Abstract
Immunoglobulin A (IgA), as the most secreted immunoglobulin in the intestine, plays an irreplaceable role in mucosal immunity regulation. Previous studies have indicated that Lactobacillus showed strain specificity in stimulating the secretion of IgA through intestinal mucosal lymphocytes. The reason for this phenomenon is not clear. The current studies have been aimed at exploring the effect of a strain on the secretion of IgA in the host’s intestine, but the mechanism behind it has not been seriously studied. Based on this, we selected five strains of Lactobacillus fermentum isolated from different individuals to determine whether there are intraspecific differences in stimulating the secretion of IgA from the intestinal mucosa. It was found that IgA concentrations in different intestinal segments and faeces induced by L. fermentum were different. 12-1 and X6L1 strains increased the secretion of IgA by the intestine significantly. In addition, different strains of L. fermentum were also proven to have different effects on the host gut microbiota but no significant effects on IgA-coated microbiota. Besides, it was speculated that different strains of L. fermentum may act on different pathways to stimulate IgA in a non-inflammatory manner. By explaining the differences of IgA secretion in the host’s intestine tract stimulated by different strains of L. fermentum, it is expected to provide a theoretical basis for the stimulation of intestinal secretion of IgA by Lactobacillus and a new direction for exploring the relationship between Lactobacillus and human immunity.
1. Introduction
There are approximately 1014 microorganisms in the intestinal cavity, including bacteria, fungi, viruses and protozoa [1]. The gut microbiota promotes the development and response of the host mucosal immune system, enhances the close association between intestinal epithelial cells and antagonises pathogens [2]. The intestinal mucus layer is composed of mucin secreted by goblet cells, antimicrobial peptides secreted by intestinal epithelial cells and secretory immunoglobulin A (IgA) produced by B cells. IgA can effectively inhibit the adhesion and colonization of some bacteria in the intestinal epithelium [3].
The intestine is not only the site of food digestion and absorption but is also an important immune organ [4]. Through coevolution, the mammalian host and microorganism have formed a mutually beneficial symbiotic relationship. The host provides nutrition and a living environment for microorganisms, while microorganisms promote the steady-state balance of the intestine by participating in and regulating a series of physiological activities of the host [5]. Evidence suggests that a balance between intestinal bacteria and the mucosal immune system is an important factor in the development of intestinal mucosal inflammation [6,7]. The mucosal immune system elicits an effective defence against pathogens that invade the mucosa. Furthermore, proper tolerance needs to be developed against microbes that live in symbiosis with organisms in the mucosa [8].
IgA is the most commonly produced immunoglobulin in mammals [9]. The specific immune response in the gut is mainly through the production of IgA, IgM and IgG. Among them, IgA can inhibit the colonization and proliferation of some microorganisms in the mucus layer. Also, IgA can combine with some microorganisms to varying degrees to regulate the gut microbiota. There is no substitute for the role of IgA in intestinal immunity. At present, there have been many reports of probiotics that can regulate the level of IgA in the intestinal tract. It is known that L. plantarum YU could stimulate the secretion of IgA in Peyer’s patches by stimulating Th1 immune responses [10]. Pediococcus acidilactici K15 promoted the secretion of IgA in the oral mucosa, and this response was mainly induced via IL-10 [11]. L. fermentum CECT5716 enhanced IgA secretion in rats during pregnancy [12]. L. fermentum UCO-979C increased intestinal IgA levels while activating TLR4 [13]. The current studies mainly focus on how pathogenic bacteria stimulate host immunity and then affect the production of IgA. There is a lack of in-depth mechanism studies on how Lactobacillus promotes IgA secretion. In addition, it has been reported that the content of IgA in human faeces can be increased by diets containing high concentrations of acetic acid [14]. However, it is not known whether the SCFAs produced by the host’s gut microbiota are responsible for stimulating the host’s gut to secrete IgA. Most studies on the effect of Lactobacillus on intestinal IgA secretion remain on inter-species differences, and there are few studies on whether there are intra-species differences. In the previous study, we used forty strains of different species of Lactobacillus to conduct in vitro cell experiments and animal experiments (unpublished data). The results showed that among the various species of Lactobacillus, L. fermentum had the best potential for stimulating intestinal secretion of IgA. In this study, we selected five strains of L. fermentum derived from different human donors to investigate whether the hosts had differences in their corresponding response to IgA.
2. Materials and Methods
2.1. Bacterial Treatment
The five strains of L. fermentum (L. fermentum X6L1 [CCFM1225], L. fermentum 12-1 [CCFM1226], L. fermentum S24-1, L. fermentum 24M1 and L. fermentum 20M5) used in this study were isolated from faeces of healthy individuals and stored at the culture collection of food microorganisms in Jiangnan University (Wuxi, Jiangsu, China). The sources of these strains are shown in Table 1. The study did not involve human experiments, and the faecal samples containing the isolated strains were collected from healthy volunteers and did not cause any foreseeable risk or discomfort to the participants. The volunteers signed a written informed consent form or obtained the consent of their legal guardian. This study used previously isolated and preserved strains. Strain isolation was not involved in this study. All bacterial strains were cultured in modified de Man, Rogosa and Sharpe broth and incubated at 37 °C under anaerobic conditions (400TG; Electrotek, West Yorkshire, UK). Bacterial cells were collected by centrifugation at 6000× g for 5 min and suspended in a small volume of 30% glycerol solution to prepare a stock, and were stored at −80 °C for further treatment. The stock was diluted with sterilised phosphate-buffered saline (PBS) to yield a final concentration of 109 colony-forming units (CFU)/mL for oral administration.

Table 1.
Strains used in this study.
2.2. Animal Experiments
The animal experiments involved in this study were carried out in the Animal Centre of Jiangnan University under environmentally controlled conditions (12-h light–dark cycle at 22 °C ± 3 °C and humidity of 55% ± 10%). All experiments were approved by the Animal Ethics Committee of Experimental Animal at Jiangnan University (JN. No:20210315c1080601 [012]). Three-week-old C57BL/6J female mice were purchased from the Model Animal Research Centre of Vital River (Shanghai, China). Standard food and sterile water were provided ad libitum. After acclimatisation for 7 days, 36 mice were randomly divided into six groups (n = 6/group). The control group was gavaged with 0.2 mL PBS solution. The X6L1, S24-1, 12-1, 20M5 and 24M1 groups were gavaged with 0.2 mL bacterial suspension (109 CFU/mL) every day for three weeks. The experimental procedure timeline is shown in Figure 1. Mice were randomly assigned to the control group and different experimental condition groups using simple randomisation.
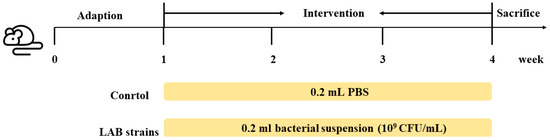
Figure 1.
Schematic of the animal experiment. Five strains were included in the lactic acid bacteria (LAB) groups, Lactobacillus strains X6L1, S24-1, 12-1, 24M1 and 20M5.
2.3. Tissue Processing
On the 7th, 14th and 21st days of the experiment, the faeces of mice were collected, placed on ice immediately and transferred to a freezer at −80 °C for storage. On the last day of the experiment, the mice were intraperitoneally injected with isoflurane after fasting for 12 h. One hour after intraperitoneal injection, the mice were sacrificed and their blood was collected. The blood was centrifuged at 2000× g for 15 min. The supernatant was collected as serum and stored in a freezer at −80 °C for later use. The mice were dissected, and the duodenum, jejunum, ileum, caecum, colon and other tissues were collected. Part of the intestinal tissues were stored in 40 g/L paraformaldehyde for later immunofluorescence staining. Of the remaining intestinal tissue, the caecal and colon contents were scraped, and the remaining tissue samples and caecal and colon contents were immediately placed into liquid nitrogen for rapid freezing. These samples were then stored at −80 °C for later use [15].
2.4. Real-Time Polymerase Chain Reaction
Approximately 20 mg of mouse colon that was preserved at −80 °C were placed together with high-temperature inactivated zirconia beads into an enzyme-free centrifuge tube. We added 1 mL of TRIzol and fully crushed the sample with a high-throughput crusher (SCIENTZ-48, Ningbo, China). The supernatant was collected, protein impurities and DNA were precipitated with chloroform and isopropanol was added for static precipitation. After discarding the supernatant, 75% pre-cooled ethanol was added to clean the extracted RNA twice, the solvent was left to air dry and the sample was re-dissolved with diethylpyrocarbonate-treated water. The purity and integrity of the extracted RNA were tested by measuring its optical density at 260/280 nm. In accordance with the instructions provided by the manufacturer of the Vazyme kit, complementary DNA was synthesised with the extracted total RNA as a template for real-time fluorescence quantitative polymerase chain reaction (qPCR) detection (Bio-Rad, Berkeley, CA, USA). The PCR system was prepared in accordance with the instructions of the qPCR mix, and the real-time qPCR program was run [16]. The transcription levels of genes encoding the polymeric immunoglobulin receptor (pIgR), myeloid differentiation factor 88 (MyD88), B-cell activating factor receptor (BAFFR), epidermal growth factor receptor (EGFR) and activation induced cytidine deaminase (ACIDA) in the mouse colon were measured by real-time qPCR. The primer sequences of mouse pIgR, MyD88, BAFFR, EGFR and ACIDA were found on the PrimerBank website (accessed on 19 January 2022, https://pga.mgh.harvard.edu/primerbank/index.html). Sonny Biotechnology Co., Ltd. (Shanghai, China) synthesised these primers. The specific primer information is shown in Table 2.

Table 2.
Primer sequence.
2.5. Determination of Short Chain Fatty Acids
Freeze-dried stool samples (50 mg) were homogenised in 500 μL of saturated NaCl solution and acidified with 40 μL of 10% sulphuric acid. Diethyl ether (1 mL) was added to the samples to extract short-chain fatty acids, after which the samples were centrifuged at 14,000× g for 15 min at 4 °C. Each supernatant (1 μL) was injected into an Rtx-WAX capillary column for gas chromatography–mass spectrometry analysis (QP2010 Ultra; Shimadzu, Kyoto, Japan). The initial oven temperature (100 °C) was increased to 140 °C at a rate of 7.5 °C min−1. The temperature was then further increased to 200 °C at a rate of 60 °C min−1 and maintained for 3 min. Helium was used as the carrier gas (flow rate: 0.89 mL min−1; column head pressure: 62.7 kPa). The injector temperature was set at 240 °C. The mass spectrometer was set at an ion source temperature of 220 °C, an interface temperature of 250 °C and a scan range of 2–100 m/z [17].
2.6. Enzyme-Linked Immunosorbent Assay
The faeces, duodenum, jejunum, ileum and colon, which were preserved at −80 °C, were thawed and the adipose tissue was removed. Pre-cooled sterile PBS solution was added according to a weight ratio of 1:9. The samples were then placed into a centrifuge tube together with cleaned and sterilised zirconia beads, broken down with a high-throughput crusher and then centrifuged at 4 °C for 2000× g for 10 min [18].
The supernatant was collected for assay measurement. Interleukin (IL)-1β, IL-6 and IL-17 concentrations were measured using mouse enzyme-linked immunosorbent assay kits according to the manufacturer’s protocol (R&D, Minneapolis, MN, USA). IgA and IgG concentrations were measured using mouse enzyme-linked immunosorbent assay kits according to the manufacturer’s protocol (Elabscience Biotechnology Co., Ltd., Wuhan, China). Protein was measured in intestinal tissue and faeces using the bicinchoninic acid assay (Beyotime Biotechnology, Shanghai, China).
2.7. Immunofluorescence of IgA in Plasma Cells in Mouse Intestinal Segments
After sacrificing the mice, approximately 1 cm of mouse intestinal tissue was carefully cut to avoid mechanical damage to the colon tissue as much as possible. After removing other tissues attached to the intestinal tissue, the residual faeces were removed by rinsing with pre-cooled normal saline, and then 4% paraformaldehyde solution was immediately added for fixation. The samples were embedded within 48 h: after washing the samples with clean water to remove excess fixative, they were successively treated with an ethanol gradient and xylene for dehydration and immersed in paraffin wax. The wax-soaked colon tissue was embedded using a Leica tissue embedding machine. After cooling, sections were cut with a hand wheel Leica tissue slicer. The sections were spread on slides and dried for subsequent staining [19]. The sections were stained overnight using the following reagents for immunofluorescence analysis: goat anti-mouse IgA alpha chain antibody (1:500, ab97231, Abcam, Shanghai, China), an FITC immunofluorescence detection kit (E670007, BBI, Shanghai, China) and 4′,6-diamidino-2-phenylindol dihydrochloride (Fcmacs, Nanjing, China).
2.8. Isolation and Identification of IgA-Coated Bacteria
IgA-coated bacteria were collected from faeces using magnetic bead-based enrichment. Briefly, faeces were suspended at 20% in pre-reduced PBS containing 0.5% Tween 20 (PBST) and protease inhibitors (1 mg/mL leupeptin, 1.6 mg/mL aprotinin; Sigma-Aldrich, St. Louis, MO, USA). The faeces were homogenised and centrifuged at 400× g to remove large debris. The supernatant was then centrifuged at 8000× g to pellet bacteria and washed with PBST three times. The bacterial pellet was resuspended in pre-reduced PBS supplemented with 0.25% bovine serum albumin, 5% goat serum and biotinylated goat anti-mouse IgA (ab97233, Abcam, Shanghai, China). After being washed, the biotinylated samples were mixed with streptavidin-coated magnetic beads to allow crosslinking (D110557; BBI, Shanghai, China). IgA-coated bacteria were separated from the suspension with the aid of a magnet. The collected bacteria were washed three times with PBST. DNA was quantified and pooled at equal concentrations by following the instructions of the Qubit dsDNA Assay Kit (Life Technologies, Carlsbad, CA, USA). All isolated strains were further typed by 16S rDNA high-throughput sequencing [20,21].
2.9. 16 S rDNA High-Throughput Sequencing
The total DNA of bacteria in fresh stool was extracted using the Fast DNA Stool Kit (MP Biomedicals, Carlsbad, CA, USA). PCR amplification of the 16S rDNA gene was performed using universal primers (341 forward: 5′-CCTAYGGGRBGCASCAG-3′ and 806 reverse: 5′-GGACTACNNGGGTATCTAAT-3′). The PCR products were purified using the TIANgel Mini Purification Kit (Tiangen, Beijing, China).
DNA was quantified and pooled at equal concentrations by following the instructions of the Qubit dsDNA Assay Kit (Life Technologies, Carlsbad, CA, USA). Samples were barcoded and finally paired-end sequenced on the Illumina MiSeq PE300 platform by following the manufacturer’s protocol. A gene sequencing analysis using 16S rDNA was performed with Quantitative Insights into Microbial Ecology version 2 (QIIME2) [22]. Amplicon sequence variants were rarefied to 10,000 according to the sampling depth.
The composition of Lactobacillus species was analysed and modified to run on QIIME2 [22]. The amplicon sequence variants were rarefied to 3000 according to the sampling depth. Subsequent processing was similar to that for 16S rDNA gene analysis unless otherwise noted.
2.10. Statistical Analysis
All data in this study are expressed as the mean ± standard deviation and were plotted with Prism 7 and Origin Pro 2021. We used one-way analysis of variance or Welch’s t test. The Spearman correlation coefficient was calculated. The results were corrected by False Discovery Rate (FDR) and with p less than 0.05 were retained. p < 0.05 indicated significance of the data (compared with the control group), and 95% confidence intervals are shown. CaseViewer was used to intercept the visual field of photographs after a slice scanner was used. Image J was used for immunofluorescence analysis, Xcalibur was used for gas chromatography–mass spectrometry off-machine data analysis and Origin Pro 2021 was used for correlation analysis. Statistical Analysis of Metagenomic (and other) Profiles (STAMP) was used for the Welch’s t test [23].
3. Results
3.1. L. fermentum Affects Intestinal Secretion of IgA
After gavage for 1 week, compared with the control group, IgA concentrations in mouse faeces changed by varying degrees according to the Lactobacillus strain. The 12-1 and X6L1 strains significantly increased IgA concentrations in the faeces, but there were no significant changes in the S24-1, 20M5 or 24M1 groups (Figure 2a). During continuous gavage for three weeks, IgA concentrations in the faeces showed a downward trend and gradually approached those in the control group. IgA concentrations in each mouse intestinal segment were detected after three weeks of gavage. There were differences in IgA concentrations in the duodenum and ileum between the Lactobacillus strain groups (Figure 2b,d). IgA concentrations in the X6L1, S24-1 and 20M5 groups were significantly higher than those in the control group in the duodenum. However, IgA concentrations in the 12-1, X6L1 and 20M5 groups were significantly lower than those in the control group in the ileum. After gavage for three weeks, IgA concentrations were significantly higher in the X6L1 group than in the control group in the colonic contents, but this difference was not significant in the colon (Figure 2e).
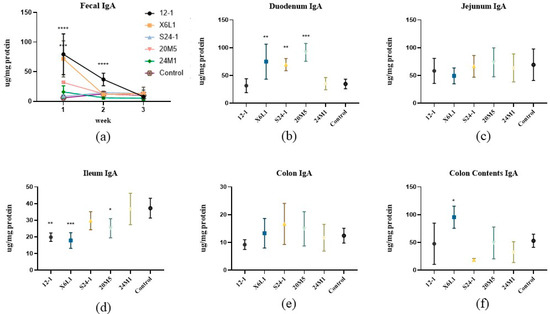
Figure 2.
Effect of L. fermentum on IgA concentration in faeces and intestinal tract of normal mice (a) IgA in one to three week faeces; (b–f) Duodenum, jejunum, ileum, colon and colon contents were detected after three weeks by ELISA, “*” indicates p < 0.05, and “**” indicates p < 0.01, “***” indicates p < 0.001, “****” indicates p < 0.0001 (compared with control group).
An immunofluorescence assay on paraffin sections of mouse intestinal segments showed that IgA in plasma cells was sporadically distributed in the duodenum and colon, compared with the control group. IgA was also observed in the jejunum and ileum, but the number of plasma cells was low (Figure 3a,b). There was no difference in the fluorescence intensity of IgA and plasma cells in the jejunum. The IgA content in the intestinal segments was different among the groups and decreased with the direction of intestinal peristalsis. As shown in Figure 3b, compared with the control group, the fluorescence intensity of IgA in different intestinal segments of Lactobacillus-treated mice were different. The IgA plasma cell content in the duodenum was significantly higher than that in the other intestinal segments. X6L1 and 20M5 significantly increased the immunofluorescence intensity of IgA plasma cells in the duodenum. In the ileum, 12-1 and X6L1 decreased the fluorescence intensity. X6L1, S24-1, 20M5 also reduced the immunofluorescence intensity of IgA plasma cells in the colon.
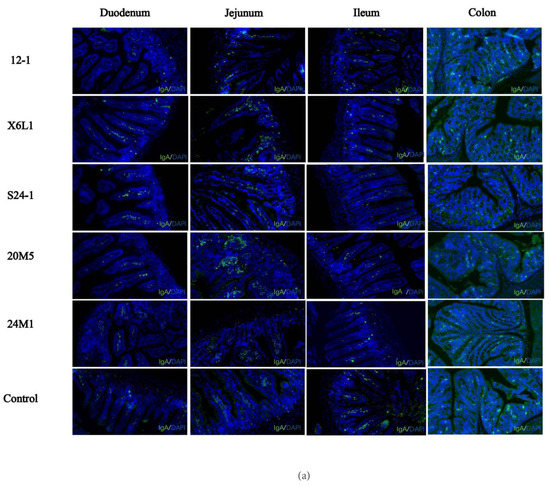
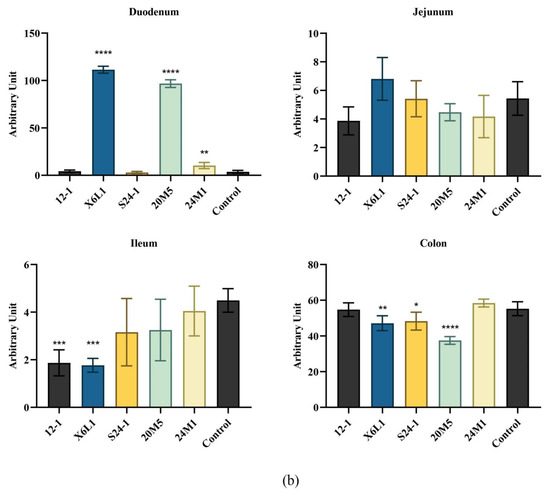
Figure 3.
Effect of L. fermentum on IgA+ plasma cell in intestinal tract of normal mice.(a) The IgA immunofluorescence map of each intestinal segment of mice was obtained from the visual field of the photos intercepted by the CaseViewer (10×, green-IgA, blue-DAPI); (b) The average fluorescence intensity of immunofluorescence images of mouse intestinal tissues treated by Image J “*” indicates p < 0.05, and “**” indicates p < 0.01, “***” indicates p < 0.001, “****” indicates p < 0.0001 (compared with the control group).
3.2. L. fermentum Affects the Host Gut Microbiota and IgA-Coated Bacteria
The microbiota of the mouse colon was examined by IgA immunomagnetic beads and 16S rDNA analysis. Firmicutes and Bacteroidetes comprised the main part of the microbiota on the whole. However, there were some differences in Actinomycetes, Tenericutes and Verrucous in the microbiota. Most IgA-binding bacteria were Proteobacteria and Firmicutes (Figure 4a,b). The overall changes in each group tended to be similar, but the proportion of IgA-bound Actinomycetes was significantly higher in the 12-1 group (Figure 4c).
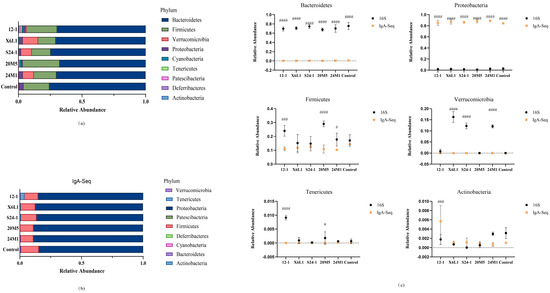
Figure 4.
Effect of L. fermentum on gut microbiota in mice at phylum level. (a) Mouse colon contents 16S rDNA sequencing, phylum level relative abundance; (b) Mouse colon contents IgA−coated bacteria 16S rDNA sequencing, phylum level relative abundance; (c) Results of comparison between IgA−coated bacteria and colon contents microbiota on gate level (“#”indicates p < 0.05, “###” indicates p < 0.001, “####” indicates p < 0.0001 (compared with 16S)).
The Chao1 index and operational taxonomic unit calculation and analysis showed that the 12-1 strain reduced the overall diversity of the host colon intestinal microbiota, but it did not affect the diversity of IgA-coated bacteria (Figure 5a,b). At the level of Lactobacillus species, the relative abundance of L. johnsonii, L. reuteri, L. acidophilus, L. murinus and L. fermentum was high. Different strains of L. fermentum had different effects on the host genus and species levels (Figure 5c,d).
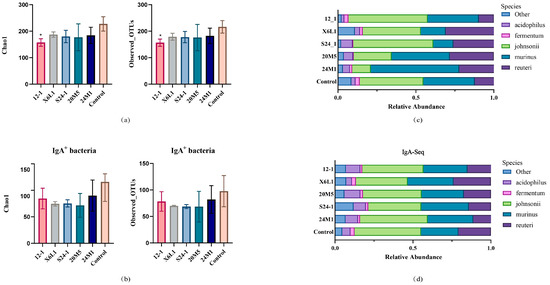
Figure 5.
Effect of L. fermentum on gut microbiota in mice at α diversity and species level. (a) Mouse colon contents bacterial α-diversity calculated by Chao1 and observed OTUs; “*” indicates p < 0.05. (Compared with control group). (b) IgA-coated bacterial α−diversity calculated by Chao1 and observed OTUs; (c) 16S rDNA sequencing of mouse colon contents, relative abundance of Lactobacillus species level; (d) IgA−coated bacteria 16S rDNA sequencing of mouse colon contents, relative abundance of Lactobacillus species level.
Welch’s t-test showed no significant difference in the S24-1 strain at the genus level compared with control group (data not shown; only groups with significant differences at the genus level are shown in Figure 6). Gavage with the 24M1 strain significantly increased the proportion of Lactobacillus species. Gavage with the 12-1 strain reduced the relative abundance of Bacteroides, Escherichia and Shigella. Gavage with the 20M5 strain increased the proportions of Eubacterium xylanophilum and Turicibacter, but decreased the proportion of Bilophila. Gavage with the X6L1 strain improved the relative abundance of Prevotellaceae UCG-001, Candidatus gastranaerophilales bacteriom zag_ 1 and Turicibacter. (Figure 6a,b).
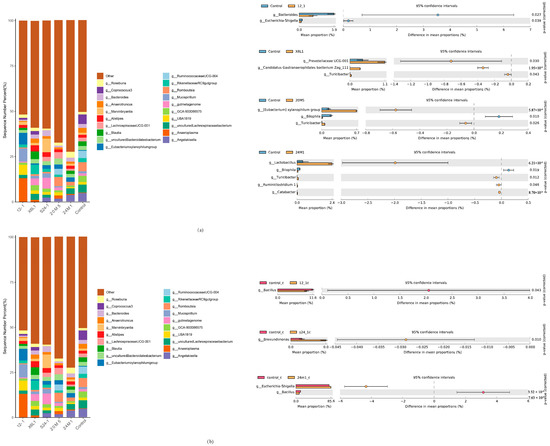
Figure 6.
Effect of L. fermentum on gut microbiota in mice at genera level. (a) Relative abundance of bacteria in mouse colon and bacteria with differences by Welch’s t test at genus level; (b) Relative abundance of IgA-coated bacteria in mouse colon and bacteria with differences by Welch’s t test at genus level.
In the correlation analysis between the genus level and IgA, Negativibacillus, Oscillibacter, Ruminiclostridium 5 and UBA1819 were significantly correlated with IgA (Figure 7a). The proportions of Escherichia and Shigella in the 24M1 strain were higher and the abundance of Bacillus was lower than those in the control group. The 12-1 strain only reduced the abundance of Bacillus. There were no significant differences among the other species in the S24-1 strain except Brevundimonas increased. At the genus level, the X6L1 and 20M5 strains did not affect the relative abundance of IgA-bound bacteria.
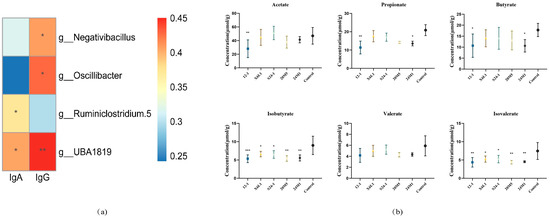
Figure 7.
Analysis of L. fermentum on gut microbiota in mice and cecum SCFAs. (a) The correlation between IgA, IgG and colonic microbiota. “*” indicates p < 0.05, and “**” indicates p < 0.01 (compared with control group); (b) The content of short chain fatty acids in cecum. “*” indicates p < 0.05, and “**” indicates p < 0.01, “***” indicates p < 0.001 (compared with control group).
All strains decreased the isobutyrate and isovalerate concentrations in the intestines. The 12-1 strain reduced the acetate, propionate and butyrate concentrations. The 24M1 strain also reduced the propionate and butyrate concentrations (Figure 7b).
3.3. L. fermentum May Stimulate Intestinal Production of IgA in Different Ways
The expression of genes encoding several reported IgA-related proteins in the mouse colon is shown in Figure 8. Of the two strains of L. fermentum, 12-1 and X6L1, that can increase the IgA content in faeces, only 12-1 upregulated BAFFR expression in the colon. The 20M5 strain upregulated pIgR expression, but the IgA concentration did not significantly change. There was no difference in gene expression related to IgA antibody class conversion among the five strains of Lactobacillus (Figure 8a).
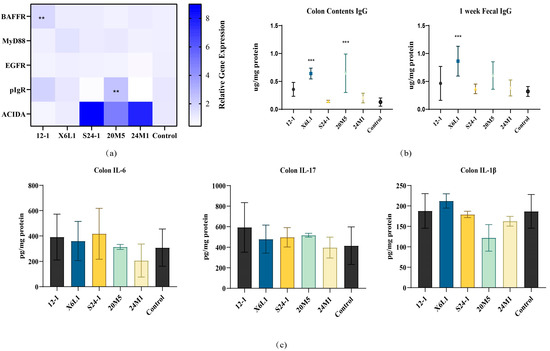
Figure 8.
Effect of L. fermentum on IgA related protein gene expression and inflammatory factor in the colon of normal mice. (a) The relative gene expression of IgA related proteins, “**” indicates p < 0.01(compared with control group); (b) The content of IgG in feces and colonic contents in one week, “***” indicates p < 0.001 (compared with control group); (c) The content of colitis indicators.
3.4. L. fermentum May Produce IgA in a Non-Inflammatory Manner
To examine the effects of different strains of L. fermentum on IgA secretion from intestinal mucosa, we measured IgG concentrations in the faeces after gavage of one week and colon contents after gavage of three weeks, when IgA concentrations were high. Only the X6L1 strain increased IgG concentrations (Figure 8b). There were no differences in IL-6, IL-17 or IL-1β concentrations among the strains (Figure 8c).
4. Discussion
Host intestinal mucosal immunity is related to intestinal symbiotic bacteria [24]. IgA is the most secreted immunoglobulin in the intestine. However, there have been few studies on the mechanism by which common bacteria, especially lactic acid bacteria, affect host intestinal IgA [25]. Three problems need to be solved to determine how an antibody reaction is related to common microorganisms in the intestine. First, whether the IgA response is specific to the symbiotic bacteria that stimulate it is unknown. Second, how the IgA reaction adapts to the current bacteria in the intestine is unclear. Third, whether all bacteria can effectively induce IgA in an equal manner is unknown [13,26,27].
In this study, we used five strains of L. fermentum from different sources to examine whether there are intraspecific differences in the effect of inducing IgA. We found that not all L. fermentum strains stimulated IgA production in the gut. The increase in free IgA content in faeces may be due to (1) an increase in IgA plasma cells (2) and an increase in IgA transported from the intestinal lamina propria to the intestinal cavity. With regard to an increase in the free IgA content in faeces, Lactobacillus may promote the proliferation of B cells or stimulate antibody class switch recombination (CSR) in the B cells. Either of these possibilities could increase the number of IgA plasma cells at the corresponding effector site in mice. We performed a series of experiments to investigate this possibility. As shown in Figure 2 and Figure 3, compared with the control group, the concentrations of IgA in different intestinal segments of Lactobacillus-treated mice were different, suggesting that different L. fermentum strains had different effector sites in the intestine of mice. Immunofluorescence staining showed that the duodenum and colon had the most plasma cells. There were differences in the duodenum and ileum. Considering the results of IgA content in various intestinal segments in Figure 2b–f, it is speculated that X6L1, S24-1, and 20M5 first induced IgA-specific immunity in the duodenum and stimulated the differentiation of IgA plasma cells in the lamina propria. However, there was no difference in the fluorescence intensity of IgA plasma cells in the jejunum, so the effect sites of X6L1 and 12-1 may not be in the jejunum. The fluorescence intensity of X6L1 and 12-1 in the ileum were significantly lower than that of the control group and other Lactobacillus intervention groups. In addition, the ileum was the site with the lowest IgA content in both groups. X6L1, S24-1, and 20M5 decreased the fluorescence intensity of IgA plasma cells in the colon, while there was no difference in IgA concentration in colon tissue. However, from the content of IgA in colonic contents, it was significantly increased in the X6L1 group, significantly decreased in S24-1 group, and not significantly changed in the 20M5 group. This further indicates that different strains of L. fermentum have different effects on intestinal stimulating IgA. In the faeces, the IgA levels of the 12-1, X6L1 group were significantly higher than that of the control group, which means that the two groups of L. fermentum increased the content of IgA from the overall level of the intestinal tract. Notably, as shown in Figure 3b, 12-1 did not increase the number of IgA plasma cells in the gut. Therefore, it is speculated that 12-1 might alter the transport efficiency of IgA from the lamina propria, rather than promote the proliferation of IgA-producing cells. This indicated that different strains of Lactobacillus of the same species had differences in the way of stimulating the intestinal secretion of IgA. The small intestine may play an important role in the production of IgA.
In Figure 2a, during continuous gavage for 3 weeks, IgA concentrations in the faeces showed a downward trend and gradually approached those in the control group. With extended contact time between the intestinal mucosa and bacteria, IgA concentrations could not be maintained at a high level. Whether the immunogenicity of lactic acid bacteria is related to this issue is unclear [28]. After a period of adaptation in this study, the gut microbiota reached a new balance. The mucosal immunity developed tolerance to the experimental strains and no longer secreted too much IgA [29]. As a result, IgA concentrations in the faeces decreased with an increase in the experimental duration.
Considering an increase in IgA transported from the intestinal lamina propria to the intestinal cavity, Lactobacillus may stimulate B cell associated pathways protein gene expression in mouse intestinal epithelial cells [30]. This stimulation could result in a higher rate of transport of IgA produced in the lamina propria out of the intestinal cavity, which could improve the transport efficiency [31]. BAFF is involved in B cell homeostasis and survival including promoting B cell to plasma cell transformation, making it an important pathway for regulating CSR and antibody production [32]. As a BAFF receptor, the content of BAFFR directly reflects the number of plasma cells [32]. In Figure 8a, the up-regulation of BAFFR levels suggested that L. fermentum 12-1 may increase the number of plasma cells by stimulating colonic secretion of BAFF. As a transmembrane receptor, EGFR can widely regulate cell proliferation, differentiation, migration and survival, and participate in various physiological and biochemical reactions [33]. However, L. fermentum treatments showed no effect on EGFR levels. It is suggested that this species of Lactobacillus does not activate the EGFR pathway. MyD88 acts as a linker molecule in the TLR pathway. It is involved in mediating the activation of the NF-κB pathway, stimulating the secretion of cytokines, and transmitting inflammatory signals [34]. In this study, the gene transcription level of this protein in the colon did not change, which indicated that L. fermentum may not stimulate colon inflammation. pIgR located on the basal surface of intestinal epithelial cells can bind to IgA in the lamina propria, allowing it to pass through the epithelial cells to the mucosal surface to exert its protective effect [35]. Only the expression of pIgR was up-regulated in the 20M5 group, but there was no significant change in the concentration of IgA. It is speculated that other subclasses of antibodies such as IgG (Figure 8b) may be transported from the lamina propria to the intestinal lumen. As a subclass of immunoglobulin, IgA needs to complete complex biochemical reactions through a special antibody CSR in order to exert its corresponding efficacy. Antigens are transported to B cells through antigen-presenting cells [36]. After B cells are stimulated, activation-induced cytidine deaminase (AID) is activated accordingly and begins to transcribe specific gene regions on the immunoglobulin heavy chain [36]. The expression level of ACIDA is an important indicator to detect whether antibody CSR occurs [36]. The ACIDA transcription levels were up-regulated in the colon tissue of mice treated with S24-1, 24M1 and 20M5. But the data showed that these three strains did not up-regulate the level of IgA in the intestine. Therefore, it is presumed that B cells at this site undergo antibody CSR but have not matured into IgA+ plasma cells. They may be transformed into plasma cells that secrete other subclasses of antibodies such as IgG. From this, it is not difficult to infer that Lactobacillus that affects different levels of intestinal secretion of IgA may cause IgA-specific immunity in different ways. These pathways are not limited to BAFFR, MyD88, EGFR, pIgR, or ACIDA-related pathways.
An increase in IgA concentrations in the faeces may not only be the result of the direct action of L. fermentum but also could be due to a new balance of the intestinal microbiota. The relative abundance of Lactobacillus and L. fermentum did not increase at the genus or species level in this study (Figure 6a,b). However, L. fermentum affected the relative abundance of other members of the intestinal microbiota. The change in IgA concentrations in the intestine is a dynamic and comprehensive result. To further study the effect of stimulation of a single bacterium on host intestinal IgA, germ-free mice need to be colonised with single species of bacteria.
IgA plays different roles by binding to different bacteria [29]. IgA combines with pathogenic bacteria to achieve the effect of immune exclusion, and with symbiotic bacteria to help colonisation [22,25,26]. However, how IgA identifies pathogenic bacteria and symbiotic bacteria is unknown. Using the immunomagnetic beads combined with 16S rDNA analysis in this study, L. fermentum was showed to have different effects on the host colonic microbiota and IgA-coated bacteria. There were differences in Escherichia, Shigella, Bacillus and Brevundimonas at the genus level (Figure 6a,b). The effect of IgA on these genera may be different [27]. IgA can specifically target lipopolysaccharide in Escherichia and Shigella species to limit it to the intestinal mucus layer and reduce intestinal inflammation [35]. However, IgA induced by Bacteroides fragilis helps the bacterium cluster and anchor on the surface of the intestinal epithelium, thus providing it with a competitive advantage [28]. In this study, differences in IgA-coated bacteria were not related to IgA concentrations. It is doubtful whether the difference of microbiota caused by different L. fermentum has any effect on IgA concentration. At present, whether this result is a two-way effect caused by IgA and the gut microbiota is unclear. Exploring the causes of this phenomenon will require more in-depth research.
In recent years, there has been a debate on whether short-chain fatty acids are related to IgA. As a metabolite of gut microbiota, acetic acid has been formally known to stimulate the secretion of IgA [37]. L. fermentum is a heterologous fermentation strain which produces lactic acid and large amounts of acetic acid, ethanol and CO2 after fermentation [37]. However, acetic acid concentrations in the caecum did not increase in this study (Figure 7b). There was no correlation between IgA and short-chain fatty acids in the caecum. Short-chain fatty acids are mainly produced in the large intestine [38]. Indeed, IgA has been confirmed in the existing studies to be produced in the small intestine and enriched in the colon [38]. However, this conclusion is mainly proposed for pathogenic bacteria or invasive and strong bacteria that can cause IgA-T cell-dependent-PPs pathways, such as Segmented filamentous bacteria [4]. Whether all the bacteria only stimulate the small intestine to secrete IgA is unknown. Therefore, the causal relationship between IgA and short-chain fatty acids cannot be determined. Short-chain fatty acids concentrations in the small intestine might be strongly correlated with IgA concentrations. Initially, we would like to explore the effector site of L. fermentum to stimulate the intestinal secretion of IgA. It was not clear from the previous experiments that L. fermentum would stimulate IgA secretion in the small intestine, since the bacteria is mainly concentrated in the colon. Therefore, we detected the IgA-coated bacteria in the colon in order to explore the effect of L. fermentum on the gut microbiota. The path through which the 12-1 and X6L1 strains stimulate intestinal lymphocytes to secrete IgA will be the focus of a future study. However, we speculate that these two strains affect different reaction pathways to a great extent.
Intestinal IgA may provide immune protection and rejection in a non-inflammatory manner, which promotes the establishment of the host microbial interaction mechanism [39]. Interleukins are a class of cytokines that are produced by cells and have direct or indirect stimulatory effects on immune cells [39]. It plays a special role in innate and adaptive immunity. Macrophages, Th2 cells, vascular endothelial cells, and fibroblasts are the main sources of interleukins [39]. IL-6 can stimulate the proliferation and differentiation of B cells, and regulate the inflammatory response by releasing antibodies [39]. IL-17 is an early initiator of T cell-induced inflammatory response and can release pro-inflammatory cytokines to amplify the inflammatory response [40]. IL-1β is a cytokine and regulator secreted by macrophages to activate cellular immunity [40]. However, the contents of IL-6, IL-17 and IL-1β in the colon of all L. fermentum treated mice did not change significantly (Figure 8c). X6L1 and 12-1, which induced IgA, might affect the intrinsic and specific immunity of intestinal mucosa through their special epitopes or secondary metabolites, and then promote the secretion of IgA. Intestinal IgA might provide immune protection for the host in a non-inflammatory manner, promoting the interaction between the host’s gut microbiota and mucosal immunity, and maintaining immune balance. The cell walls of different strains of L. fermentum need to be investigated in further studies to determine whether the difference in antigenic determinants in the cell wall lead to a difference in the IgA response [41], whether different surface antigens stimulate IgA in the same manner and whether other types of lactic acid bacteria also have intraspecific differences in stimulating the secretion of IgA from the intestinal mucosa [41]. At present, the two-way effect between symbiotic bacteria and IgA is not well understood. IgA may have an undiscovered response mechanism in humoral immunity.
Notably, among the five tested strains of L. fermentum, two were from infants and three were from older people (Table 1). The strains that increased IgA concentrations in faeces were from the infants. Whether the bacteria that cause changes in IgA in the intestine are related to age and the physical condition of the provider are unknown [42]. An infant’s intestinal environment might be different from that of an adult, which may lead to differences in genes of the same bacteria and thus to different characteristics of the bacteria [42]. The intestinal microbiota and immune system in infants are in a process of development [43]. During this process, the production of IgA may be different from that in adults, which is stimulated by a stable intestinal microbiota [43]. Additionally, the induction of IgA by bacteria in different intestinal environments may be different between infants and adults. This difference may be at the levels of the gut microbiota and of different genotypes and physiological characteristics of the same species. This possibility would indicate a complicated interaction between the host gut microbiota and IgA [44]. There have been no relevant reports on whether the age or sex of the provider affects their intestinal IgA concentration. Therefore, this lack of knowledge could point to a new direction of IgA research.
5. Conclusions
Different strains of L. fermentum show differences in stimulating the secretion of IgA in the host intestine. Some of these strains increase the number of IgA plasma cells in the duodenum, which increases IgA concentrations, while some stimulate BAFFR expression to induce high IgA concentrations. Furthermore, IgA concentrations are not significantly correlated with short-chain fatty acid concentrations in the caecum. L. fermentum, from different sources, has different effects on the host intestinal microbiota, but it does not affect the diversity of IgA-coated microbiota. Additionally, L. fermentum may stimulate intestinal secretion of IgA through different reaction pathways in a non-inflammatory manner.
Author Contributions
L.M.: investigation, writing—original draft, visualization; Y.C.: methodology, investigation; J.W.: software, formal analysis; J.Z.: validation, resources, data curation; H.Z.: methodology, validation, resources; J.L. and G.W.: conceptualization, validation, writing—review and editing, supervision, project administration, funding acquisition; W.C.: resources, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31972052, 32021005, 31820103010), the Fundamental Research Funds for the Central Universities (JUSRP22006, JUSRP51501), “Light of Taihu Lake” science and technology research project sponsored by Science and Technology Bureau of Wuxi City (Y20212030), the Program of Collaborative Innovation Centre of Food Safety and Quality Control in Jiangsu Province.
Institutional Review Board Statement
The study was conducted according to the Guidelines for Care and Use of Laboratory Animals of Jiangnan University (SYXK 2016-0045) and approved by the Animal Ethics Committee of Experimental Animals at Jiangnan University (qualified number: JN. No:20210315c1080601 [012]).
Informed Consent Statement
Not applicable.
Acknowledgments
Thanks to Yihan Li, Mengshu Xu, Luyao Wang and Zheng Wang for their help in the experiment.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Feifei, X.; Newby, J.M.; Schiller, J.L.; Schroeder, H.A.; Wessler, T.; Chen, A.; Forest, M.G.; Lai, S.K. Modeling Barrier Properties of Intestinal Mucus Reinforced with IgG and Secretory IgA against Motile Bacteria. ACS Infect. Dis. 2019, 5, 1570–1580. [Google Scholar] [CrossRef]
- Steinhoff, U. Who controls the crowd? New findings and old questions about the intestinal microflora. Immunol. Lett. 2005, 99, 12–16. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive Immune Regulation in the Gut: T Cell–Dependent and T Cell–Independent IgA Synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar] [CrossRef]
- Liu, H.Y.; Seignez, C.; Ahl, D.; Giraud, A.; Guo, F.; Sedin, J.; Walden, T.; Roos, S.; Holm, L.; Bertilsson, S.; et al. Bacteria-immune cell interactions at the intestinal Peyer’s Patches. Free. Radic. Biol. Med. 2019, 139, S3. [Google Scholar]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Shroff, K.E.; Meslin, K.; Cebra, J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 1995, 63, 3904–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, A.J.; Hunziker, L.; McCoy, K.; Lamarre, A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001, 3, 1021–1035. [Google Scholar] [CrossRef]
- Gupta, S.; Basu, S.; Bal, V.; Rath, S.; George, A. Gut IgA abundance in adult life is a major determinant of resistance to dextran sodium sulfate-colitis and can compensate for the effects of inadequate maternal IgA received by neonates. Immunology 2019, 158, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci. Rep. 2018, 8, 5065. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Tres, A. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects mammary milk composition. J. Dairy Sci. 2020, 103, 2982–2992. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Komatsu, R. Evaluation of the Immunomodulatory Activities of the Probiotic Strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1376. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Miyauchi, E. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 2021, 595, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; McCoy, K.D. The function of secretory IgA in the context of the intestinal continuum of adaptive immune responses in host-microbial mutualism. Semin. Immunol. 2012, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Caixin, N.; Xing, J. Evaluation of antioxidant activity of Lactobacillus in vivo using Caenorhabditis elegans and comparison with its antioxidant parameters in vitro. Food Ferment. Ind. 2019, 3, 21–27. [Google Scholar]
- Choi, Y.; Jeon, B.Y.; Shim, T.S.; Jin, H.; Cho, S.N.; Lee, H. Development of a highly sensitive one-tube nested real-time PCR for detecting Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 2014, 80, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L. A randomised, double-blind, placebo-controlled trial of Bifidobacterium bifidum CCFM16 for manipulation of the gut microbiota and relief from chronic constipation. Food Funct. 2022, 13, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, C.A.; Tomasi, T.B. The life span of IgA plasma cells from the mouse intestine. J. Exp. Med. 1973, 138, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Qi, C. IgA-Targeted Lactobacillus jensenii Modulated Gut Barrier and Microbiota in High-Fat Diet-Fed Mice. Front. Microbiol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, C.; Ding, M. Sex-dependent modulation of immune development in mice by secretory IgA-coated Lactobacillus reuteri isolated from breast milk. J. Dairy Sci. 2021, 104, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y. Pediococcus acidilactici CCFM6432 mitigates chronic stress-induced anxiety and gut microbial abnormalities. Food Funct. 2021, 12, 11241–11249. [Google Scholar] [CrossRef] [PubMed]
- Huus, K.E.; Bauer, K.C. Commensal Bacteria Modulate Immunoglobulin A Binding in Response to Host Nutrition. Cell Host Microbe 2020, 27, 909–921.e5. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Yilmaz, B. IgA Function in Relation to the Intestinal Microbiota. Annu. Rev. Immunol. 2018, 36, 359–381. [Google Scholar] [CrossRef]
- Yang, C.; Mogno, I. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host Microbe 2020, 27, 467–475.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, H.; Markus, A.; Diard, M.; Loverdo, C.; Slack, E. Growing, evolving and sticking in a flowing environment: Understanding IgA interactions with bacteria in the gut. Immunology 2020, 159, 52–62. [Google Scholar]
- Bunker, J.J.; Flynn, T.M. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 2015, 43, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, G.P.; Ladinsky, M.S. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadlallah, J.; El Kafsi, H.; Sterlin, D.; Juste, C.; Parizot, C.; Dorgham, K.; Autaa, G.; Gouas, D.; Almeida, M.; Lepage, P.; et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 2018, 10, eaan1217. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Bautista, E.R.; Suzuki, K. Bacteria-immune cells dialog and the homeostasis of the systems. Curr. Opin. Immunol. 2020, 66, 82–89. [Google Scholar] [CrossRef]
- Liang, Z.-Q.; Tu, P. Gu-Ben-Fang-Xiao attenuates allergic airway inflammation by inhibiting BAFF-mediated B cell activation. Biomed. Pharmacother. 2020, 132, 110801. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.W. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanan, D.; Sefik, E. An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint. Cell 2020, 181, 1276–1290. [Google Scholar] [CrossRef]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients Mediate Intestinal Bacteria-Mucosal Immune Crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Bendelac, A. IgA Responses to Microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Schwab, C.; Ganzle, M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Fujihashi, K.; McGhee, J.R. Cytoking specific elispot assay single cell analysis of IL-2, IL-4 and IL-6 production cells. J. Immunol. Methods 1993, 160, 181–189. [Google Scholar] [CrossRef]
- Isailovic, N.; Daigo, K. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Bellis, K.; Faal, A.; Birt, J. SIgA, TGF-beta1, IL-10, and TNF alpha in Colostrum Are Associated with Infant Group B Streptococcus Colonization. Front. Immunol. 2017, 8, 1269. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F. Association between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O. Potential role of the intestinal microbiota in programming health and disease. Nutr. Rev. 2015, 73 (Suppl. S1), 32–40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).