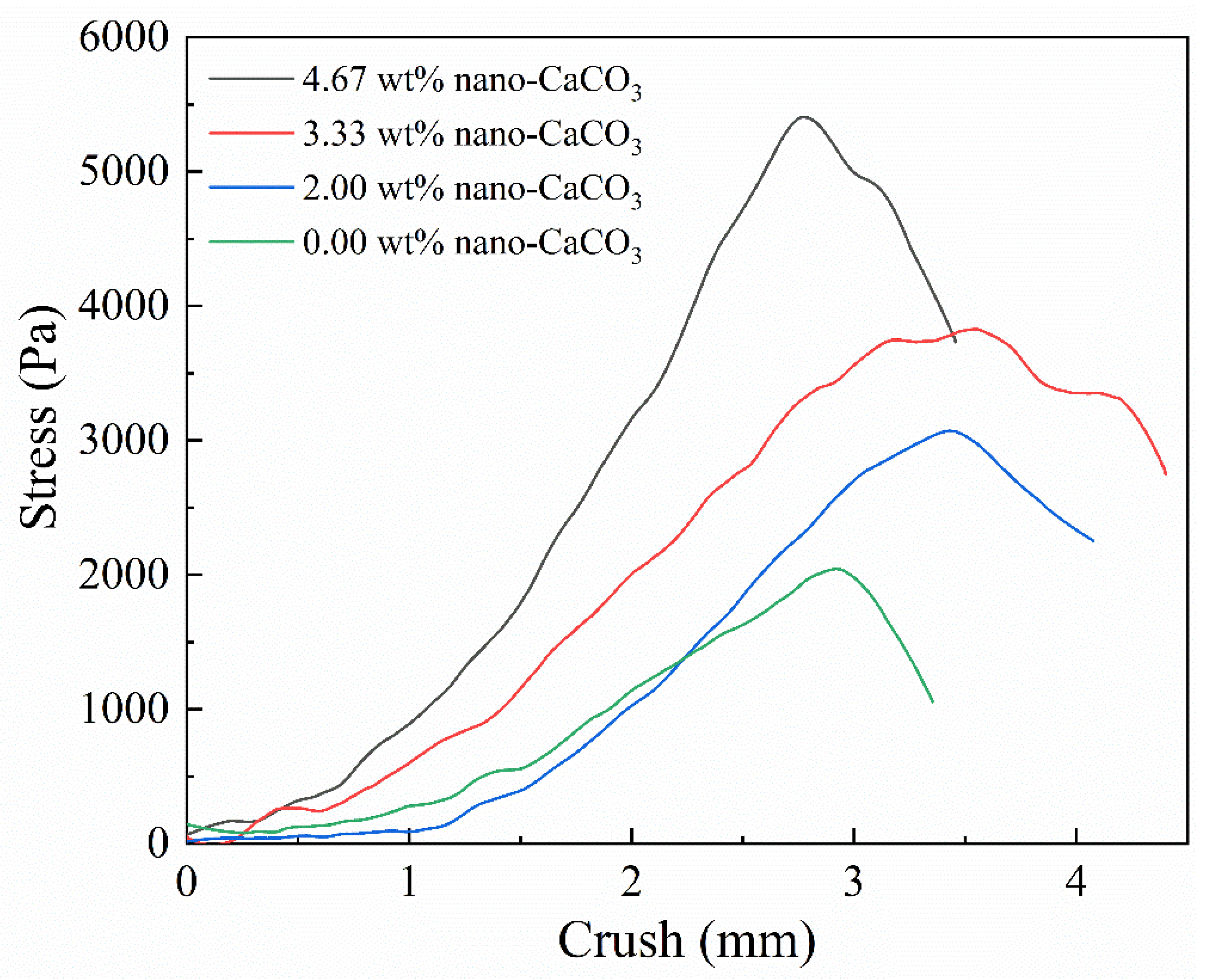
3.3.4. Effect of MNC Content on the Gel Strength of CAAMC after Water Absorption and Saturation
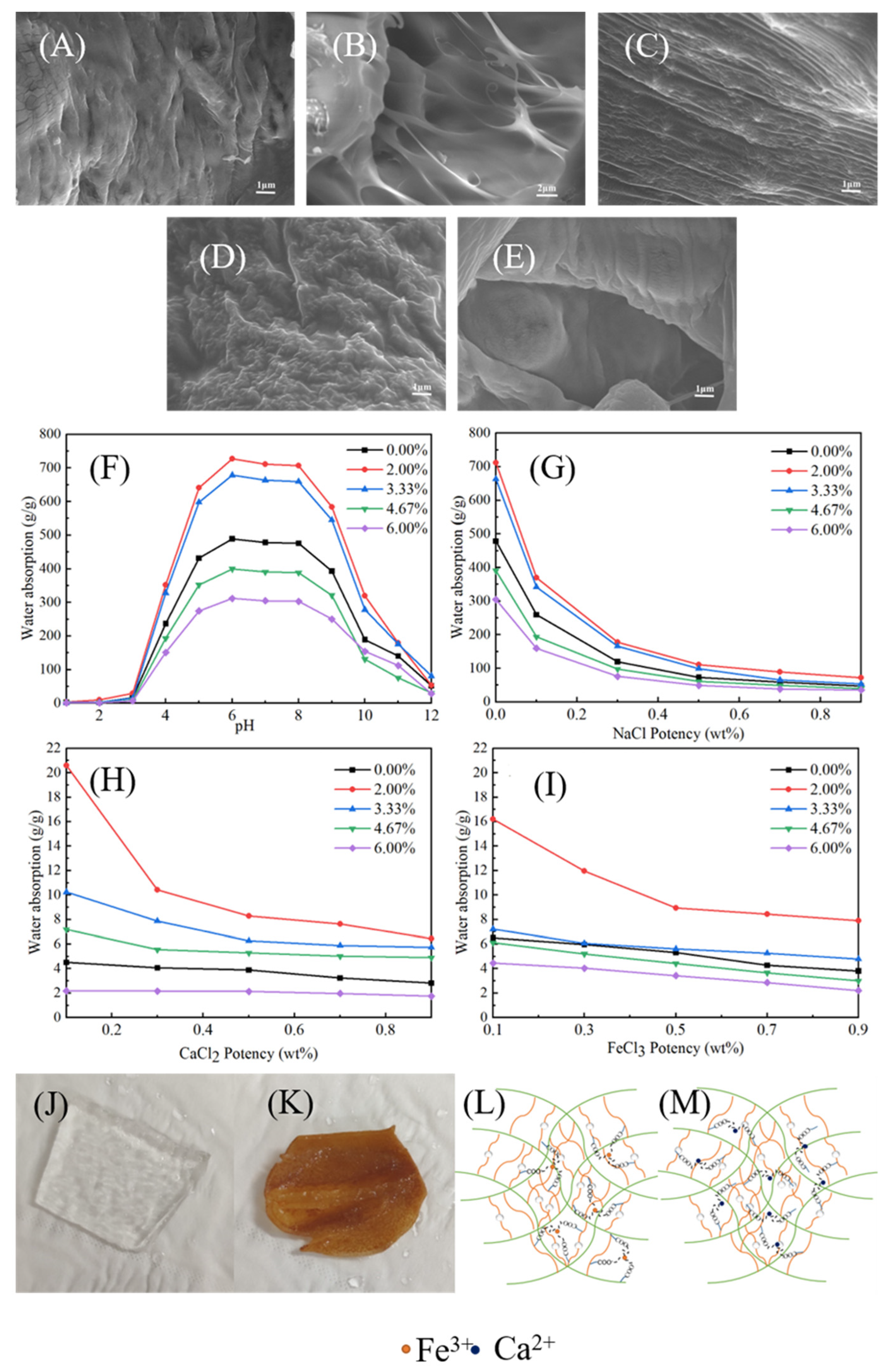
3.3.5. Effect of MNC Content on the CAAMC Morphology
3.3.6. Water Absorbency of CAAMC in Different pH Solutions and Different Salt Solutions
3.4. Water Retention in Soil
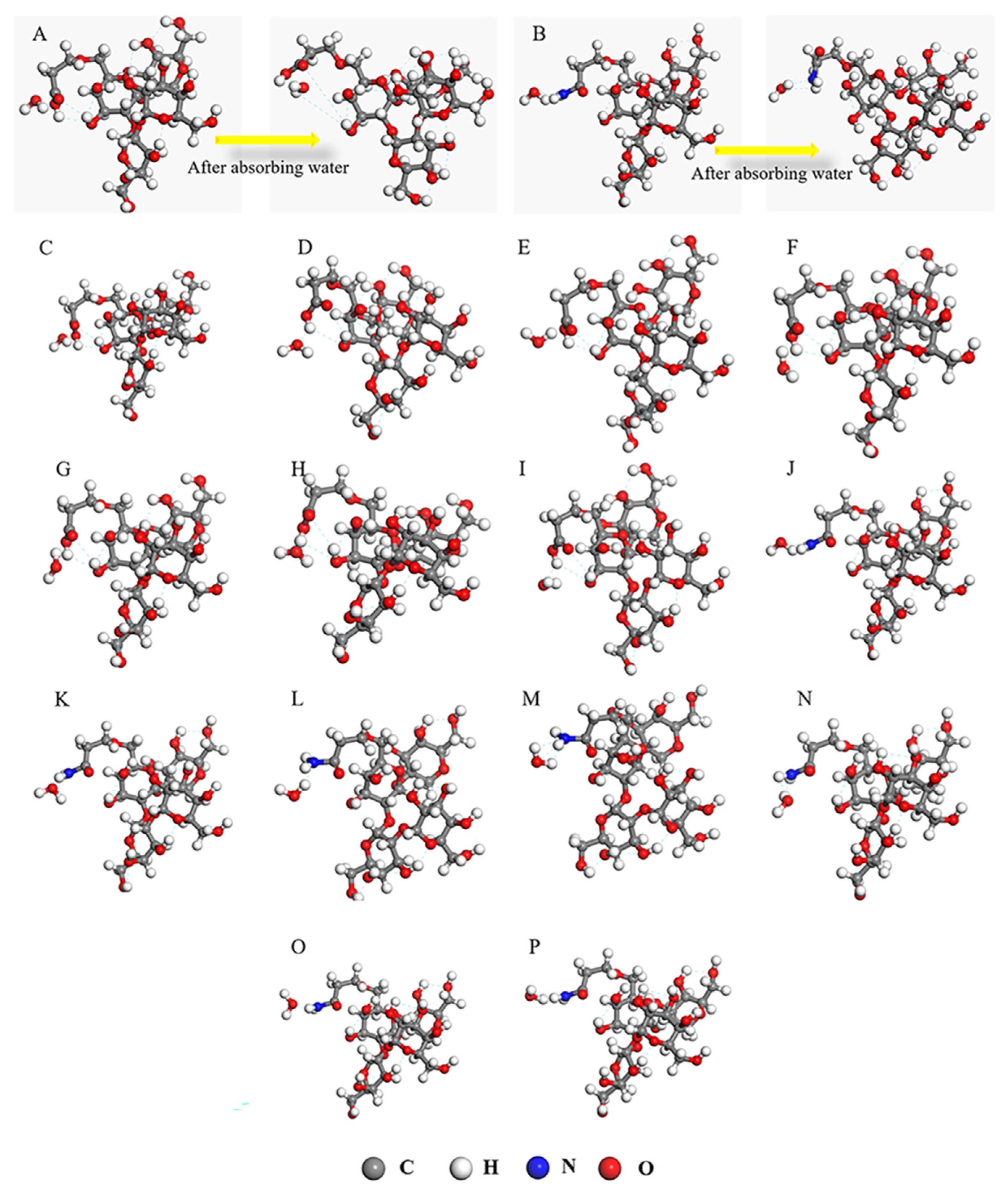
3.5. Simulation Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Wang, Q.; Liu, Q.; Li, Z.; Sun, G. Villus-like nanocomposite hydrogels with a super-high water absorption capacity. J. Mater. Chem. A 2020, 8, 12613–12622. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takacs, E.; Wojnarovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef]
- Teli, M.D.; Mallick, A. Utilization of Waste Sorghum Grain for Producing Superabsorbent for Personal Care Products. J. Polym. Environ. 2018, 26, 1393–1404. [Google Scholar] [CrossRef]
- Gregorio, I.; Zapata, F.; Garcia-Ruiz, C. Analysis of human bodily fluids on superabsorbent pads by ATR-FTIR. Talanta 2017, 162, 634–640. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Gao, B. A biodegradable biomass-based polymeric composite for slow release and water retention. J. Environ. Manag. 2019, 230, 190–198. [Google Scholar] [CrossRef]
- Qin, S.; Wu, Z.; Rasool, A.; Li, C. Synthesis and characterization of slow-release nitrogen fertilizer with water absorbency: Based on poly(acrylic acid-acrylic amide)/Na-bentonite. J. Appl. Polym. Sci. 2012, 126, 1687–1697. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Wang, Z.; Yin, G. Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr. Polym. 2009, 77, 131–135. [Google Scholar] [CrossRef]
- Krul, L.P.; Nareiko, E.I.; Matusevich, Y.I.; Yakimtsova, L.B.; Matusevich, V.; Seeber, W. Water super absorbents based on copolymers of acrylamide with sodium acrylate. Polym. Bull. 2000, 45, 159–165. [Google Scholar] [CrossRef]
- Hao, Q.; An, Y.; Wang, C.; Teng, L. Research Progress of Performance Defects and Modification Strategies of High Water Absorbent Resin. Polym. Bull. 2018, 3, 40–45. [Google Scholar]
- Ren, J.; Kong, W.; Sun, R. Preparation of Sugarcane Bagasse/Poly(Acrylic Acid-co-Acrylamide) Hydrogels and their Application. Bioresources 2014, 9, 3290–3303. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Huang, Z.; Zhang, Y.; Hu, H.; Liu, Z. Synthesis and properties of novel superabsorbent hydrogels with mechanically activated sugarcane bagasse and acrylic acid. Polym. Bull. 2013, 70, 1781–1794. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Sawut, A.; Yimit, M.; Sun, W.; Nurulla, I. Photopolymerisation and characterization of maleylatedcellulose-g-poly(acrylic acid) superabsorbent polymer. Carbohydr. Polym. 2014, 101, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Oshima, T.; Taguchi, S.; Ohe, K.; Baba, Y. Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr. Polym. 2011, 83, 953–958. [Google Scholar] [CrossRef]
- Xiao, S.; Gao, R.; Lu, Y.; Li, J.; Sun, Q. Fabrication and characterization of nanofibrillated cellulose and its aerogels from natural pine needles. Carbohydr. Polym. 2015, 119, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M. Biopolymers and Biocomposites: Chemistry and Technology. Curr. Anal. Chem. 2020, 16, 500–503. [Google Scholar] [CrossRef]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Nurazzi, N.M.; Lee, S.H.; Lee, C.H. A Comprehensive Review on Advanced Sustainable Woven Natural Fibre Polymer Composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Abd El-Rehim, H.A. Radiation synthesis of kappa-carrageenan/acrylamide graft copolymers as superabsorbents and their possible applications. J. Polym. Res. 2009, 16, 63–72. [Google Scholar] [CrossRef]
- Yano, B.B.R.; Silva, S.A.M.; Almeida, D.H.; Aquino, V.B.M.; Christoforo, A.L.; Rodrigues, E.F.C.; Lahr, F.A.R. Use of Sugarcane Bagasse and Industrial Timber Residue in Particleboard Production. Bioresources 2020, 15, 4753–4762. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Pandare, K.V.; Nair, G.; Varma, A.J. Utilization of sugarcane bagasse cellulose for producing cellulose acetates: Novel use of residual hemicellulose as plasticizer. Carbohydr. Polym. 2009, 76, 23–29. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Aita, G.M.; Wu, Q. Cellulose fibers isolated from energycane bagasse using alkaline and sodium chlorite treatments: Structural, chemical and thermal properties. Ind. Crops Prod. 2015, 76, 355–363. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Pourkhiyabi, M.; Gharekhani, H.; Doustdar, F. Semi-IPN superabsorbent nanocomposite based on sodium alginate and montmorillonite: Reaction parameters and swelling characteristics. Carbohydr. Polym. 2018, 190, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R. Fabrication of novel polyetherimide-fluorinated silica organic-inorganic composite hollow fiber membranes intended for membrane contactor application. J. Membr. Sci. 2013, 443, 170–180. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Zhang, M.; Zhang, H.; Luan, Q.; Zhou, W.; Huang, F. Wet-spun nanoTiO2/chitosan nanocomposite fibers as efficient and retrievable absorbent for the removal of free fatty acids from edible oil. Carbohydr. Polym. 2019, 210, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125133. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Liang, Q.; Pang, J.; Qin, Y. Optimization of extracting cellulose from sugarcane bagasse using ultrasound-assisted alkaline hydrogen peroxide. J. South. Agric. 2013, 44, 1008–1013. [Google Scholar]
- Rehman, S.; Khan, K.; Mujahid, M.; Nosheen, S. Synthesis of nano-hydroxyapatite and its rapid mediated surface functionalization by silane coupling agent. Mater. Sci. Eng. C 2016, 58, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Seggiani, M.; Cinelli, P.; Elnaby, H.M.H.; Azaam, M.M. Swelling capacity of sugarcane bagasse-g-poly(acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020, 133, 109769. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Zhang, A.; Yang, S. Water retention and fertilizer slow release integrated superabsorbent synthesized from millet straw and applied in agriculture. Ind. Crops Prod. 2021, 160, 113126. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Bai, H.; Li, Z.; Zhang, S.; Wang, W.; Dong, W. Interpenetrating polymer networks in polyvinyl alcohol/cellulose nanocrystals hydrogels to develop absorbent materials. Carbohydr. Polym. 2018, 200, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Fernando, T.N.; Ariadurai, S.A.; Disanayaka, C.K.; Kulathunge, S.; Aruggoda, A.G.B. Development of Radiation Grafted Super Absorbent Polymers for Agricultural Applications. Energy Procedia 2017, 127, 163–177. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, Y.; Yang, Y.; Shin, H.S.; Kwon, S. Induced Infiltration of Hole-Transporting Polymer into Photocatalyst for Staunch Polymer-Metal Oxide Hybrid Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 25915–25922. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Cheng, G.; Chen, Y.; Yu, X.; Wang, H. Characteristics evaluation of calcium carbonate particles modified by surface functionalization. Adv. Powder Technol. 2014, 25, 1618–1623. [Google Scholar] [CrossRef]
- Tai, Y.; Qian, J.; Zhang, Y.; Huang, J. Study of surface modification of nano-SiO2 with macromolecular coupling agent (LMPB-g-MAH). Chem. Eng. J. 2008, 141, 354–361. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-poly-(acrylamide-co-acrylic acid) polymeric bioadsorbent for the removal of toxic inorganic pollutants from wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and response of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/graphene oxide hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.-f.; Lau, Y.-T.R.; Lin, Y.; Chan, C.-m. Preparation and characterization of coverage-controlled CaCO3 nanoparticles. J. Colloid Interface Sci. 2010, 345, 168–173. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, G.; Singh, V.; Rattan, S. A dual stimuli responsive natural polymer based superabsorbent hydrogel engineered through a novel cross-linker. Polym. Chem. 2021, 12, 2404–2420. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.-h.; Zhang, N.; Wang, Y.; Zhou, Z.-W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II nanocrystals produced by sulfuric acid hydrolysis of Tetra pak cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hirata, G.A.; Farias, M.H.; Castillon, F.F. Synthesis and characterization of (3-Aminopropyl)trimethoxy-silane (APTMS) functionalized Gd2O3:Eu3+ red phosphor with enhanced quantum yield. Nanotechnology 2016, 27, 065601. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Pethick, K.; McCormick, P.G. Synthesis of CaCO3 Nanoparticles by Mechanochemical Processing. J. Nanoparticle Res. 2000, 2, 375–380. [Google Scholar] [CrossRef]
- Chen, J.; He, T.; Wu, W.; Cao, D.; Yun, J.; Tan, C.K. Adsorption of sodium salt of poly(acrylic) acid (PAANa) on nano-sized CaCO3 and dispersion of nano-sized CaCO3 in water. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 163–168. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Gharedaghi, H.; Dousti, A.; Eshraghi, J.; Hanafizadeh, P.; Ashjaee, M. A novel numerical approach for investigation of the gas bubble characteristics in stagnant liquid using Young-Laplace equation. Chem. Eng. Sci. 2017, 173, 37–48. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; Abd El-Hai, F.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Du, R.; Gao, B.; Men, J.; An, F. Characteristics and advantages of surface-initiated graft-polymerization as a way of “grafting from” method for graft-polymerization of functional monomers on solid particles. Eur. Polym. J. 2020, 127, 109479. [Google Scholar] [CrossRef]
- He, R.; Tan, Y.; Chen, H.; Wang, Z.; Zhang, J.; Fang, J. Preparation and properties of novel superabsorbent polymer (SAP) composites for cementitious materials based on modified metakaolin. Constr. Build. Mater. 2020, 258, 119575. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Zhao, G.; Xiang, Y.; Liu, Y. Novel Semi-IPN Nanocomposites with Functions of both Nutrient Slow-Release and Water Retention part 1: Microscopic Structure, Water Absorbency, and Degradation Performance. J. Agric. Food Chem. 2019, 67, 7587–7597. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, A.; Xie, P.; Gao, G.; Xie, L.; Li, X.; Song, A. Synthesis and swelling behaviors of carboxymethyl cellulose-based superabsorbent resin hybridized with graphene oxide. Carbohydr. Polym. 2017, 157, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujita, S.; Oeda, I. Comparative study of homogeneous solvents for the esterification crosslinking of cellulose with 1,2,3,4-butanetetracarboxylic dianhydride and water absorbency of the reaction products. J. Appl. Polym. Sci. 2013, 127, 478–486. [Google Scholar] [CrossRef]
- Wen, P.; Wu, Z.; He, Y.; Ye, B.-C.; Han, Y.; Wang, J.; Guan, X. Microwave-Assisted Synthesis of a Semi-interpenetrating Polymer Network Slow-Release Nitrogen Fertilizer with Water Absorbency from Cotton Stalks. ACS Sustain. Chem. Eng. 2016, 4, 6572–6579. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Zhang, A.; Yang, Z. Preparation and properties of novel corn straw cellulose–based superabsorbent with water-retaining and slow-release functions. J. Appl. Polym. Sci. 2020, 137, 48951. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and characterization of biomass lignin-based PVA super-absorbent hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef]
- Chen, M.; Shen, Y.; Xu, L.; Xiang, G.; Ni, Z. Synthesis of a super-absorbent nanocomposite hydrogel based on vinyl hybrid silica nanospheres and its properties. RSC Adv. 2020, 10, 41022–41031. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Azaam, M.M.; El-nshar, E.M. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/montmorillonite superabsorbent composite as a slow-release urea fertilizer. Polym. Adv. Technol. 2018, 29, 2072–2079. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, P.; Zhang, J.; Wang, A. Study on superabsorbent composite XVI. Synthesis, characterization and swelling behaviors of poly(sodium acrylate)/vermiculite superabsorbent composites. Eur. Polym. J. 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef] [PubMed]




| Raw Materials | Ratio of Monomers (Monomer:Natural Polymer Material) | Water Absorbency in Deionized Water (g/g) | Water Absorbency in 0.9 wt% NaCl (g/g) | Ref. |
|---|---|---|---|---|
| Cellulose, AA, AM | 10:1 | 240 | - | [64] |
| Carboxymethyl cellulose, PAA, Graphene oxide | 25:3 | 750 | 85 | [65] |
| Cellulose, 1,2,3,4-butanetetracarboxylic dianhydride | 3:1 | 987 | - | [66] |
| cotton stalk, bentonite, polyvinylpyrrolidone | 9:1 | 1018 | 71 | [67] |
| Corn straw cellulose, ammonium polyphosphate, AA | 3:1 | 303 | - | [68] |
| Starch, AA, AM, polyvinyl alcohol, cellulose nanocrystals | 17.5:1 | 922 | 52 | [12] |
| (2-pyridyl) acetyl chitosan chloride, AA, AM | 10:1 | 615 | 44 | [48] |
| Lignin, PVA | 20:1 | 456 | - | [69] |
| Bagasse cellulose, AA, AM, CaCO3 | 8:1 | 712 | 72 | This work |
| Configuration | Pre-Adsorption Energy (kJ/mol) | Post-Adsorption Energy (kJ/mol) | Adsorption Energy (kJ/mol) |
|---|---|---|---|
| a | 32.613 | 31.265 | −1.348 |
| b | 32.613 | 31.443 | −1.170 |
| c | 32.613 | 31.368 | −1.245 |
| d | 32.613 | 31.062 | −1.551 |
| e | 32.613 | 31.512 | −1.101 |
| f | 32.613 | 30.373 | −2.240 |
| g | 32.613 | 31.847 | −0.766 |
| h | 33.010 | 32.452 | −0.558 |
| i | 33.010 | 31.200 | −1.81 |
| j | 33.010 | 30.918 | −2.092 |
| k | 33.010 | 31.576 | −1.434 |
| l | 33.010 | 31.992 | −1.018 |
| m | 33.010 | 30.597 | −2.413 |
| n | 33.010 | 32.056 | −0.954 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Ma, L.; Chen, Y.; Luo, X.; Long, M.; Ji, H.; Chen, J. Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy. Nanomaterials 2022, 12, 1459. https://doi.org/10.3390/nano12091459
Xie X, Ma L, Chen Y, Luo X, Long M, Ji H, Chen J. Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy. Nanomaterials. 2022; 12(9):1459. https://doi.org/10.3390/nano12091459
Chicago/Turabian StyleXie, Xinling, Li Ma, Yongmei Chen, Xuan Luo, Minggui Long, Hongbing Ji, and Jianhua Chen. 2022. "Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy" Nanomaterials 12, no. 9: 1459. https://doi.org/10.3390/nano12091459
APA StyleXie, X., Ma, L., Chen, Y., Luo, X., Long, M., Ji, H., & Chen, J. (2022). Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy. Nanomaterials, 12(9), 1459. https://doi.org/10.3390/nano12091459