Synergistic Plant-Microbe Interactions between Endophytic Actinobacteria and Their Role in Plant Growth Promotion and Biological Control of Cotton under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Screening for Plant Beneficial Traits and Antifungal Activities
2.1.1. Determination of Nitrogenase Activity
2.1.2. Indole Acetic Acid (IAA) Production
2.1.3. Detection of Siderophores
2.1.4. Determination of Phosphate Solubilization Ability
2.1.5. Assays for Proteolytic, Lipolytic, Cellulolytic, and Chitinolytic Activity
2.1.6. Screening for Antifungal Activities In Vitro
2.2. In Vivo Salt Stress Experiment under Greenhouse Conditions
2.2.1. Plant Growth-Promoting Activity in Saline Soil
2.2.2. Biological Control of V. dahliae under Salt Stress
2.2.3. Disease Assessment
2.3. Plant-Microbe Defense Response to Pathogens and Photosynthetic Pigments under Salt Stress
2.3.1. Determination of Antioxidant Enzymatic Activity
2.3.2. Photosynthetic Pigments
2.4. Extraction and Identification of Metabolites
2.4.1. Isolation and Purification of Bioactive Compounds
2.4.2. Identification of Bioactive Compounds
2.5. Statistical Analysis
3. Results
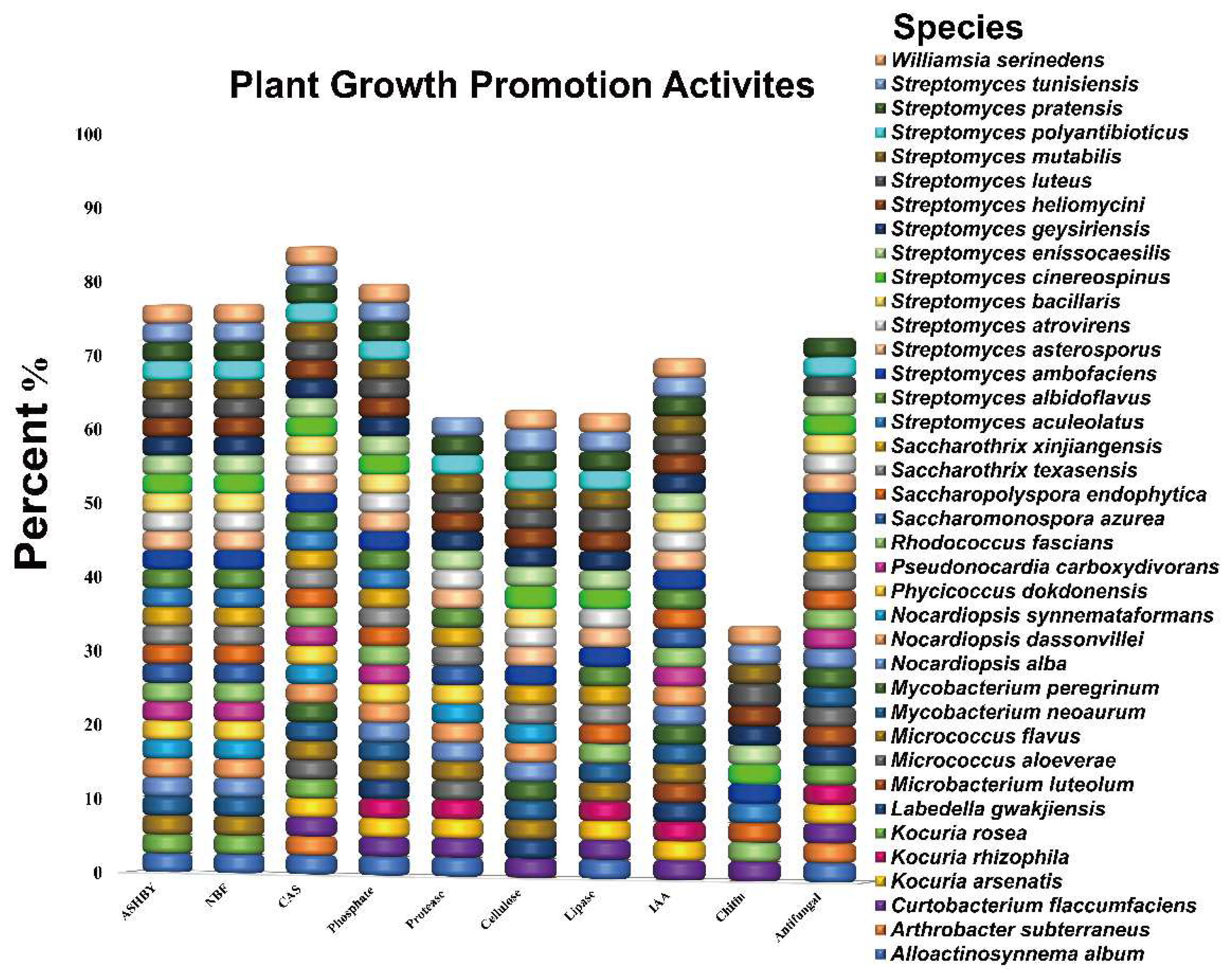
3.1. In Vitro Screening of Endophytic Actinobacteria Strains for Plant Growth-Promoting Traits
3.2. Stimulation of Cotton Growth by Endophytes under Salt Stress in a Pot Experiment
3.3. Determination of Antioxidant Enzyme Activity
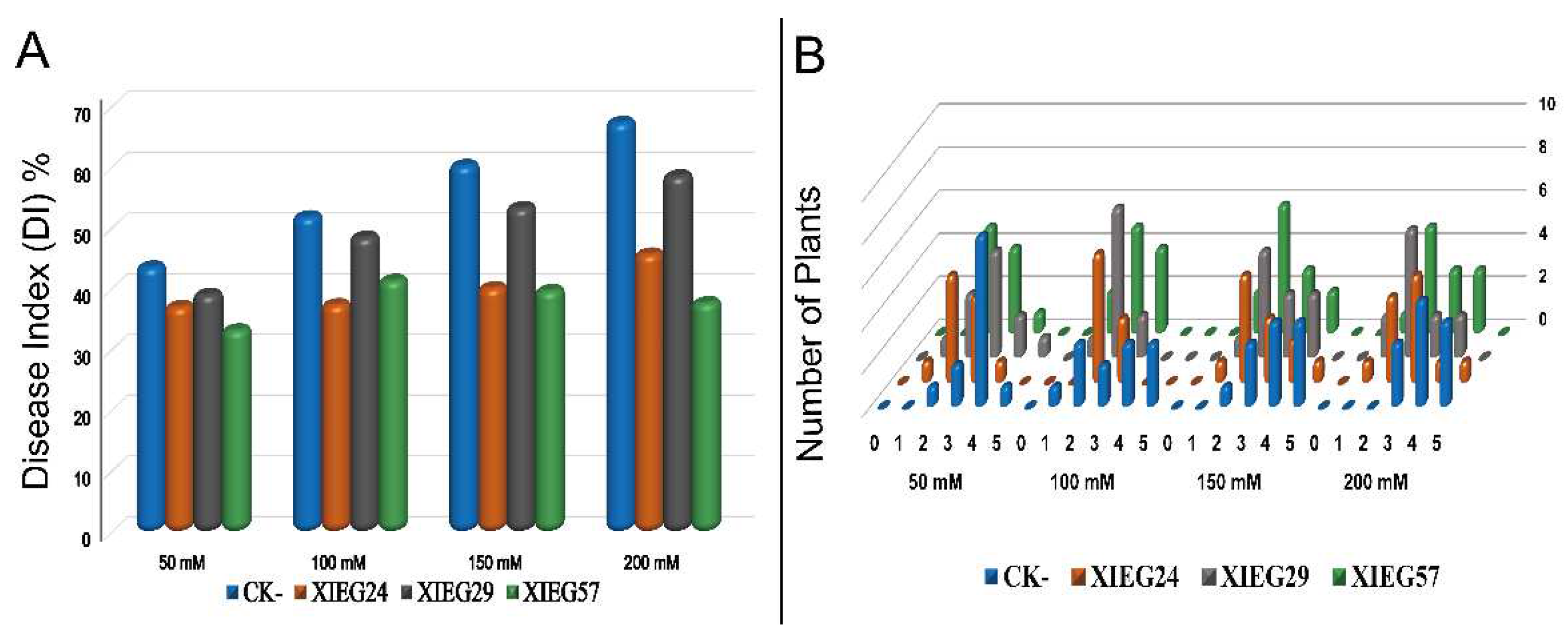
3.4. Biological Control of V. dahliae In Vivo under Salt Stress
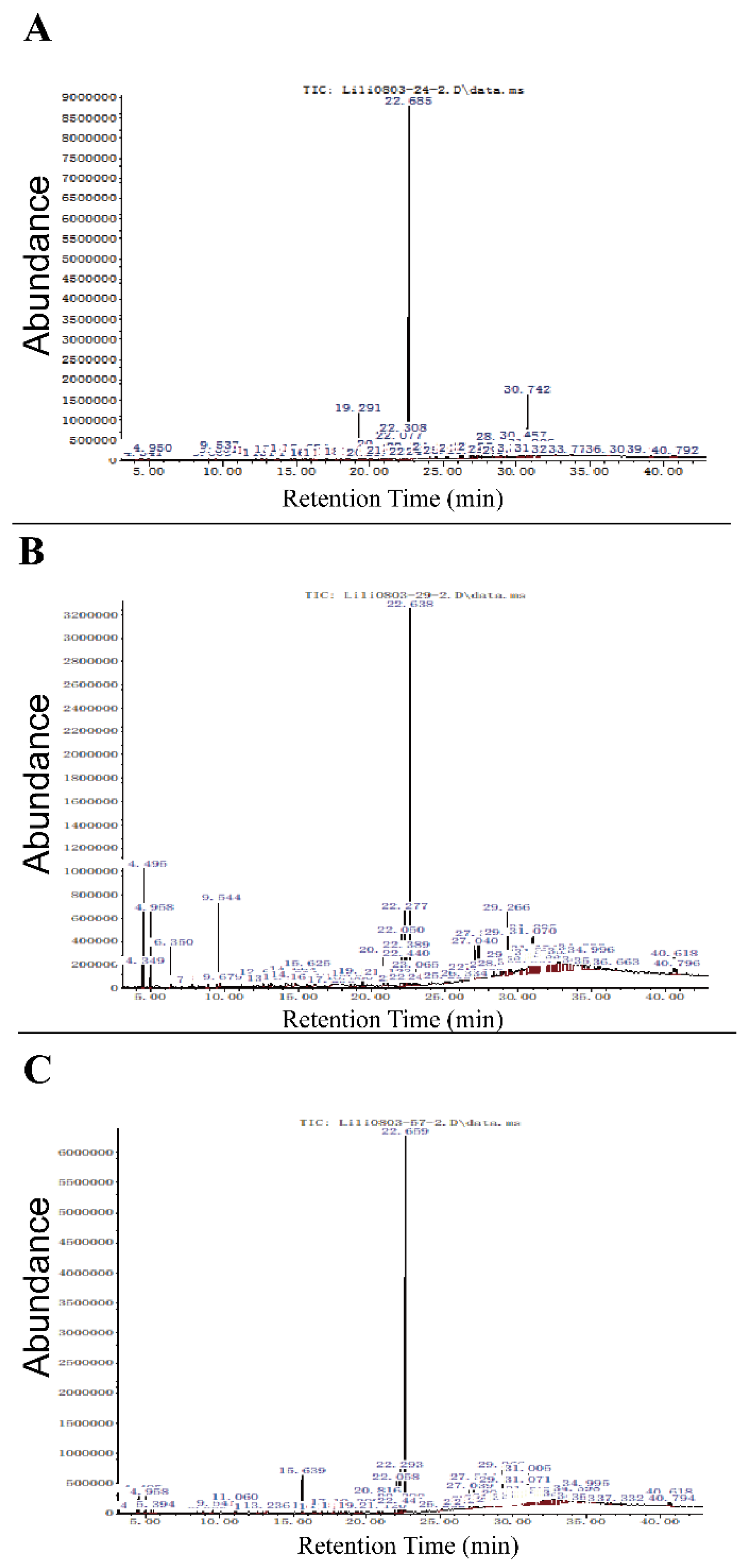
3.5. Identification of Bioactive Compound by Gas-Chromatography/Mass-Spectrometry (GC-MS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Lade, H. Plant-Growth-Promoting Rhizobacteria to Improve Crop Growth in Saline Soils: A Review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature: London, UK, 2018; p. 164. [Google Scholar]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Ghanati, F. Effect of Salinity on Growth, Xylem Structure and Anatomical Characteristics of Soybean. Not. Sci. Biol. 2011, 3, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Jabborova, D.; Mamadalieva, N. Salt Tolerant Pseudomonas extremorientalis able to Stimulate Growth of Silybum marianum under Salt Stress. Med. Aromat. Plant Sci Biotechnol 2013, 7, 7–10. [Google Scholar]
- Abeer, H.; Abd_Allah, E.; Alqarawi, A.; El-Didamony, G.; Alwhibi, M.; Egamberdieva, D.; Ahmad, P. Alleviation of Adverse Impact of Salinity on Faba Bean (Vicia faba L.) by Arbuscular Mycorrhizal Fungi. Pak. J. Bot. 2014, 46, 2003–2013. [Google Scholar]
- Hameed, A.; Dilfuza, E.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity Stress and Arbuscular Mycorrhizal Symbiosis in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: Berlin, Germany, 2014; Volume 1, pp. 139–159. [Google Scholar]
- Wild, A. Soils, Land and Food: Managing the Land during the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2003; Volume 93, pp. 785–786. [Google Scholar]
- Solaw, F. The State of the World’s Land and Water Resources for Food and Agriculture; Earthscan: Rome, Italy, 2011; pp. 19–59. [Google Scholar]
- Nair, D.; Padmavathy, S. Impact of Endophytic Microorganisms on Plants, Environment and Humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Molina, G.; Dionísio, A.; Maróstica, M.R., Jr.; Pastore, G.M. The Use of Endophytes to Obtain Bioactive Compounds and Their Application in Biotransformation Process. Biotechnol. Res. Int. 2011, 2011, 576286. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Lodewyckx, C.; Vangronsveld, J.; Porteous, F.; Moore, E.R.; Taghavi, S.; Mezgeay, M.; der Lelie, D.V. Endophytic Bacteria and their Potential Applications. Crit. Rev. Plant Sci. 2002, 21, 583–606. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial Endophytes: Recent Developments and Applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedler, H.-P.; Bruntner, C.; Riedlinger, J.; Bull, A.T.; Knutsen, G.; Goodfellow, M.; Jones, A.; Maldonado, L.; Pathom-Aree, W.; Beil, W. Proximicin A, B and C, Novel Aminofuran Antibiotic and Anticancer Compounds Isolated from Marine Strains of the Actinomycete Verrucosispora. J. Antibiot. 2008, 61, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimnoi, P.; Pongsilp, N.; Lumyong, S. Endophytic Actinomycetes Isolated from Aquilaria Crassna Pierre Ex Lec and Screening of Plant Growth Promoters Production. World J. Microbiol. Biotechnol. 2010, 26, 193–203. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Finnie, J.F.; Van Staden, J. The Role of Endophytes in Secondary Metabolites Accumulation in Medicinal Plants under Abiotic Stress. S. Afr. J. Bot. 2020, 134, 126–134. [Google Scholar] [CrossRef]
- Arunachalam Palaniyandi, S.; Yang, S.H.; Damodharan, K.; Suh, J.W. Genetic and Functional Characterization of Culturable Plant-Beneficial Actinobacteria Associated with Yam Rhizosphere. J. Basic Microbiol. 2013, 53, 985–995. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic Actinobacteria of Medicinal Plants: Diversity and Bioactivity. Anton. Leeuw. Int. J. 2015, 108, 267–289. [Google Scholar] [CrossRef] [Green Version]
- Taechowisan, T.; Lu, C.; Shen, Y.; Lumyong, S. Secondary Metabolites from Endophytic Streptomyces aureofaciens CMUAc130 and their Antifungal Activity. Microbiology 2005, 151, 1691–1695. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Guo, J.; Li, L.; Asem, M.D.; Zhang, Y.; Mohamad, O.A.; Salam, N.; Li, W. Endophytic Bacteria Associated with Endangered Plant Ferula sinkiangensis KM Shen in an Arid Land: Diversity and Plant Growth-Promoting Traits. J. Arid Land 2017, 9, 432–445. [Google Scholar] [CrossRef]
- Abdelshafy Mohamad, O.A.; Li, L.; Ma, J.-B.; Hatab, S.; Rasulov, B.A.; Musa, Z.; Liu, Y.-H.; Li, W.-J. Halophilic Actinobacteria Biological Activity and Potential Applications. In Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications; Egamberdieva, D., Birkeland, N.-K., Panosyan, H., Li, W.-J., Eds.; Springer Singapore: Singapore, 2018; pp. 333–364. [Google Scholar]
- Mohamad, O.A.A.; Ma, J.-B.; Liu, Y.-H.; Li, L.; Hatab, S.; Li, W.-J. Medicinal Plant-Associated Microbes as a Source of Protection and Production of Crops. In Medically Important Plant Biomes: Source of Secondary Metabolites; Springer: Berlin, Germany, 2019; pp. 239–263. [Google Scholar]
- Govindasamy, V.; Franco, C.M.; Gupta, V.V. Endophytic Actinobacteria: Diversity and Ecology. In Advances in Endophytic Research; Springer: Berlin, Germany, 2014; pp. 27–59. [Google Scholar]
- Dinesh, R.; Srinivasan, V.; TE, S.; Anandaraj, M.; Srambikkal, H. Endophytic Actinobacteria: Diversity, Secondary Metabolism and Mechanisms to Unsilence Biosynthetic Gene Clusters. Crit. Rev. Microbiol. 2017, 43, 546–566. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, A.K. Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microb. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018; pp. 13–30. [Google Scholar]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-Derived Bioactive Compounds Produced by Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N. Phytochemical Analysis of Biologically Active Constituents of Medicinal Plants. Main Group Chem. 2014, 13, 7–21. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635. [Google Scholar] [CrossRef] [Green Version]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef] [Green Version]
- Vaičiulytė, V.; Ložienė, K. Impact of Chemical Polymorphism of Thymus Pulegioides on Some Associated Plant Species under Natural and Laboratory Conditions. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 154, 663–672. [Google Scholar] [CrossRef]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus proximus Serg. Essential Oil and Its Major Constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial Relationships between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 758. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.; Zhao, S.; Yin, X.; Chen, J.; Wang, M.; Cai, Y.; Niu, Q. A Novel Peroxiredoxin from the Antagonistic Endophytic Bacterium Enterobacter sp. V1 Contributes to Cotton Resistance against Verticillium dahliae. Plant Soil 2020, 454, 395–409. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zhang, C.; Zhang, Q.; Sun, Y.; Zhu, H.; Tang, C. Pectin Lyase Enhances Cotton Resistance to Verticillium Wilt by Inducing Cell Apoptosis of Verticillium dahliae. J. Hazard. Mater. 2021, 404, 124029. [Google Scholar] [CrossRef]
- Peng, J.; Liu, J.; Zhang, L.; Luo, J.; Dong, H.; Ma, Y.; Zhao, X.; Chen, B.; Sui, N.; Zhou, Z. Effects of Soil Salinity on Sucrose Metabolism in Cotton Leaves. PLoS ONE 2016, 11, e0156241. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, A.; Fatima, M. Salt Tolerance in Zea mays (L). Following Inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-Resistant Plant Growth Promoting Rhizobacteria Ameliorates Sodium Chloride Stress on Tomato Plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Jong Yim, W.; Sa, T. Enhancement of Growth and Salt Tolerance of Red Pepper Seedlings (Capsicum annuum L.) by Regulating Stress Ethylene Synthesis with Halotolerant Bacteria Containing 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Arshad, M. The Combined Application of Rhizobial Strains and Plant Growth Promoting Rhizobacteria Improves Growth and Productivity of Mung Bean (Vigna radiata L.) under Salt-Stressed Conditions. Ann. Microbiol. 2012, 62, 1321–1330. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Abdelshafy Mohamad, O.A.; Abaydulla, G.; Liu, Y.; Li, W.-J.; Li, L. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus Roseus. Front. Microbiol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Li, L.; Mohamad, O.A.A.; Ma, J.; Friel, A.D.; Su, Y.; Wang, Y.; Musa, Z.; Liu, Y.; Hedlund, B.P.; Li, W. Synergistic Plant–Microbe Interactions between Endophytic Bacterial Communities and the Medicinal Plant Glycyrrhiza uralensis F. Anton. Leeuw. Int. J. 2018, 111, 1735–1748. [Google Scholar] [CrossRef]
- Abdelshafy Mohamad, O.A.; Ma, J.-B.; Liu, Y.-H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.-J.; Li, L. Beneficial Endophytic Bacterial Populations Associated with Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front. Plant Sci. 2020, 11, 47. [Google Scholar] [CrossRef]
- He, Y.; Pantigoso, H.A.; Wu, Z. Co-inoculation of Bacillus sp. and Pseudomonas Putida at Different Development Stages Acts as a Biostimulant to Promote Growth, Yield and Nutrient Uptake of Tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, S.N. Isolation and Characterization of Phosphate Solubilizing Bacterium Pseudomonas aeruginosa KUPSB12 with Antibacterial Potential from River Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Tiru, M.; Muleta, D.; Berecha, G.; Adugna, G. Antagonistic Effects of Rhizobacteria against Coffee Wilt Disease Caused by Gibberella xylarioides. Asian J. Plant Pathol. 2013, 7, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, T.; Kotasthane, A.S. Chitinolytic Assay of Indigenous Trichoderma Isolates Collected from Different Geographical Locations of Chhattisgarh in Central India. Springer Plus 2012, 1, 73. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, O.A.A.; Li, L.; Ma, J.-B.; Hatab, S.; Xu, L.; Guo, J.-W.; Rasulov, B.A.; Liu, Y.-H.; Hedlund, B.P.; Li, W.-J. Evaluation of the Antimicrobial Activity of Endophytic Bacterial Populations from Chinese Traditional Medicinal Plant Licorice and Characterization of the Bioactive Secondary Metabolites Produced by Bacillus atrophaeus Against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo Red-Polysaccharide Interactions in Enumeration and Characterization of Cellulolytic Bacteria from the Bovine Rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Yao, T.; Feng, C.; Chen, L.; Li, J.; Wang, L. Identification and Biocontrol Potential of Antagonistic Bacteria Strains against Sclerotinia sclerotiorum and Their Growth-Promoting Effects on Brassica napus. Biol. Control 2016, 104, 35–43. [Google Scholar] [CrossRef]
- Kilani-Feki, O.; Culioli, G.; Ortalo-Magné, A.; Zouari, N.; Blache, Y.; Jaoua, S. Environmental Burkholderia cepacia Strain Cs5 Acting by Two Analogous Alkyl-Quinolones and a Didecyl-Phthalate against a Broad Spectrum of Phytopathogens Fungi. Curr. Microbiol. 2011, 62, 1490–1495. [Google Scholar] [CrossRef]
- Qin, L.; Hu, S.; Cui, Q.; Tian, P.; Xie, C.; Jian, W.; Yang, X.; Shen, H. Bacillus circulans GN03 Alters the Microbiota, Promotes Cotton Seedling Growth and Disease Resistance, and Increases the Expression of Phytohormone Synthesis and Disease Resistance-Related Genes. Front. Plant Sci. 2021, 12, 648. [Google Scholar] [CrossRef]
- Botta, A.L.; Santacecilia, A.; Ercole, C.; Cacchio, P.; Del Gallo, M. In Vitro and in Vivo Inoculation of Four Endophytic Bacteria on Lycopersicon esculentum. New Biotechnol. 2013, 30, 666–674. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial Soil Bacterium Pseudomonas frederiksbergensis OS261 Augments Salt Tolerance and Promotes Red Pepper Plant Growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tian, T.; Tan, X.; Zheng, Y.; Xie, C.; Xu, Y.; Yang, X. VdNPS, a Nonribosomal Peptide Synthetase, is Involved in Regulating Virulence in Verticillium dahliae. Phytopathology 2020, 110, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in Mitigating NaCl Stress in Indian Mustard (Brassica juncea L) through Antioxidative Defense System. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, L.M.; Stewart, D.W.; Gregorich, E.; Anderson, A.M.; Ma, B.L.; Tollenaar, M. Quantifying the Nonlinearity in Chlorophyll Meter Response to Corn Leaf Nitrogen Concentration. Can. J. Plant Sci. 1995, 75, 179–182. [Google Scholar] [CrossRef]
- Kunoh, H. Endophytic Actinomycetes: Attractive Biocontrol Agents. Jpn. J. Phytopathol. 2002, 68, 124–127. [Google Scholar] [CrossRef]
- Passari, A.K.; Mishra, V.K.; Saikia, R.; Gupta, V.K.; Singh, B.P. Isolation, Abundance and Phylogenetic Affiliation of Endophytic Actinomycetes Associated with Medicinal Plants and Screening for Their in vitro Antimicrobial Biosynthetic Potential. Front. Microbiol. 2015, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Zachow, C.; Müller, H.; Philipps, J.; Tilcher, R. Next-Generation Bio-Products Sowing the Seeds of Success for Sustainable Agriculture. Agronomy 2013, 3, 648–656. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the Plant Microbiome: Looking Back and Future Perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.-T.; Chang, H.-H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.-H. Genome Analysis of Pseudomonas fluorescens PCL1751: A Rhizobacterium That Controls Root Diseases and Alleviates Salt Stress for Its Plant Host. PLoS ONE. 2015, 10, e0140231. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic Interactions between Bradyrhizobium japonicum and the Endophyte Stenotrophomonas rhizophila and Their Effects on Growth, and Nodulation of Soybean under Salt Stress. Plant Soil. 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Lindström, K.; Räsänen, L.A. A Synergistic Interaction between Salt-Tolerant Pseudomonas and Mesorhizobium Strains Improves Growth and Symbiotic Performance of Liquorice (Glycyrrhiza uralensis Fish.) under Salt Stress. Appl. Microbiol. Biotechnol. 2016, 100, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Thakur, D. Phylogenetic and Functional Characterization of Culturable Endophytic Actinobacteria Associ-Ated with Camellia spp. for Growth Promotion in Commercial Tea Cultivars. Front. Microbiol. 2020, 11, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galletti, S.; Paris, R.; Cianchetta, S. Selected Isolates of Trichoderma gamsii Induce Different Pathways of Systemic Resistance in Maize upon Fusarium verticillioides Challenge. Microbiol. Res. 2020, 233, 126406. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Sousa, J.A.; Olivares, F.L. Plant Growth Promotion by Streptomycetes: Ecophysiology, Mechanisms and Applications. Chem. Biol. Technol. Agric. 2016, 3, 1–12. [Google Scholar]
- Gao, L.; Ma, J.; Liu, Y.; Huang, Y.; Mohamad, O.A.A.; Jiang, H.; Egamberdieva, D.; Li, W.; Li, L. Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin. Microorganisms 2021, 9, 1448. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergencein Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- El-Tarabily, K.; Nassar, A.; Hardy, G.S.J.; Sivasithamparam, K. Plant Growth Promotion and Biological Control of Pythium aphanidermatum, A Pathogen of Cucumber, by Endophytic Actinomycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef]
- Pattanapipitpaisal, P.; Kamlandharn, R. Screening of Chitinolytic Actinomycetes for Biological Control of Sclerotium rolfsii Stem Rot Disease of Chilli. Songklanakarin J. Sci. Technol. 2012, 34, 387–393. [Google Scholar]
- Barassi, C.; Ayrault, G.; Creus, C.; Sueldo, R.; Sobrero, M. Seed Inoculation with Azospirillum mitigates NaCl Effects on Lettuce. Sci. Hortic. 2006, 109, 8–14. [Google Scholar] [CrossRef]
- Coombs, J.T.; Franco, C.M. Isolation and Identification of Actinobacteria from Surface-Sterilized Wheat Roots. Appl. Environ. Microbiol. 2003, 69, 5603–5608. [Google Scholar] [CrossRef] [Green Version]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Tian, X.; Cao, L.; Tan, H.; Han, W.; Chen, M.; Liu, Y.; Zhou, S. Diversity of Cultivated and Uncultivated Actinobacterial Endophytes in the Stems and Roots of Rice. Microb. Ecol. 2007, 53, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, J.; Chen, H.-H.; Zhao, G.-Z.; Zhu, W.-Y.; Jiang, C.-L.; Xu, L.-H.; Li, W.-J. Isolation, Diversity, and Antimicrobial Activity of Rare Actinobacteria from Medicinal Plants of Tropical Rain Forests in Xishuangbanna, China. Appl. Environ. Microbiol. 2009, 75, 6176–6186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Zhang, Y.-J.; Yuan, B.; Xu, P.-Y.; Xing, K.; Wang, J.; Jiang, J.-H. Isolation of ACC Deaminase-Producing Habitat-Adapted Symbiotic Bacteria Associated with Halophyte Limonium Sinense (Girard) Kuntze and Evaluating Their Plant Growth-Promoting Activity under Salt Stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Rungin, S.; Indananda, C.; Suttiviriya, P.; Kruasuwan, W.; Jaemsaeng, R.; Thamchaipenet, A. Plant Growth Enhancing Effects by a Siderophore-Producing Endophytic Streptomycete Isolated from a Thai Jasmine Rice Plant (Oryza sativa L. cv. KDML105). Anton. Leeuw. Int. J. 2012, 102, 463–472. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) With Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Baek, D.; Rokibuzzaman, M.; Khan, A.; Kim, M.C.; Park, H.J.; Yun, D.-J.; Chung, Y.R. Plant-Growth Promoting Bacillus oryzicola YC7007 Modulates Stress-Response Gene Expression and Provides Protection from Salt Stress. Front. Plant Sci. 2020, 10, 1646. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic Bacteria in Plant Salt Stress Tolerance: Current and Future Prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of Water Stress and Inoculation with Plant Growth Promoting Rhizobacteria (PGPR) On Antioxidant Status and Photosynthetic Pigments in Basil (Ocimum basilicum L.). J. Agric. Biol. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Heidari, M.; Mousavinik, S.M.; Golpayegani, A. Plant Growth Promoting Rhizobacteria (PGPR) Effect on Physiological Parameters and Mineral Uptake in Basil (Ociumum basilicm L.) under Water Stress. J. Agric. Biol. Sci. 2011, 6, 6–11. [Google Scholar]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-Solubilizing and Auxin-Producing Rhizobacteria Promote Plant Growth Under Saline Conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Nasraoui-Hajaji, A.; Gouia, H.; Carrayol, E.; Haouari-Chaffei, C. Ammonium Alleviates Redox State in Solanum Seedlings under Cadmium Stress Conditions. J. Environ. Anal. Toxicol. 2012, 2, 141. [Google Scholar]
- Inderbitzin, P.; Subbarao, K.V. Verticillium Systematics and Evolution: How Confusion Impedes Verticillium Wilt Management and How to Resolve It. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial Endophytes as Potential Biocontrol Agents of Vascular Wilt Diseases–Review and Future Prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, L.; Yang, Y.; Guan, Q.; Gong, M. Potential of Endophytic Bacteria Isolated from 'Sophora alopecuroides' Nodule in Biological Control against Verticillium Wilt Disease. Aust. J. Crop Sci. 2013, 7, 139–146. [Google Scholar]
- Sunish, K.; Sreedharan, P.; Daniel, S.; Biji, M.; Rosamma, P.; Sukumaran, V.; Mohandas, A.; Singh, I.B. A Novel Substituted Derivative of Sterol from Marine Actinomycetes Nocardiopsis alba MCCB 110 Antagonistic to the Aquaculture Pathogen Vibrio harveyi. Microb. Pathog. 2021, 157, 104967. [Google Scholar] [CrossRef]
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two Strains of Airborne Nocardiopsis alba Producing Different Volatile Organic Compounds (VOCs) as Biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138. [Google Scholar] [CrossRef]
- Goudjal, Y.; Toumatia, O.; Yekkour, A.; Sabaou, N.; Mathieu, F.; Zitouni, A. Biocontrol of Rhizoctonia Solani Damping-off and Promotion of Tomato Plant Growth by Endophytic Actinomycetes Isolated from Native Plants of Algerian Sahara. Microbiol. Res. 2014, 169, 59–65. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.; Kashalkar, R. Antibacterial Activity of Dibutyl Phthalate: A Secondary Metabolite Isolated from Ipomoea carnea Stem. J. Pharm. Res. 2012, 5, 150–152. [Google Scholar]
- Motshudi, M.; Olaokun, O.; Mkolo, N. Evaluation of GC× GC-TOF-MS Untargeted Metabolomics, Cytotoxicity and Antimicrobial Activity of Leaf Extracts of Artemisia afra (Jacq.) Purchased from Three Local Vendors. J. King Saud Univ. Sci. 2021, 33, 101422. [Google Scholar] [CrossRef]
- Fernandes, P.; Ferreira, B.S.; Cabral, J.M.S. Solvent Tolerance in Bacteria: Role of Efflux Pumps and Cross-Resistance with Antibiotics. Int. J. Antimicrob. Agents 2003, 22, 211–216. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile Antimicrobials from Muscodor crispans, A Novel Endophytic Fungus. Microbiology 2009, 156, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpaslan, G.; Boyacioglu, B.; Demir, N.; Tümer, Y.; Yapar, G.; Yıldırım, N.; Yıldız, M.; Ünver, H. Synthesis, Characterization, Biological Activity and Theoretical Studies of a 2-Amino-6-Methoxybenzothiazole-Based Fluorescent Schiff Base. J. Mol. Struct. 2019, 1180, 170–178. [Google Scholar] [CrossRef]
- Tang, L.; Mo, J.; Guo, T.; Huang, S.; Li, Q.; Ning, P.; Hsiang, T. In Vitro Antifungal Activity of Dimethyl Trisulfide against Colletotrichum gloeosporioides from Mango. World J. Microbiol. Biotechnol. 2020, 36, 4. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Senapati, A.; Kumar, U.; Sharma, L.; Lepcha, P.; Prabhukarthikeyan, S.; Jahan, A.; Parameshwaran, C.; Govindharaj, G.P.P.; Lenka, S. Antagonistic and Plant-Growth Promoting Novel Bacillus Species from Long-Term Organic Farming Soils from Sikkim, India. 3 Biotech 2019, 9, 416. [Google Scholar] [CrossRef]
- Kannabiran, K. Bioactivity of Pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro-3-(phenylmethyl)-Extracted from Streptomyces sp. VITPK9 Isolated from the Salt Spring Habitat of Manipur, India. Asian J. Pharm. 2016, 10, 4. [Google Scholar]
- Albratty, M.; Alhazmi, H.; Meraya, A.; Najmi, A.; Alam, M.; Rehman, Z.; Moni, S. Spectral Analysis and Antibacterial Activity of the Bioactive Principles of Sargassum tenerrimum J. Agardh Collected from the Red Sea, Jazan, Kingdom of Saudi Arabia. Braz. J. Biol. 2021, 83, e249536. [Google Scholar] [CrossRef]
- Priyanka, R.; Nakkeeran, S. Ochrobactrum ciceri Mediated Induction of Defence Genes and Antifungal Metabolites Enhance the Biocontrol Efficacy for the Management of Botrytis Leaf Blight of Lilium under Protected Conditions. J. Plant Pathol. 2019, 101, 323–337. [Google Scholar] [CrossRef]
- Yogeswari, S.; Ramalakshmi, S.; Neelavathy, R.; Muthumary, J.y. Identification and Comparative Studies of Different Volatile Fractions from Monochaetia kansensis by GCMS. Glob. J. Pharmacol. 2012, 6, 65–71. [Google Scholar]
- Awla, H.K.; Kadir, J.; Othman, R.; Rashid, T.S.; Wong, M.-Y. Bioactive Compounds Produced by Streptomyces sp. Isolate UPMRS4 and Antifungal Activity against Pyricularia oryzae. Am. J. Plant Sci. 2016, 7, 1077. [Google Scholar] [CrossRef] [Green Version]
- Dos Reis, C.M.; da Rosa, B.V.; da Rosa, G.P.; do Carmo, G.; Morandini, L.M.B.; Ugalde, G.A.; Kuhn, K.R.; Morel, A.F.; Jahn, S.L.; Kuhn, R.C. Antifungal and Antibacterial Activity of Extracts Produced from Diaporthe schini. J. Biotechnol. 2019, 294, 30–37. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, O.A.A.; Liu, Y.-H.; Li, L.; Ma, J.-B.; Huang, Y.; Gao, L.; Fang, B.-Z.; Wang, S.; El-Baz, A.F.; Jiang, H.-C.; et al. Synergistic Plant-Microbe Interactions between Endophytic Actinobacteria and Their Role in Plant Growth Promotion and Biological Control of Cotton under Salt Stress. Microorganisms 2022, 10, 867. https://doi.org/10.3390/microorganisms10050867
Mohamad OAA, Liu Y-H, Li L, Ma J-B, Huang Y, Gao L, Fang B-Z, Wang S, El-Baz AF, Jiang H-C, et al. Synergistic Plant-Microbe Interactions between Endophytic Actinobacteria and Their Role in Plant Growth Promotion and Biological Control of Cotton under Salt Stress. Microorganisms. 2022; 10(5):867. https://doi.org/10.3390/microorganisms10050867
Chicago/Turabian StyleMohamad, Osama Abdalla Abdelshafy, Yong-Hong Liu, Li Li, Jin-Biao Ma, Yin Huang, Lei Gao, Bao-Zhu Fang, Shuang Wang, Ashraf F. El-Baz, Hong-Chen Jiang, and et al. 2022. "Synergistic Plant-Microbe Interactions between Endophytic Actinobacteria and Their Role in Plant Growth Promotion and Biological Control of Cotton under Salt Stress" Microorganisms 10, no. 5: 867. https://doi.org/10.3390/microorganisms10050867
APA StyleMohamad, O. A. A., Liu, Y.-H., Li, L., Ma, J.-B., Huang, Y., Gao, L., Fang, B.-Z., Wang, S., El-Baz, A. F., Jiang, H.-C., & Li, W.-J. (2022). Synergistic Plant-Microbe Interactions between Endophytic Actinobacteria and Their Role in Plant Growth Promotion and Biological Control of Cotton under Salt Stress. Microorganisms, 10(5), 867. https://doi.org/10.3390/microorganisms10050867








