Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Biomass
2.2. Adsorption Tests
2.3. Adsorption Kinetics
2.4. Adsorption Equilibrium
2.5. Thermodynamic Parameters
3. Results
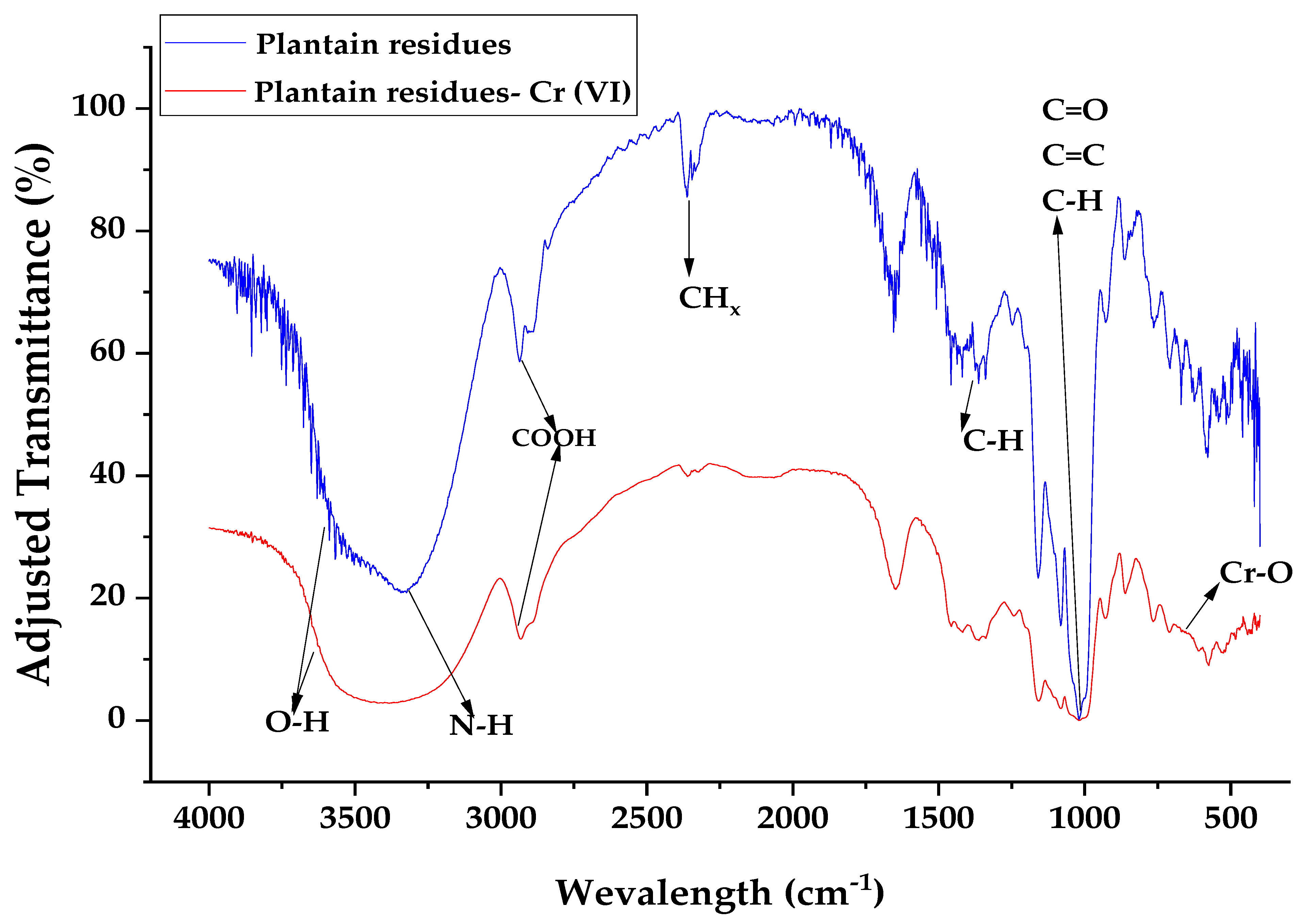
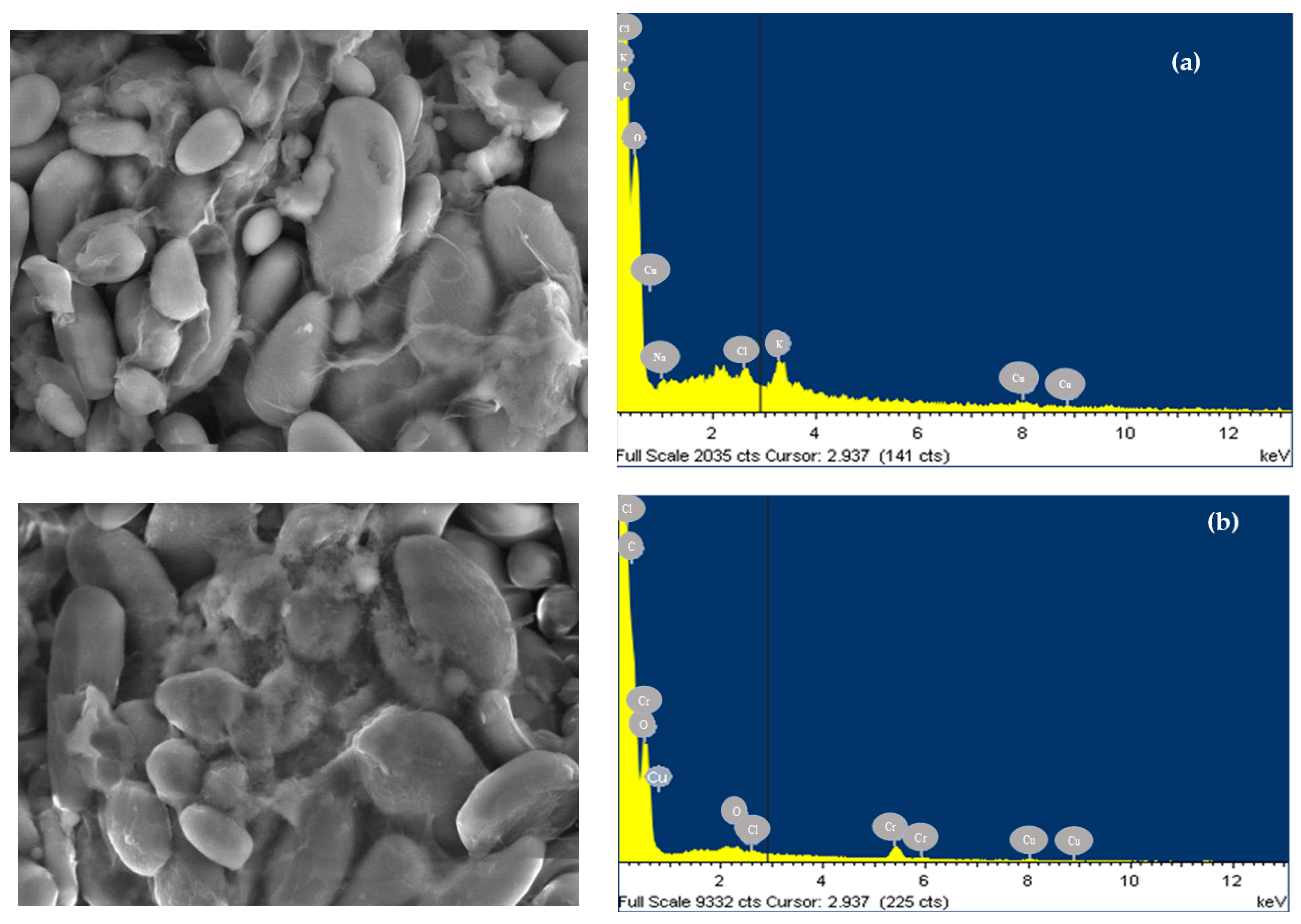
3.1. Characterization of the Bioadsorbent
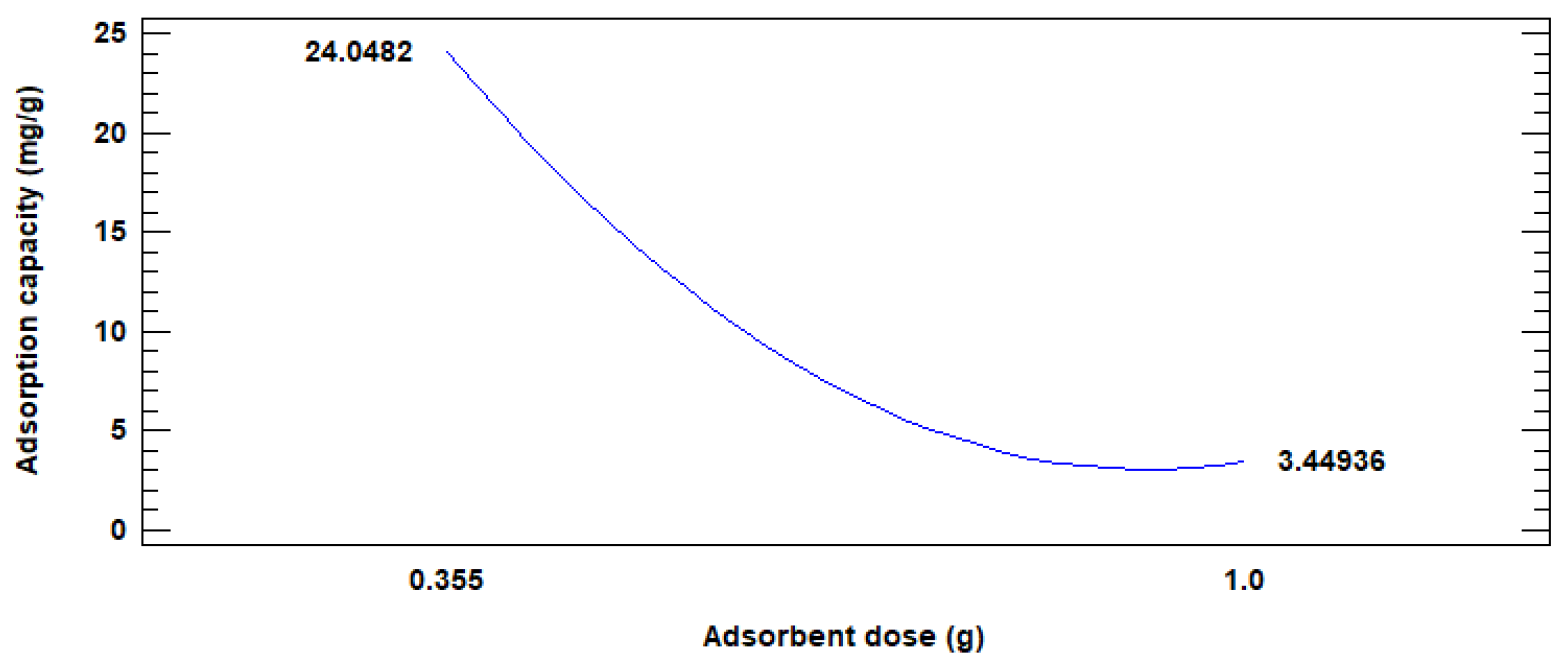
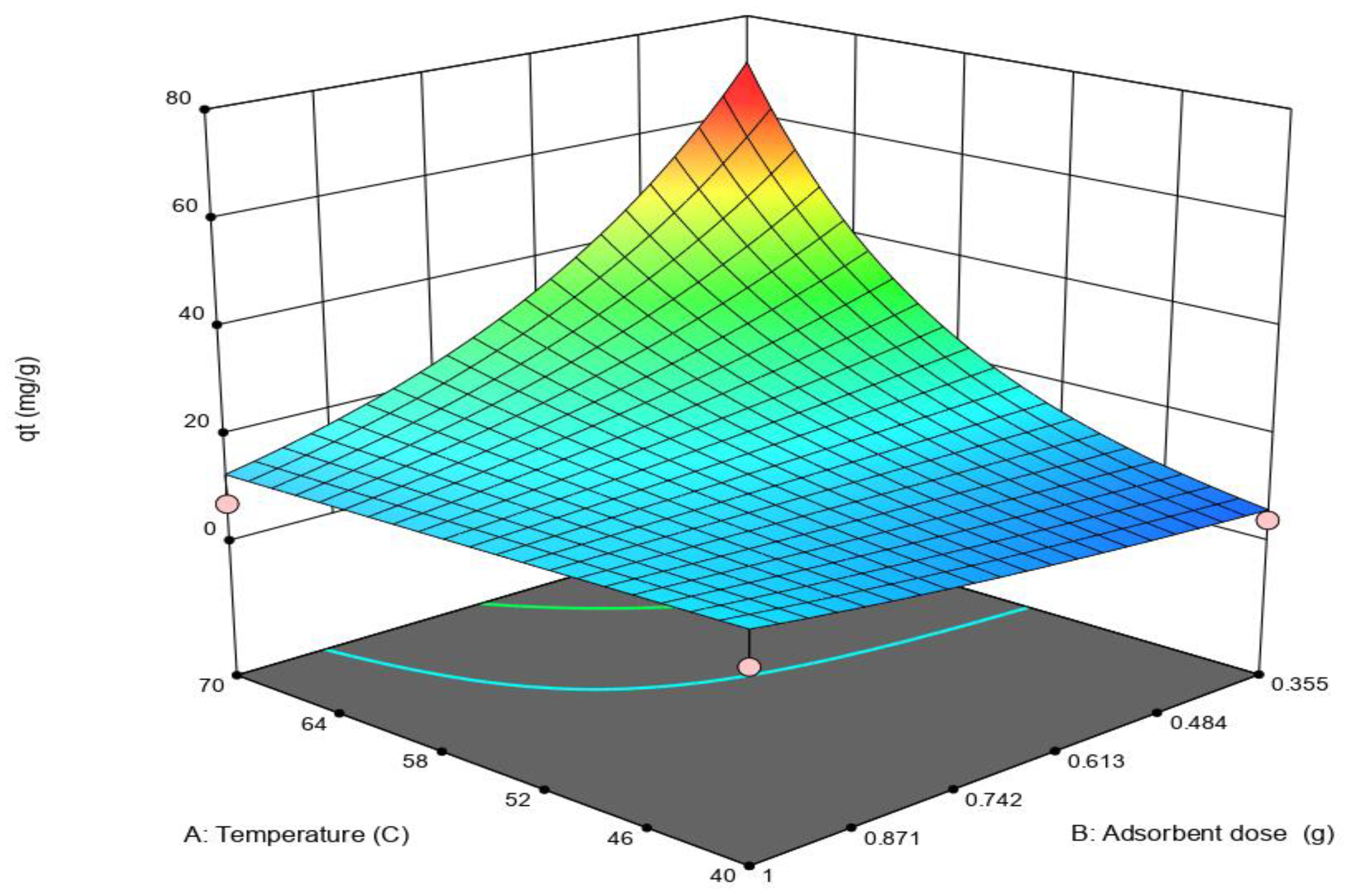
3.2. Adsorption Test and Statistical Analysis
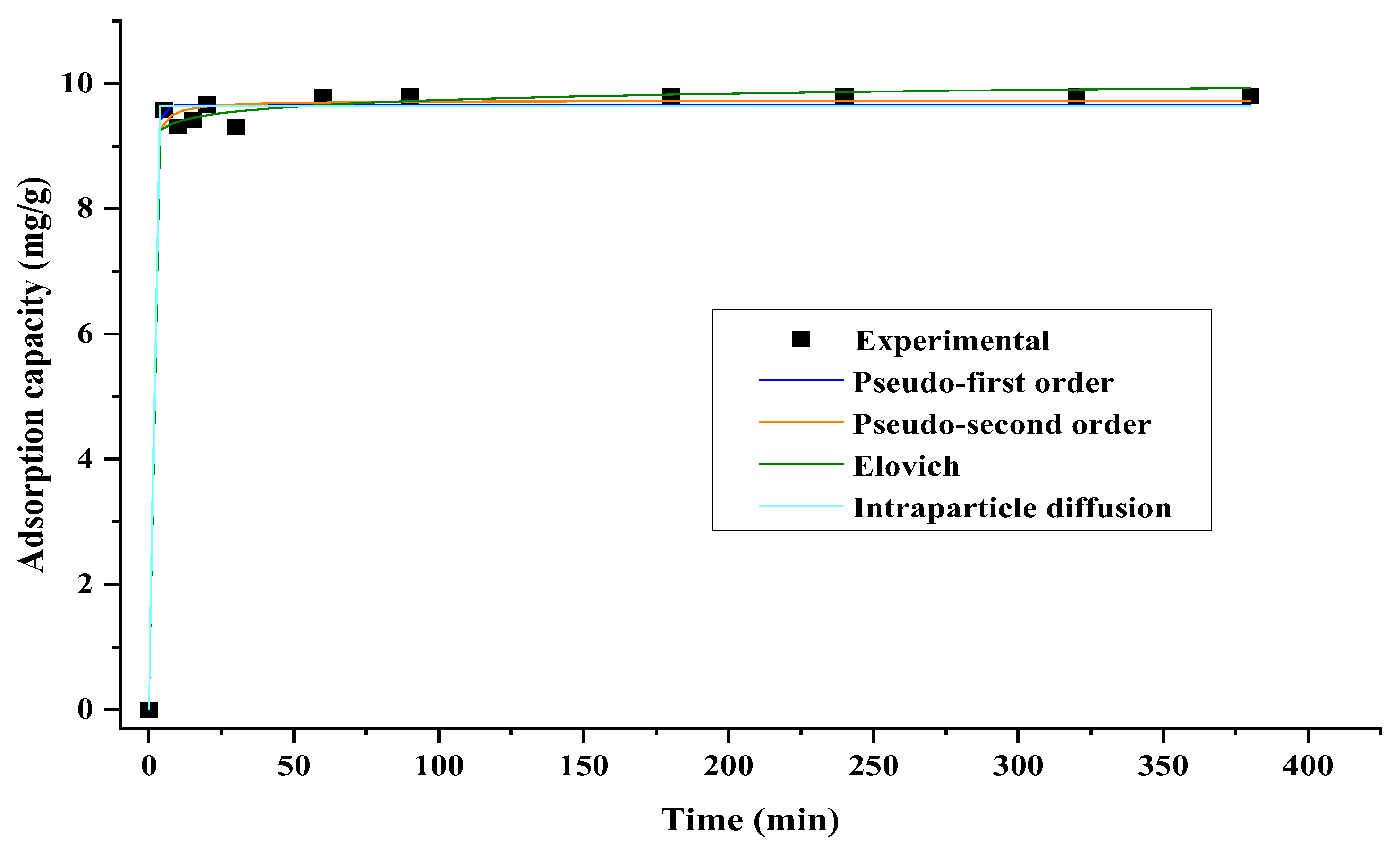
3.3. Cr (VI) Adsorption Kinetics
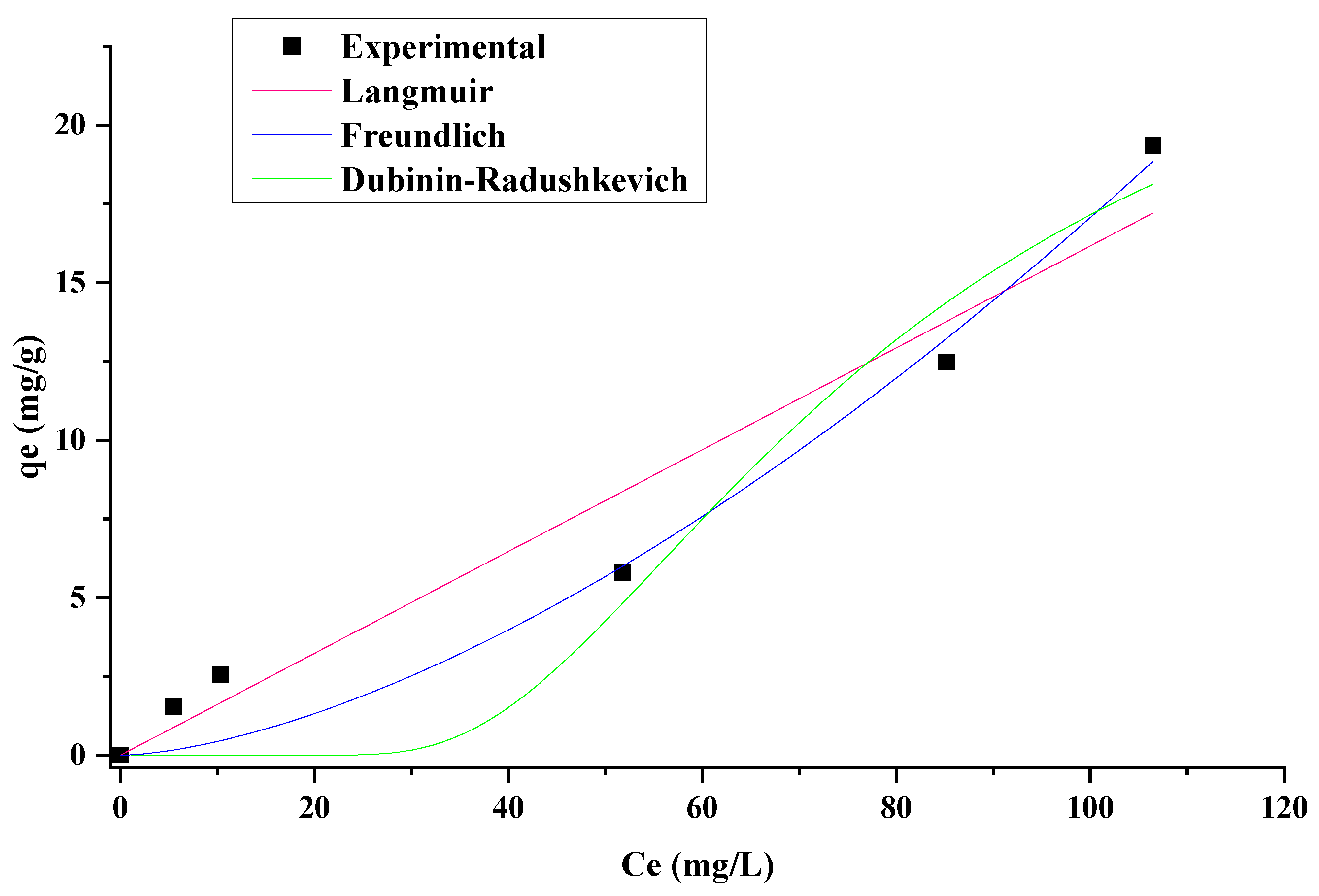
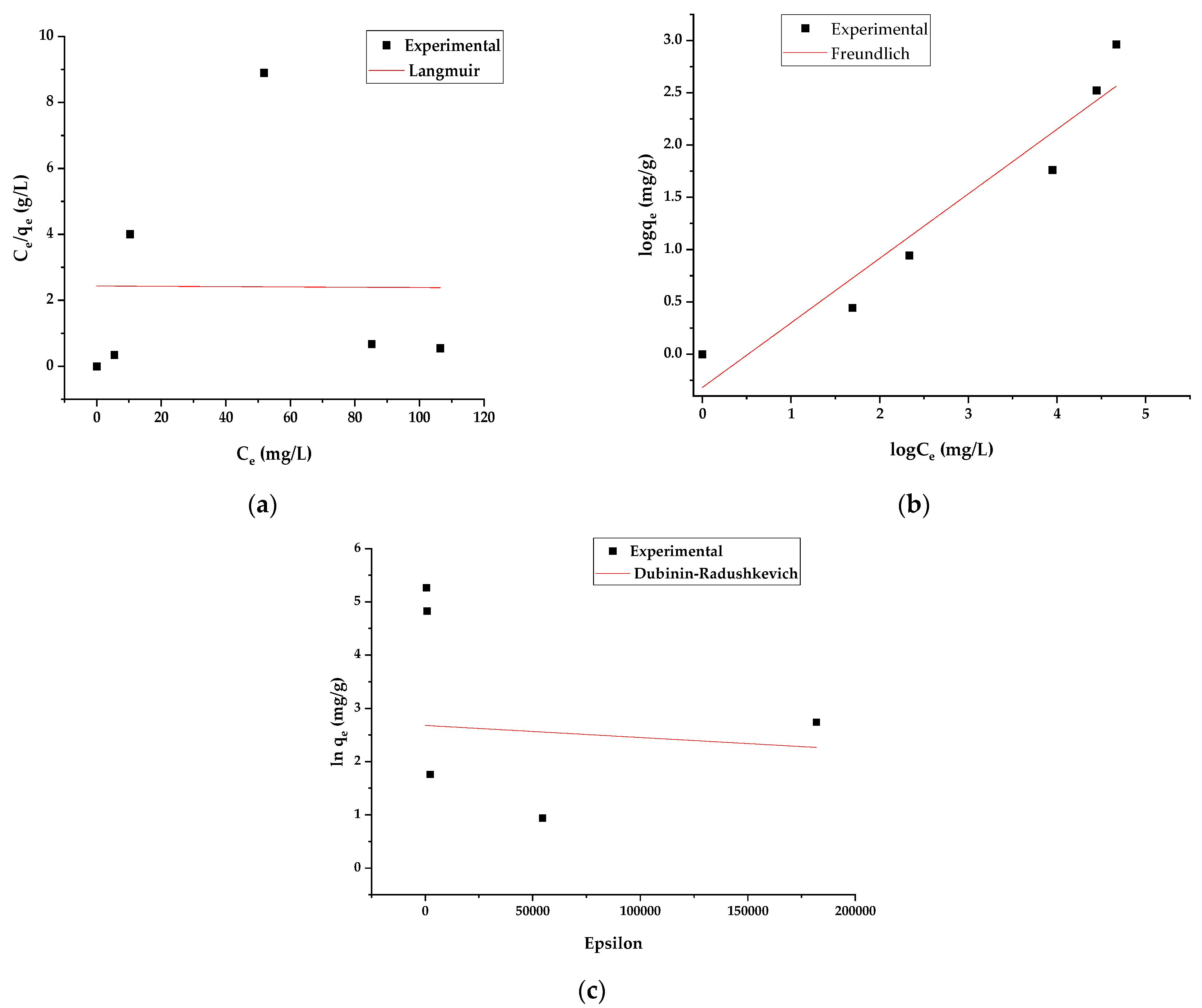
3.4. Adsorption Equilibrium
3.5. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Manjuladevi, M.; Anitha, R.; Manonmani, S. Kinetic study on adsorption of Cr(VI), Ni(II), Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl. Water Sci. 2018, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Parlayici, Ş.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Mullick, A.; Moulik, S.; Bhattacharjee, S. Removal of Hexavalent Chromium from Aqueous Solutions by Low-Cost Rice Husk-Based Activated Carbon: Kinetic and Thermodynamic Studies. Indian Chem. Eng. 2018, 60, 58–71. [Google Scholar] [CrossRef]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.K.; Samanta, A.; Chakraborty, S.; Shaikh, W.A. Enhanced chromium(VI) removal using banana peel dust: Isotherms, kinetics and thermodynamics study. Sustain. Water Resour. Manag. 2017, 4, 489–497. [Google Scholar] [CrossRef]
- Lee, C.-G.; Lee, S.; Park, J.-A.; Park, C.; Lee, S.J.; Kim, S.-B.; An, B.; Yun, S.-T.; Choi, J.-W. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 2017, 166, 203–211. [Google Scholar] [CrossRef]
- Corral-Escárcega, M.C.; Ruiz-Gutiérrez, M.G.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; Lardizabal-Gutiérrez, D.; Campos-Venegas, K. Use of biomass-derived from pecan nut husks (Carya illinoinensis) for chromium removal from aqueous solutions. column modeling and adsorption kinetics studies. Rev. Mex. Ing. Qumica 2017, 16, 939–953. [Google Scholar]
- Gómez Aguilar, D.L.; Rodríguez Miranda, J.P.; Esteban Muñoz, J.A.; Betancur, J.F. Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters. Processes 2019, 7, 403. [Google Scholar] [CrossRef] [Green Version]
- Tejada-Tovar, C.; Herrera-Barros, A.; Villabona-Ortíz, A. Assessment of Chemically Modified Lignocellulose Waste for the Adsorption of Cr (VI). Rev. Fac. Ing. 2020, 29, e10298. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 244–271. [Google Scholar] [CrossRef]
- Al-Jubory, F.K.; Mujtaba, I.M.; Abbas, A.S. Preparation and characterization of biodegradable crosslinked starch ester as adsorbent. AIP Conf. Proc. 2020, 2213, 020165. [Google Scholar] [CrossRef]
- Villabona-Ortíz, A.; Tejada-Tovar, C.; Ruiz-Paternina, E.; Frías-González, J.D.; Blanco-García, G.D. Optimization of the Effect of Temperature and Bed Height on Cr (VI) Bioadsorption in Continuous System. Rev. Fac. Ing. 2020, 29, e10477. [Google Scholar] [CrossRef]
- Ruiz-Paternina, E.B.; Villabona-Ortiz, Á.; Tejada-Tova, C.; Ortega-Toro, R. Estudio Termodinámico de la Remoción de Níquel y Cromo en Solución Acuosa usando Adsorbentes de Origen Agroindustrial. Inf. Tecnol. 2019, 30, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Maniglia, B.; Tapia-Blácido, D. Food Hydrocolloids Isolation and characterization of starch from babassu mesocarp. Food Hydrocoll. 2016, 55, 47–55. [Google Scholar] [CrossRef]
- Villabona-Ortíz, A.; De Cartagena, U.; Tejada-Tovar, C.N.; Ortega-Toro, R. Modelling of the adsorption kinetics of Chromium (VI) using waste biomaterials. Rev. Mex. Ing. Química 2019, 19, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Peng, S.-H.; Wang, R.; Yang, L.-Z.; He, L.; He, X.; Liu, X. Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicol. Environ. Saf. 2018, 165, 61–69. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Ruiz-Paternina, E.; Herrera-Barros, A.; Ortega-Toro, R. Characterization and use of agroindustrial by-products in the removal of metal ions in aqueous solution. J. Teknol. 2019, 81, 151–158. [Google Scholar] [CrossRef]
- Schwantes, D.; Gonçalves, A.C.; Campagnolo, M.A.; Tarley, C.R.T.; Dragunski, D.C.; de Varennes, A.; dos Santos Silva, A.K.; Conradi, E. Chemical modifications on pinus bark for adsorption of toxic metals. J. Environ. Chem. Eng. 2018, 6, 1271–1278. [Google Scholar] [CrossRef]
- Medellin-Castillo, N. Bioadsorción de plomo (II) presente en solución acuosa sobre residuos de fibras naturales procedentes de la industria ixtlera (Agave lechuguilla Torr. y Yucca carnerosana (TREL.) MCKELVEY). Rev. Int. Contam. Ambient. 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Rodriguez Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Tripathi, B.D.; Rai, A.K. Packed-bed column biosorption of chromium(VI) and nickel(II) onto Fenton modified Hydrilla verticillata dried biomass. Ecotoxicol. Environ. Saf. 2016, 132, 420–428. [Google Scholar] [CrossRef]
- Chinyelu Ijeamaka, E.; Odebeatu Chinoso, C.; Obumselu Onyeka, F.; Iloamaeke Ifeoma, M.J.; Kammeje Tochukwu, J. Isotherm Studies of Adsorption of Cr (Vi) Ions onto Coconut Husk. Int. J. Biochem. Biophys. Mol. Biol. 2018, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Mutongo, F.; Kuipa, O.; Kuipa, P.K. Removal of Cr(VI) from Aqueous Solutions Using Powder of Potato Peelings as a Low Cost Sorbent. Bioinorg. Chem. Appl. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Saranya, N.; Ajmani, A.; Sivasubramanian, V.; Selvaraju, N. Hexavalent Chromium Removal from Simulated and Real Effluents Using Artocarpus Heterophyllus Peel Biosorbent—Batch and Continuous Studies, 265; Elsevier B.V: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R. Oak (Quercus robur) Acorn Peel as a Low-Cost Adsorbent for Hexavalent Chromium Removal from Aquatic Ecosystems and Industrial Effluents. Water Air Soil Pollut. 2016, 227, 62. [Google Scholar] [CrossRef]
- Ajmani, A.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 2019, 26, 32137–32150. [Google Scholar] [CrossRef]
- Azadi, F.; Saadat, S.; Karimi-Jashni, A. Experimental Investigation and Modeling of Nickel Removal from Wastewater Using Modified Rice Husk in Continuous Reactor by Response Surface Methodology. Iran. J. Sci. Technol. Trans. Civ. Eng. 2018, 42, 315–323. [Google Scholar] [CrossRef]
- Nordin, N.; Asmadi, N.A.A.; Manikam, M.K.; Halim, A.A.; Hanafiah, M.M.; Hurairah, S.N. Removal of Hexavalent Chromium from Aqueous Solution by Adsorption on Palm Oil Fuel Ash (POFA). J. Geosci. Environ. Prot. 2020, 08, 112–127. [Google Scholar] [CrossRef] [Green Version]
- Mahmood-Ul-Hassan, M.; Yasin, M.; Yousra, M.; Ahmad, R.; Sarwar, S. Kinetics, isotherms, and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust. Environ. Sci. Pollut. Res. 2018, 25, 12570–12578. [Google Scholar] [CrossRef]
- Manirethan, V.; Gupta, N.; Balakrishnan, R.M.; Raval, K. Batch and continuous studies on the removal of heavy metals from aqueous solution using biosynthesised melanin-coated PVDF membranes. Environ. Sci. Pollut. Res. 2019, 27, 24723–24737. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Arim, A.L.; Neves, K.; Quina, M.; Gando-Ferreira, L.M. Experimental and mathematical modelling of Cr(III) sorption in fixed-bed column using modified pine bark. J. Clean. Prod. 2018, 183, 272–281. [Google Scholar] [CrossRef]
- Rodrigues, E.; de Almeida, O.; Brasil, H.; Moraes, D.; Reis, M.A.L. Applied Clay Science Adsorption of chromium (VI) on hydrotalcite-hydroxyapatite material doped with carbon nanotubes: Equilibrium, kinetic and thermodynamic study. Appl. Clay Sci. 2019, 172, 57–64. [Google Scholar] [CrossRef]
- Módenes, A.N.; de Oliveira, A.P.; Espinoza-Quiñones, F.R.; Trigueros, D.E.G.; Kroumov, A.; Bergamasco, R. Study of the involved sorption mechanisms of Cr(VI) and Cr(III) species onto dried Salvinia auriculata biomass. Chemosphere 2017, 172, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zand, A.D.; Abyaneh, M.R. Adsorption of Lead, manganese, and copper onto biochar in landfill leachate: Implication of non-linear regression analysis. Sustain. Environ. Res. 2020, 30, 1–16. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Hamitouche, A.Y.E.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef] [Green Version]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Haroon, H.; Ashfaq, T.; Gardazi, S.M.H.; Sherazi, T.A.; Ali, M.; Rashid, N.; Bilal, M. Equilibrium kinetic and thermodynamic studies of Cr(VI) adsorption onto a novel adsorbent of Eucalyptus camaldulensis waste: Batch and column reactors. Korean J. Chem. Eng. 2016, 33, 2898–2907. [Google Scholar] [CrossRef]



| Independent Variables | Unit | Range and Level | ||||
|---|---|---|---|---|---|---|
| Lower Value | Low Value | Center Point | High Value | Higher Value | ||
| Temperature | °C | 29.80 | 40 | 55 | 70 | 80 |
| Adsorbent amount | g | 0.14 | 0.36 | 0.68 | 1.00 | 1.20 |
| Initial concentration | mg/L | 31.80 | 100 | 200 | 300 | 368.20 |
| Model | Parameter | Equation | |
|---|---|---|---|
| Pseudo-first order | qe (mg g−1): adsorption capacity at equilibrium | (3) | |
| qt (mg g−1): adsorption capacity at time t | |||
| k1 (min−1): the pseudo first order constant | |||
| Pseudo-second model | k2 (g−1 min−1): second order adsorption constant | (4) | |
| t (min): time | |||
| Elovich | α (mg g−1 min−1): the initial adsorption rate of the model | (5) | |
| β (g mg−1): is the constant related to the surface coverage scope and activation energy in chemisorption | |||
| Intraparticle diffusion | k3 (mg g−1 min−1/2): intraparticle diffusion constant. | (6) | |
| Model | Parameter | Equation | |
|---|---|---|---|
| Langmuir | qe (mg g−1): amount of contaminant adsorbed on the bioadsorbent surface | (7) | |
| b (L mg−1): ratio of adsorption/desorption | |||
| qmax (mg g−1): maximum adsorption corresponding to the saturation sites | |||
| Ce (min L−1): the pseudo-first order constant | |||
| Freundlich | KF (mg L g−2): Freundlich constant | (8) | |
| n: adsorption intensity | |||
| Dubinin–Radushkevich | ε: potential of Polanyi based on temperature | (9) | |
| KDR (mol2 kJ−2): is the constant of Dubinin–Radushkevich related to adsorption energy E (kJ mol−1): average adsorption energy per molecule of adsorbate required to transfer one mol of ion from solution to the surface of the adsorbent | (10) | ||
| (11) | |||
| Adsorbent Dose (g) | Temperature(°C) | Ci (mg/L) | Cf (mg/L) | ER (%) | qt (mg/g) |
|---|---|---|---|---|---|
| 1.00 | 70 | 100 | 95.08 | 4.92 | 0.492 |
| 0.68 | 55 | 31.82 | 18.33 | 10.95 | 1.99 |
| 0.68 | 80.2 | 200 | 0.64 | 99.18 | 29.43 |
| 0.68 | 29.8 | 200 | 97.37 | 51. 31 | 15.15 |
| 0.36 | 70 | 100 | 3.30 | 96.70 | 27.24 |
| 1.00 | 40 | 300 | 216.72 | 27.76 | 8.33 |
| 0.68 | 55 | 368.2 | 95.08 | 74.17 | 40.31 |
| 0.68 | 55 | 200 | 151.90 | 24.05 | 7.099 |
| 0.36 | 70 | 300 | 230.96 | 69.36 | 19.45 |
| 0.68 | 55 | 200 | 175.95 | 24.05 | 3.55 |
| 0.36 | 40 | 300 | 286.86 | 13.14 | 3.70 |
| 1.22 | 55 | 200 | 131.72 | 68.29 | 5.59 |
| 1.00 | 70 | 300 | 230.17 | 69.83 | 6.98 |
| 0.36 | 40 | 100 | 40.68 | 59.32 | 16.71 |
| 1.00 | 40 | 100 | 0.51 | 99.49 | 9.95 |
| 0.14 | 55 | 200 | 112.90 | 87.10 | 64.46 |
| Source | Sum of Squares | Ratio-F | p-Value |
|---|---|---|---|
| A: Temperature | 158.25 | 0.48 | 0.04 |
| B: Adsorbent dose | 1302.18 | 3.95 | 0.03 |
| C: Initial concentration | 126.19 | 0.38 | 0.56 |
| AA | 58.77 | 0.18 | 0.69 |
| AB | 113.16 | 0.34 | 0.58 |
| AC | 5.01 | 0.02 | 0.91 |
| BB | 456.69 | 1.39 | 0.28 |
| BC | 43.56 | 0.13 | 0.73 |
| CC | 41.47 | 0.13 | 0.74 |
| Total error | 1976.93 | ||
| Total (corr.) | 4190.36 |
| Model | Parameter | Non-Linear | Linear |
|---|---|---|---|
| Pseudo-first order | qe | 9.65 | 9.34 |
| k1 | 4.92 | 0.08 | |
| R2 | 0.99 | 0.99 | |
| SS | 9.19 | 0.16 | |
| Pseudo-second order | k2 | 2.99 | 0.24 |
| qe | 9.73 | 9.81 | |
| R2 | 0.99 | 0.99 | |
| SS | 8.19 | 0.02 | |
| Elovich | β | 1.20 × 1040 | 10.56 |
| α | 10.243 | 9.79 | |
| R2 | 0.997 | 0.53 | |
| SS | 6.92 | 0.19 | |
| Intraparticle diffusion | k3 | 9.644 | 0.02 |
| C | 9.43 | ||
| R2 | 0.995 | 0.51 | |
| SS | 7.0435 | 0.20 |
| Isotherm Model | Parameters | Non-Linear | Linear |
|---|---|---|---|
| Langmuir | qmax | 44.02 | 40.00 |
| b | 3.67 × 10−6 | 5.62 × 10−4 | |
| R2 | 0.94 | 0.26 | |
| Freundlich | Kf | 0.01 | 0.40 |
| 1/n | 1.89 | 1.30 | |
| n | 0.63 | 0.77 | |
| qF | 10.10 | 7.94 | |
| R2 | 0.97 | 0.95 | |
| Dubinin–Radushkevich | qDR | 27.46 | 9.64 |
| KDR | 6.39 × 10−9 | 1.30 × 10−4 | |
| E | 8.85 | 61.89 | |
| R2 | 0.93 | 0.70 |
| Temperature (K) | ΔH° (kJ/mol) | ΔS° (kJ × K−1) | ΔG° (kJ/mol) |
|---|---|---|---|
| 306.9 | 85.25 | −0.2 | 145.88 |
| 328.2 | - | - | 150.88 |
| 349.4 | - | - | 155.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villabona-Ortíz, A.; González-Delgado, Á.D.; Tejada-Tovar, C. Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca). Water 2022, 14, 1383. https://doi.org/10.3390/w14091383
Villabona-Ortíz A, González-Delgado ÁD, Tejada-Tovar C. Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca). Water. 2022; 14(9):1383. https://doi.org/10.3390/w14091383
Chicago/Turabian StyleVillabona-Ortíz, Angel, Ángel Darío González-Delgado, and Candelaria Tejada-Tovar. 2022. "Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca)" Water 14, no. 9: 1383. https://doi.org/10.3390/w14091383
APA StyleVillabona-Ortíz, A., González-Delgado, Á. D., & Tejada-Tovar, C. (2022). Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca). Water, 14(9), 1383. https://doi.org/10.3390/w14091383








