Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Pretreatment
2.2. Cellulose Extraction and Modification
2.3. Adsorption Assays
2.4. Adsorption Isotherms
3. Results
3.1. Characterization of the Bioadsorbents
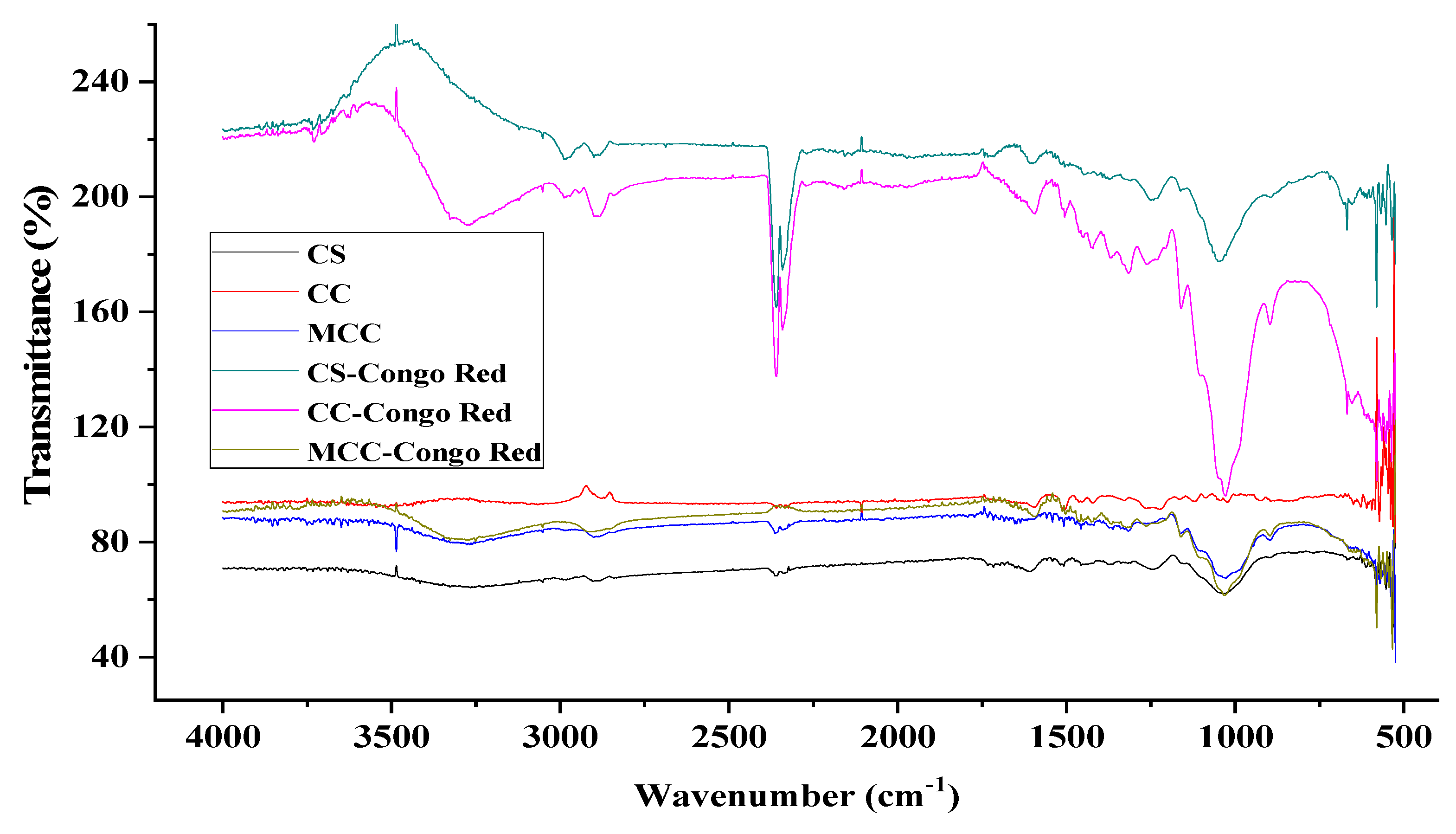
3.1.1. FTIR Spectrum
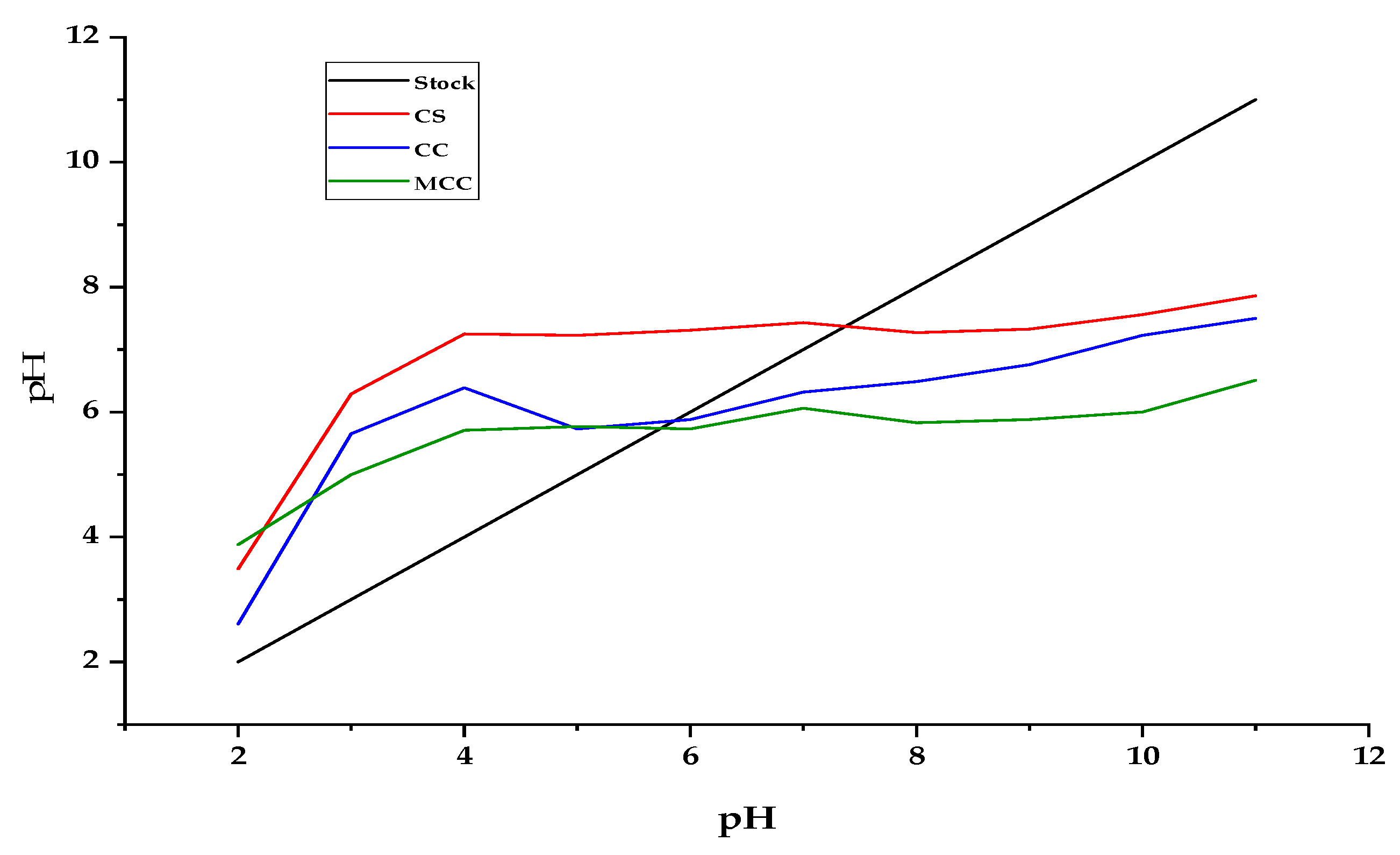
3.1.2. Point Zero Charge
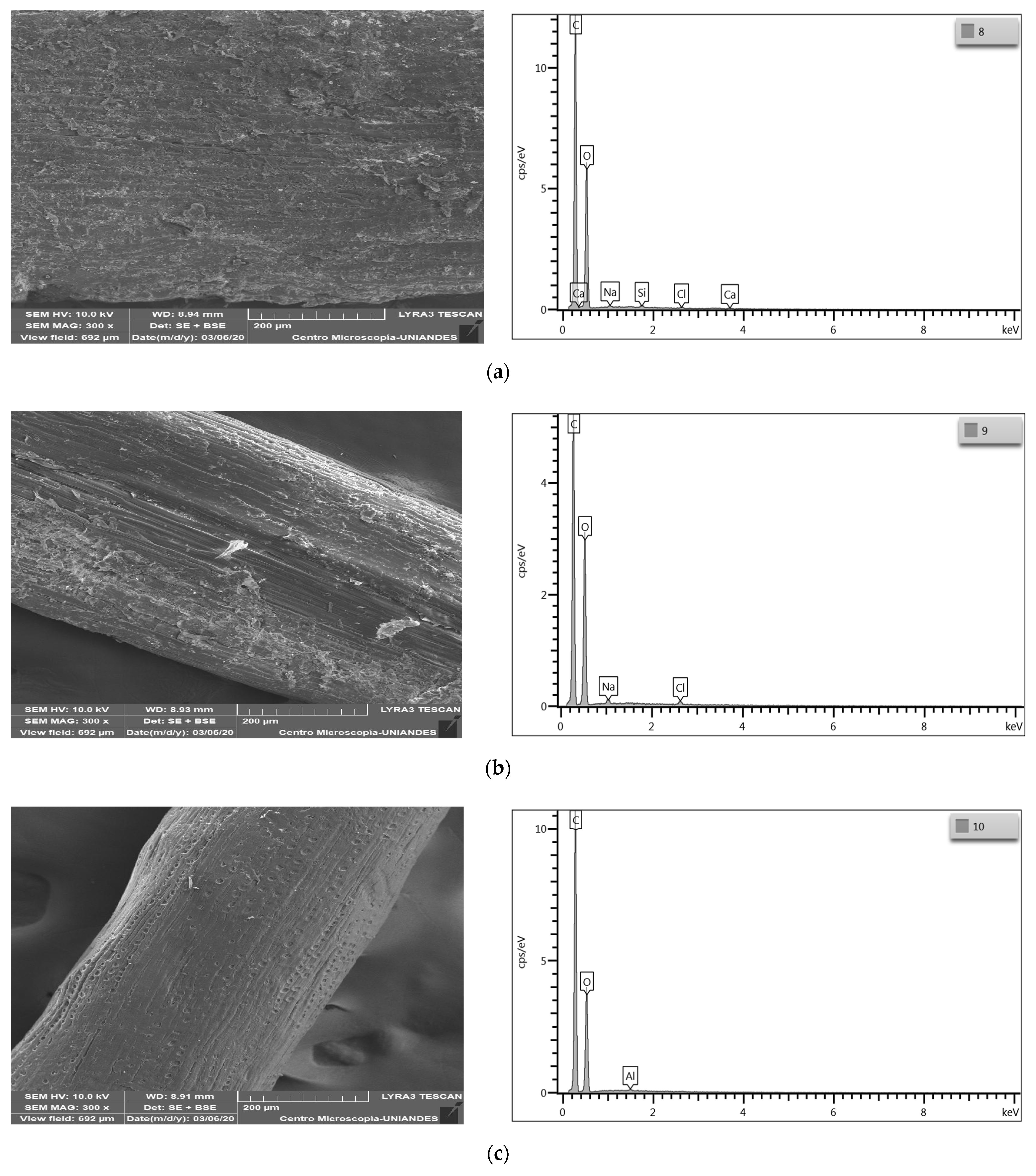
3.1.3. Bromatological and Structural Analysis
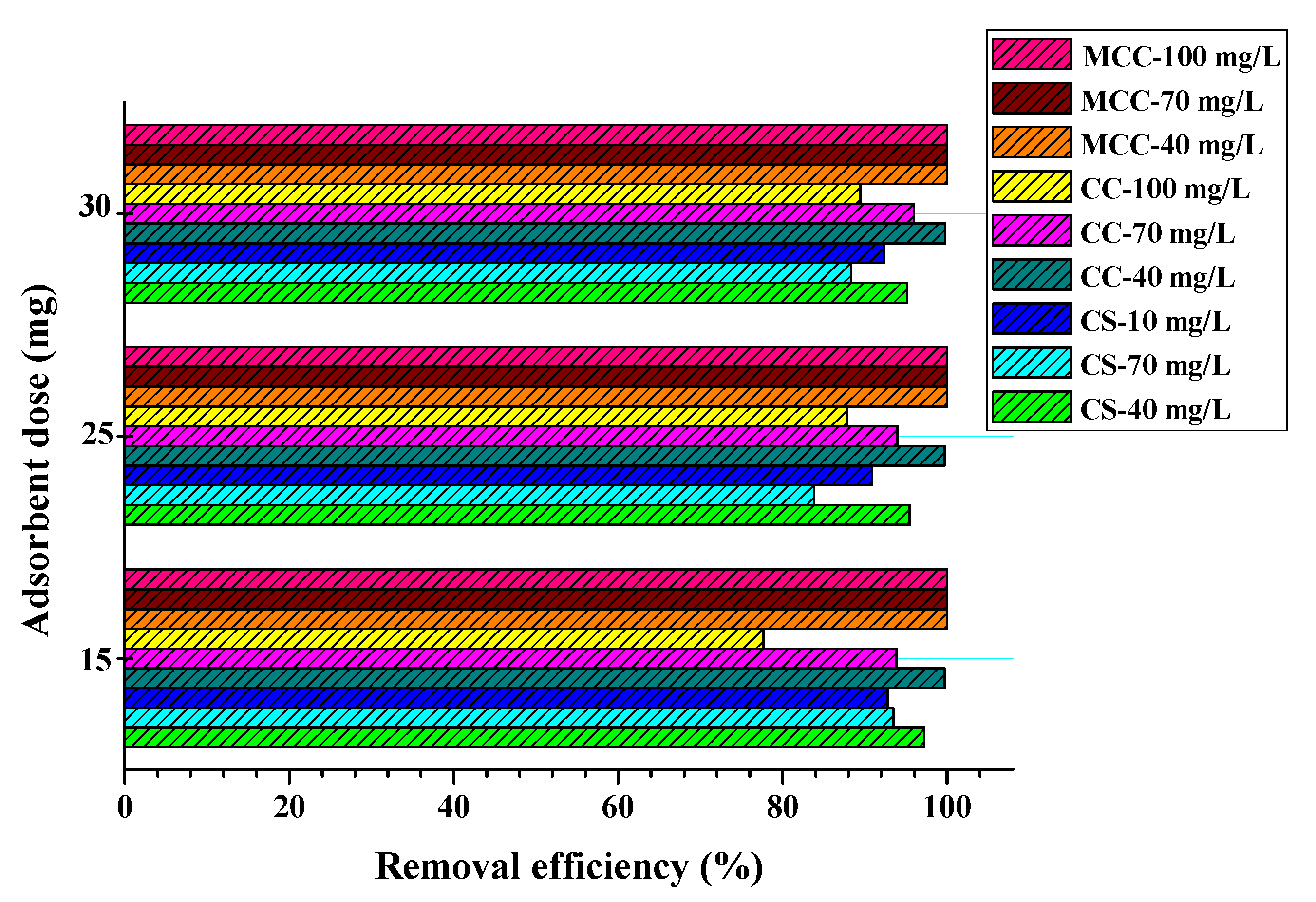
3.2. Congo Red Adsorption Tests
3.3. Statistical Analysis
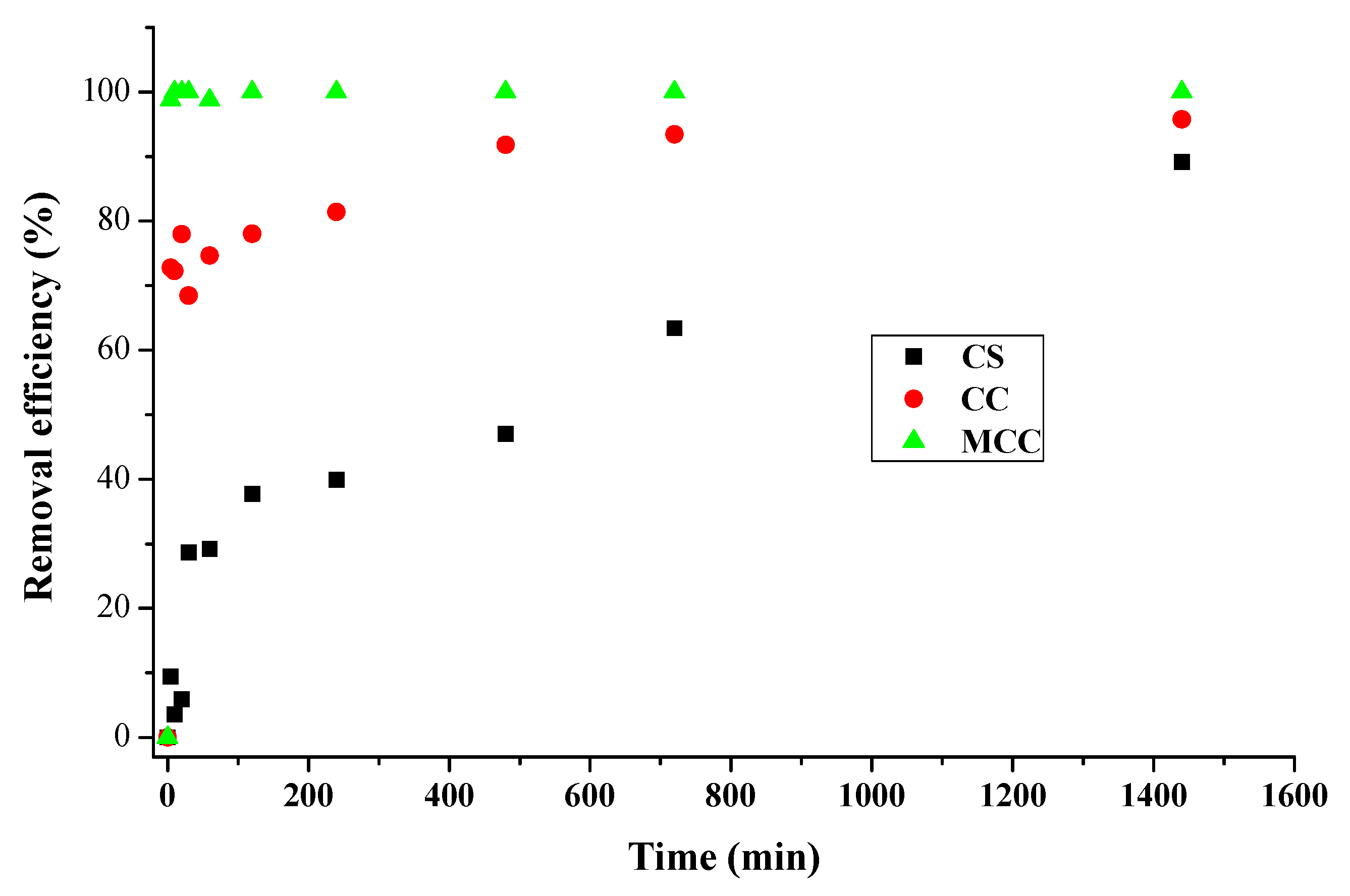
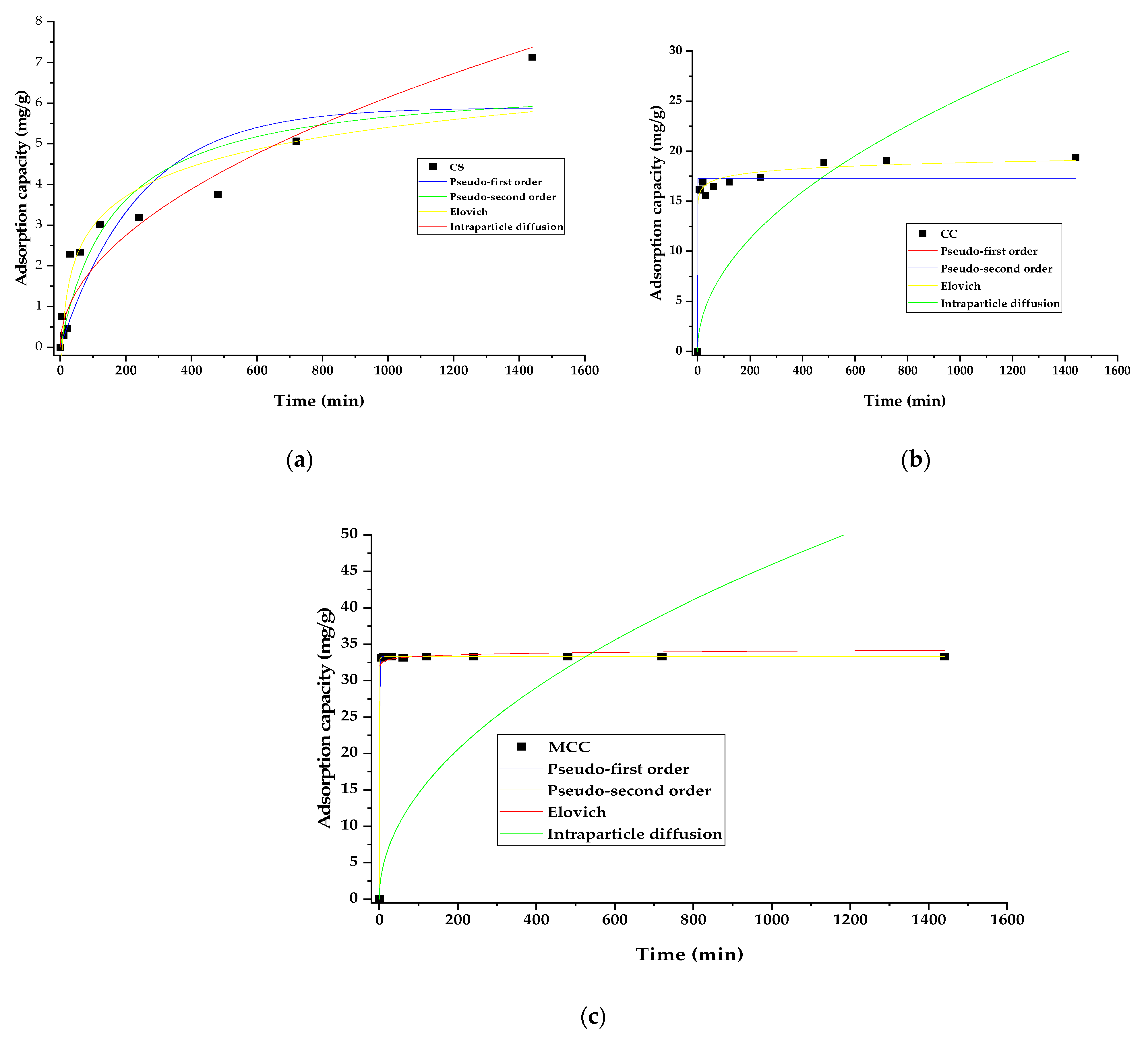
3.4. Effect of the Contact Time
3.5. Adsorption Equilibrium Isotherm
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharagava, R.N.; Chowdhary, P. Textile Wastewater Dyes: Toxicity Profile and Treatment Approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–435. [Google Scholar]
- Padhi, B. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar]
- Gray, L.E.; Ostby, J.S.; Kavlock, R.J.; Marshall, R. Gonadal effects of fetal exposure to the azo dye Congo red in mice: Infertility in female but not male offspring. Toxicol. Sci. 1992, 19, 411–422. [Google Scholar] [CrossRef]
- Kumari, S.; Mankotia, D.; Chauhan, G.S. Crosslinked cellulose dialdehyde for Congo red removal from its aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 1126–1136. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction and Analytical Methods for the Determination of Tartrazine (E 102) in Foodstuffs. Crit. Rev. Anal. Chem. 2017, 47, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ding, L.; Ren, P.; Zhong, M.; Ma, J.; Fan, X. Performances and Mechanism of Methyl Orange and Congo Red Adsorbed on the Magnetic Ion-Exchange Resin. J. Chem. Eng. Data 2020, 65, 725–736. [Google Scholar] [CrossRef]
- Adam, R.E.; Pozina, G.; Willander, M.; Nur, O. Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct.-Fundam. Appl. 2018, 32, 11–18. [Google Scholar] [CrossRef]
- Garvasis, J.; Prasad, A.R.; Shamsheera, K.O.; Jaseela, P.K.; Joseph, A. Efficient removal of Congo red from aqueous solutions using phytogenic aluminum sulfate nano coagulant. Mater. Chem. Phys. 2020, 251, 123040. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Serrano, M.; Rodríguez-Schmidt, R.; Escudero, P.A. Behaviour of polysulfone ultrafiltration membrane for dyes removal. Water Sci. Technol. 2018, 77, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-based foams for versatile applications. Nano Res. 2021, 1–10. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Marques, B.S.; Frantz, T.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Dotto, G.L. Adsorption of a textile dye onto piaçava fibers: Kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ. Sci. Pollut. Res. 2019, 26, 28584–28592. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Elango, G. Exploitation of Cocos nucifera a non-food toward the biological and nanobiotechnology field. Ind. Crops Prod. 2015, 67, 130–136. [Google Scholar] [CrossRef]
- Sadeghalvad, B.; Khorshidi, N.; Azadmehr, A.; Sillanpää, M. Sorption, mechanism, and behavior of sulfate on various adsorbents: A critical review. Chemosphere 2021, 263, 128064. [Google Scholar] [CrossRef]
- Kono, H.; Ogasawara, K.; Kusumoto, R.; Oshima, K.; Hashimoto, H.; Shimizu, Y. Cationic cellulose hydrogels cross-linked by poly (ethylene glycol): Preparation, molecular dynamics, and adsorption of anionic dyes. Carbohydr. Polym. 2016, 152, 170–180. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.; Okada, M. Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 2002, 48, 1041–1046. [Google Scholar] [CrossRef]
- Cao, W.; Dang, Z.; Zhou, X.Q.; Yi, X.Y.; Wu, P.X.; Zhu, N.W.; Lu, G.N. Removal of sulphate from aqueous solution using modified rice straw: Preparation, characterization and adsorption performance. Carbohydr. Polym. 2011, 85, 571–577. [Google Scholar] [CrossRef]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Pan, Y.; Zhang, M.; Jin, Y. Thermal and pH dual-responsive cellulose microfilament spheres for dye removal in single and binary systems. J. Hazard. Mater. 2019, 377, 88–97. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, A.D.; Benitez-Monroy, J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustain. Chem. Pharm. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VII. Update. Adv. Colloid Interface Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Koley, P.; Sakurai, M.; Takei, T.; Aono, M. Facile fabrication of silk protein sericin-mediated hierarchical hydroxyapatite-based bio-hybrid architectures: Excellent adsorption of toxic heavy metals and hazardous dye from wastewater. RSC Adv. 2016, 6, 86607–86616. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, A.; Siddiqi, Z.M. Removal of congo red dye from aqueous system using Phoenix dactylifera seeds. J. Mol. Liq. 2016, 219, 359–367. [Google Scholar] [CrossRef]
- Rani, K.C.; Naik, A.; Chaurasiya, R.S.; Raghavarao, K.S.M.S. Removal of toxic Congo red dye from water employing low-cost coconut residual fiber. Water Sci. Technol. 2017, 75, 2225–2236. [Google Scholar] [CrossRef]
- Mondal, N.K.; Kar, S. Potentiality of banana peel for removal of Congo red dye from aqueous solution: Isotherm, kinetics and thermodynamics studies. Appl. Water Sci. 2018, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Parvin, S.; Biswas, B.K.; Rahman, M.A.; Rahman, M.H.; Anik, M.S.; Uddin, M.R. Study on adsorption of Congo red onto chemically modified egg shell membrane. Chemosphere 2019, 236, 124326. [Google Scholar] [CrossRef]
- Salahuddin, N.; Abdelwahab, M.A.; Akelah, A.; Elnagar, M. Adsorption of Congo red and crystal violet dyes onto cellulose extracted from Egyptian water hyacinth. Nat. Hazards 2020, 105, 1375–1394. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Luo, Z.; Shen, J.; Ni, Q.; Yao, J. Facile preparation of a cellulose-based bioadsorbent modified by hPEI in heterogeneous system for high-efficiency removal of multiple types of dyes. React. Funct. Polym. 2018, 125, 77–83. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Saba, A.; Reza, M.T. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar] [CrossRef]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of Lead and Cadmium from Aqueous Solution in Single and Binary Systems Using Avocado Pear Exocarp: Effects of Competing Ions. Anal. Lett. 2020, 53, 2501–2516. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Beroual, M.; Mehelli, O.; Boumaza, L.; Trache, D.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Synthesis and Characterization of Microcrystalline Cellulose from Giant Reed Using Different Delignification Processes; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Mohamed Pauzan, A.S.; Ahad, N. Biomass Modification Using Cationic Surfactant Cetyltrimethylammonium Bromide (CTAB) to Remove Palm-Based Cooking Oil. J. Chem. 2018, 2018, 5059791. [Google Scholar] [CrossRef] [PubMed]
- Bakar, A.H.B.A.; Koay, Y.S.; Ching, Y.C.; Abdullah, L.C.; Choong, T.S.Y.; Alkhatib, M.; Mobarekeh, M.N.; Zahri, N.A.M. Removal of fluoride using quaternized palm kernel shell as adsorbents: Equilibrium isotherms and kinetics studies. BioResources 2016, 11, 4485–4511. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, S.; Gao, Y. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K. Bioresour. Technol. 2020, 312, 123564. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Sciences, N. A simple method for determining surface porosity based on SEM images using OriginPro software. Indones. J. Phys. 2009, 20, 2. [Google Scholar]
- Madan, S.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl. Surf. Sci. 2019, 487, 907–917. [Google Scholar] [CrossRef]
- Tan, C.H.C.; Sabar, S.; Hussin, M.H. Development of immobilized microcrystalline cellulose as an effective adsorbent for methylene blue dye removal. S. Afr. J. Chem. Eng. 2018, 26, 11–24. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Fu, Y.Q.; Zong, E.M.; Jiang, S.T.; Li, J.B.; Zhu, J.Q.; Zhu, Y.Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Siddiqui, Z.M.; Dhar, S.; Mehta, P.; Pathania, D. Adsorptive removal of congo red dye (CR) from aqueous solution by Cornulaca monacantha stem and biomass-based activated carbon: Isotherm, kinetics and thermodynamics. Sep. Sci. Technol. 2019, 54, 916–929. [Google Scholar] [CrossRef]
- Jaramillo, A.C.; Echavarría, A.M.; Hormaza, A. Diseño Box-Behnken para la optimización de la adsorción del colorante azul ácido sobre residuos de flores. Ing. Y Cienc. 2013, 9, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Pei, A.; Butchosa, N.; Berglund, L.A.; Zhou, Q. Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 2013, 9, 2047–2055. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef] [Green Version]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, U.J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Jiang, J.H.; Xiao, L.; Zeng, G.M.; Zhao, S.L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Aoopngan, C.; Nonkumwong, J.; Phumying, S.; Promjantuek, W.; Maensiri, S.; Noisa, P.; Pinitsoontorn, S.; Ananta, S.; Srisombat, L. Amine-Functionalized and Hydroxyl-Functionalized Magnesium Ferrite Nanoparticles for Congo Red Adsorption. ACS Appl. Nano Mater. 2019, 2, 5329–5341. [Google Scholar] [CrossRef]
- William Kajjumba, G.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. Advanced Sorption Process Application. 2019, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shen, H.; Shen, C.; Li, Y.; Ying, Z.; Duan, Y. Kinetics and Mechanism Study of Mercury Adsorption by Activated Carbon in Wet Oxy-Fuel Conditions. Energy Fuels 2019, 33, 1344–1353. [Google Scholar] [CrossRef]
- Das, G.K.; Chatterjee, S. Use of Kinetic Models for Correlating Adsorbate Breakthrough in a Fixed Bed of Use of Kinetic Models for Correlating Adsorbate Breakthrough in a Fixed Bed of Adsorbent. In Proceedings of the ChemCon, Kolkata, India, 27–30 December 2007. [Google Scholar]
- Gautam, R.K.; Gautam, P.K.; Banerjee, S.; Rawat, V.; Soni, S.; Sharma, S.K.; Chattopadhyaya, M.C. Removal of tartrazine by activated carbon biosorbents of Lantana camara: Kinetics, equilibrium modeling and spectroscopic analysis. J. Environ. Chem. Eng. 2015, 3, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, H.; Ma, L.; Huang, H.; Wu, J.; Zhang, Y. Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym. 2019, 19, 115599. [Google Scholar] [CrossRef]
| Model | Equation | Parameter |
|---|---|---|
| Pseudo-first order | qe and qt (mg/g): adsorption capacities at an equilibrium and at a certain time. k1 (min−1): Lagergren constant. | |
| Pseudo-second order | k2 (g−1 min−1): pseudo-second order adsorption constant. | |
| Elovich | α (mg g−1 min−1): speed of adsorption at the beginning. β (g mg−1): desorption constant. qt (mg/g): quantity of chemisorbed pollutant. | |
| Intraparticle diffusion | qt (mg/g): amount of contaminant adsorbed per mass unit of adsorbent in a time, t. t (min): time. k3 (mg g−1 min−1/2): constant intraparticular diffusion. |
| Component | Composition % | Method |
|---|---|---|
| Lignin | 48.94 ± 0.88 | TAPPI T 203 os-74 |
| Cellulose | 35.99 ± 0.45 | TAPPI T 222 om-83 |
| Hemicellulose | 10.51 ± 0.37 | TAPPI T 222 om-83 |
| Extractives | 4.56 ± 0.2 | TAPPI T 204 D-2 |
| Source | Bioadsorbent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BS | CC | MCC | |||||||
| Sum of Squares | F-Ratio | p-Value | Sum of Squares | F-Ratio | p-Value | Sum of Squares | F-Ratio | p-Value | |
| A: adsorbent dosage | 9.64 | 1.40 | 0.32 | 33.17 | 6.63 | 0.082 | 0.0 | 0 | 0 |
| B: initial concentration | 23.39 | 3.40 | 0.16 | 326.07 | 65.14 | 0.004 | 6.58 × 10−5 | 0 | 0 |
| AA | 19.73 | 2.87 | 0.19 | 2.29 | 0.46 | 0.55 | 0.0 | 0 | 0 |
| AB | 0.73 | 0.11 | 0.77 | 34.3434 | 6.86 | 0.08 | 0.0 | 0 | 0 |
| BB | 58.78 | 8.54 | 0.06 | 10.19 | 2.04 | 0.25 | 3.96 × 10−6 | 0 | 0 |
| Total error | 20.65 | 15.02 | 0.0 | ||||||
| Total (corr.) | 132.91 | 421.09 | 6.98 × 10−4 | ||||||
| Model | Parameter | CS | CC | MCC |
|---|---|---|---|---|
| Pseudo-first order | qe (mg/g) | 5.892 | 17.291 | 33.313 |
| k1 (min−1) | 0.004 | 15.187 | 1.087 | |
| R2 | 0.807 | 0.936 | 0.999 | |
| X2 | 0.939 | 1.843 | 0.003 | |
| Pseudo-second order | qe (mg/g) | 6.595 | 17.291 | 33.324 |
| k2 (g/mg × min) | 9.21 × 10−4 | 2.136 | 1.674 | |
| R2 | 0.8674 | 0.936 | 0.999 | |
| X2 | 0.646 | 1.843 | 0.003 | |
| Elovich | α (mg/g × min) | 0.948 | 1.576 | 3.206 |
| β (g/mg) | 0.177 | 4.929 | 8.049 × 1043 | |
| R2 | 0.885 | 0.987 | 0.997 | |
| X2 | 0.561 | 0.380 | 0.321 | |
| Intraparticle diffusion | k3 (mg/g × s1/2) | 0.194 | 0.797 | 1.452 |
| R2 | 0.923 | 30.543 | 15.452 | |
| X2 | 0.373 | 101.964 | 449.515 |
| Model | Parameters | Congo Red | ||
|---|---|---|---|---|
| CS | CC | MCC | ||
| Langmuir | qmax (mg/g) | 17.758 | 121.618 | 256.115 |
| b (L/mg) | 5.722 | 0.168 | 1.539 | |
| R2 | 0.6363 | 0.814 | 0.009 | |
| X2 | 58.392 | 6.821 | 90.282 | |
| Freundlich | kf (mg/g) | 0.019 | 5.402 | 15.189 |
| n | 0.588 | 2.983 | 4.572 | |
| R2 | 0.932 | 0.752 | 0.629 | |
| X2 | 2.505 | 9.137 | 84.519 | |
| Contaminant | Adsorbent | qmax (mg/g) | Reference |
|---|---|---|---|
| Congo Red | Cellulose nanocrystals modified with cetyl trimethyl ammonium bromide | 448.43 | [20] |
| NaOH-modified water hyacinth cellulose nanocrystals | 181.8 | [31] | |
| Hydrogel from pineapple peel extracted by bleaching | 138.89 | [60] | |
| MCC | 256.115 | This study | |
| CC | 121.618 | ||
| CS | 17.758 | ||
| Coconut residues | 128.94 | [28] | |
| Hydrogel from water-extracted pineapple peel | 114.19 | [60] | |
| Cornulaca-activated carbon | 78.19 | [45] | |
| Hydrogel made from pineapple peel extracted with NaOH | 77.52 | [60] | |
| Hydrogel from pineapple peel extracted with bleached NaOH | 75.19 | [60] | |
| Date palm seeds | 61.72 | [27] | |
| Cornulaca biomass | 43.42 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Delgado, A.D.; Villabona-Ortíz, A.; Tejada-Tovar, C. Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water 2022, 14, 408. https://doi.org/10.3390/w14030408
González-Delgado AD, Villabona-Ortíz A, Tejada-Tovar C. Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water. 2022; 14(3):408. https://doi.org/10.3390/w14030408
Chicago/Turabian StyleGonzález-Delgado, Angel Darío, Angel Villabona-Ortíz, and Candelaria Tejada-Tovar. 2022. "Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye" Water 14, no. 3: 408. https://doi.org/10.3390/w14030408
APA StyleGonzález-Delgado, A. D., Villabona-Ortíz, A., & Tejada-Tovar, C. (2022). Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water, 14(3), 408. https://doi.org/10.3390/w14030408








