Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of CS-Based PEC Cryogels
2.2. Characterization of PEC Cryogels
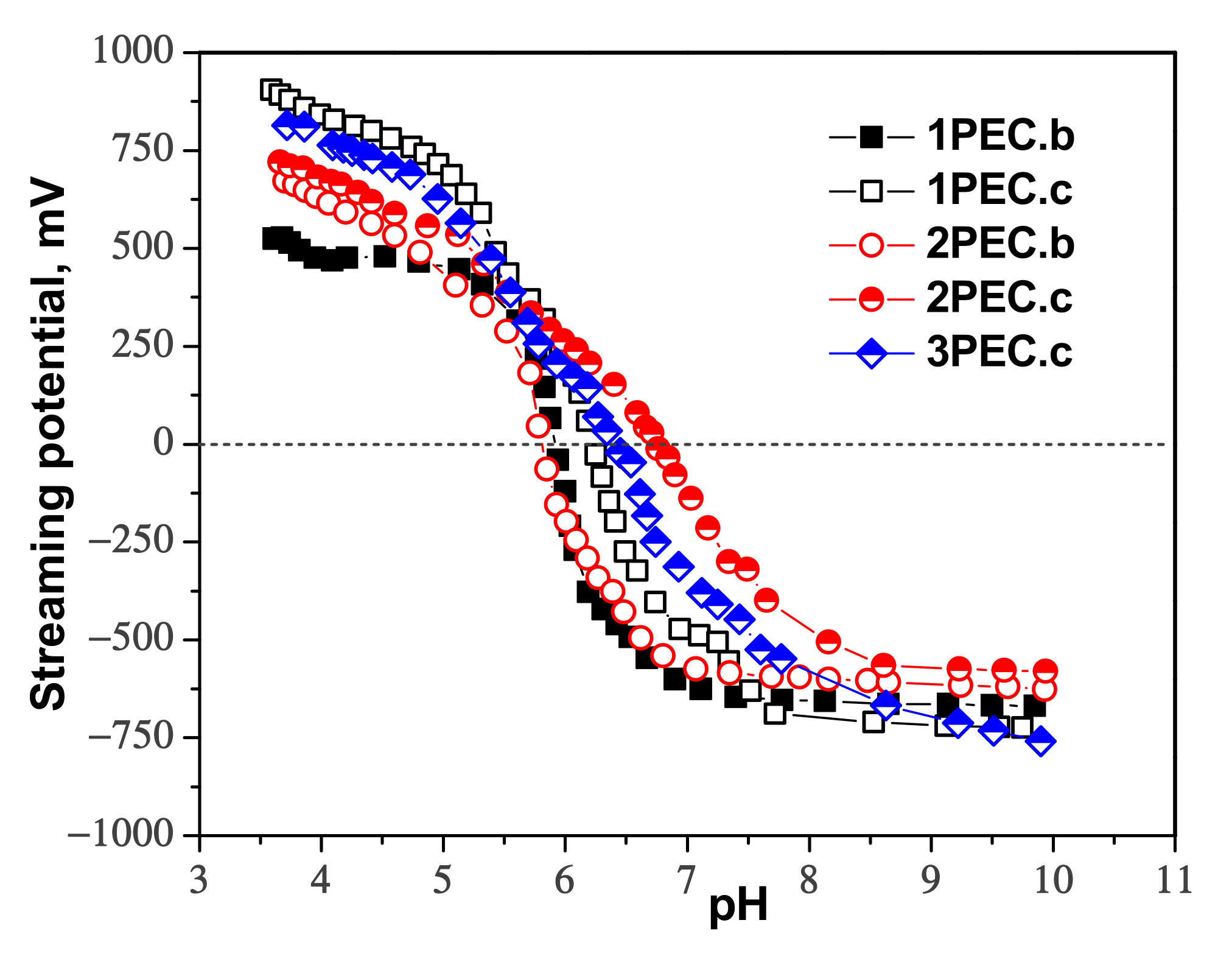
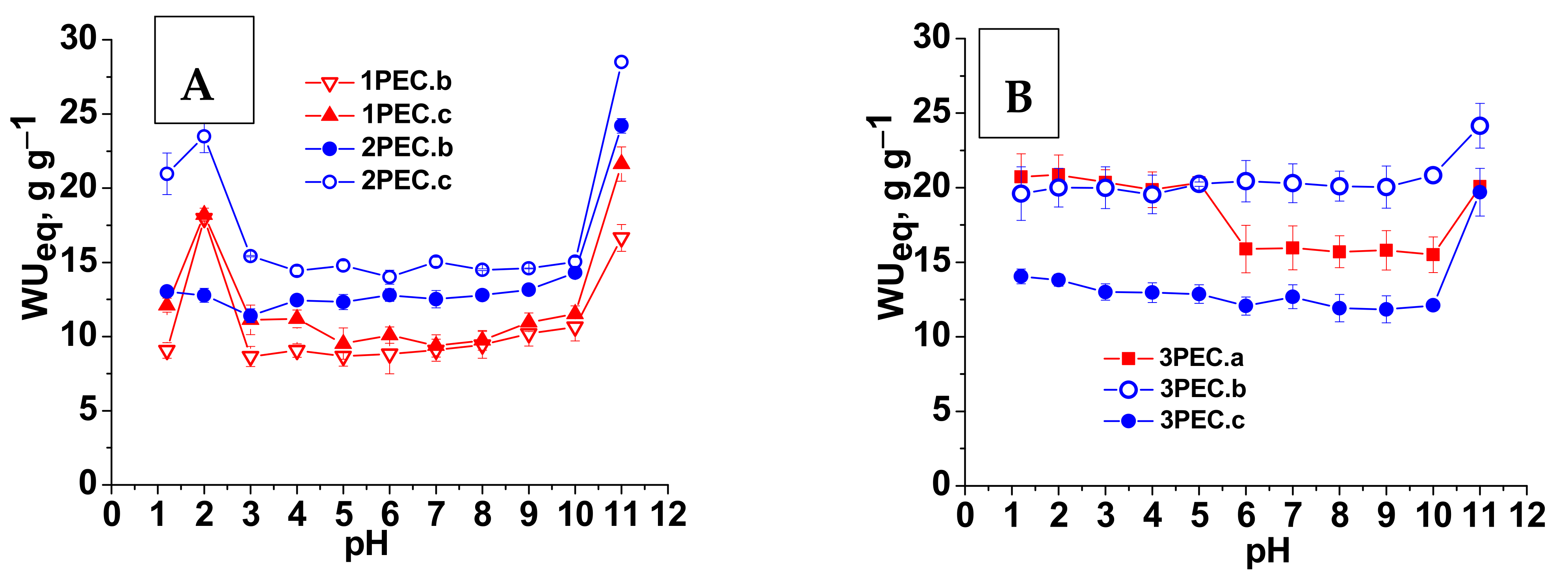
2.2.1. Structure, Morphology and Swelling
2.2.2. Elasticity and Shape Memory Performance of PEC Cryogels
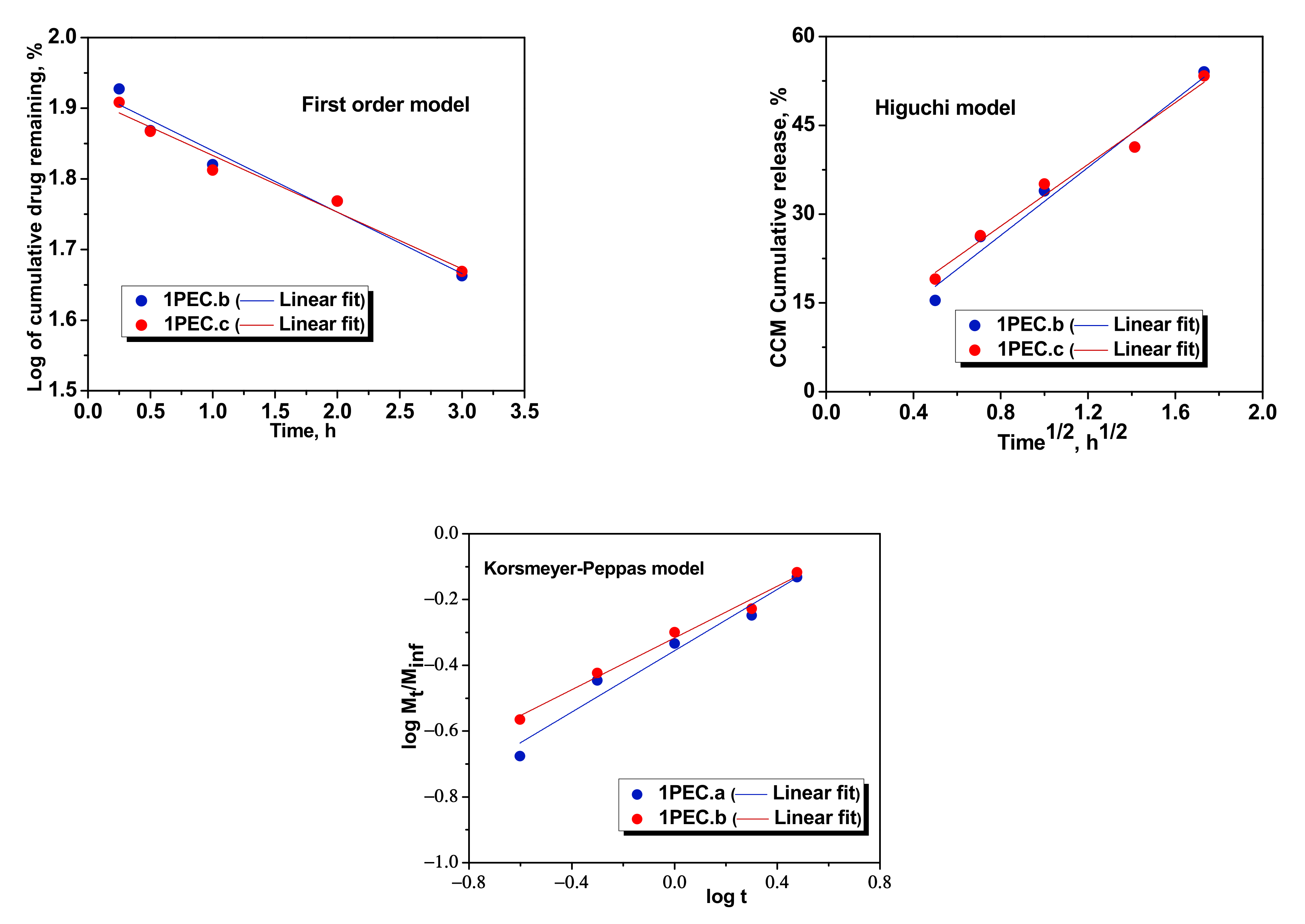
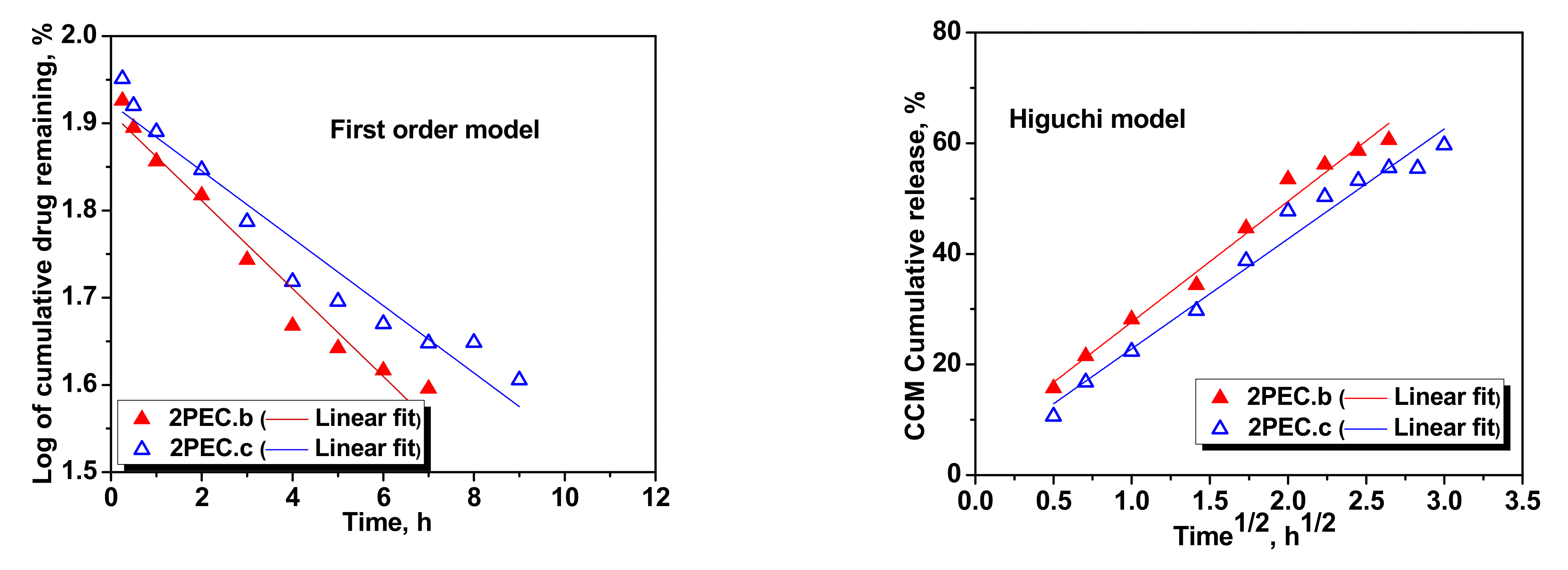
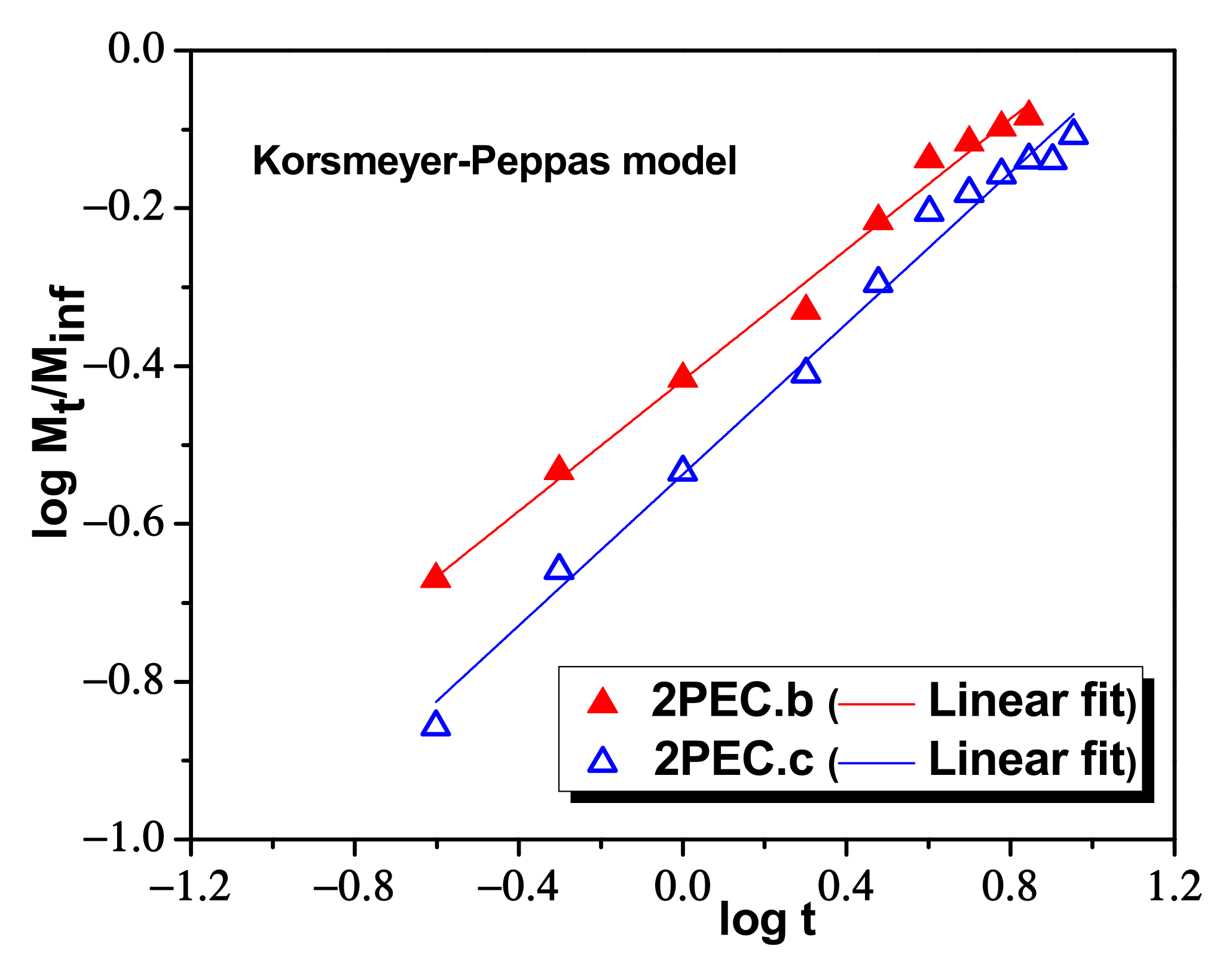
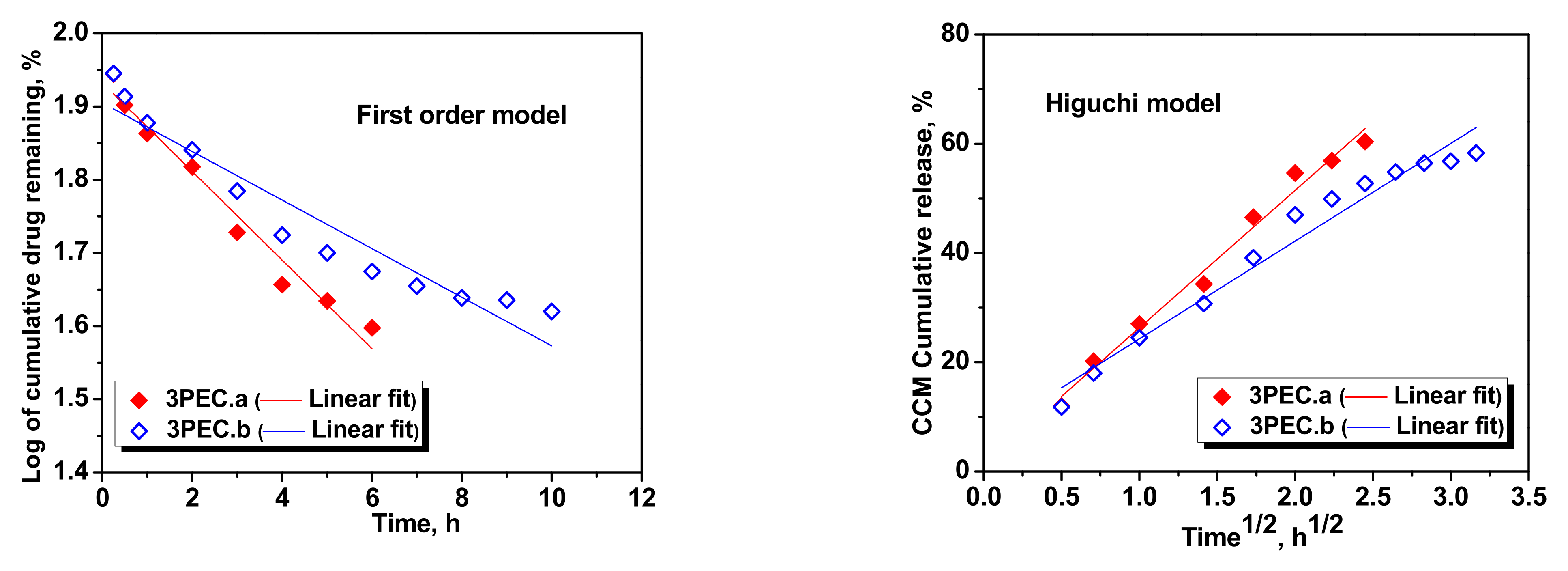
2.3. Loading and Release of CCM in/from PEC Cryogels
3. Conclusions
4. Materials and Methods
4.1. Preparation of PEC Cryogels
4.2. Characterization of PEC Cryogels
4.3. Loading and Release of CCM from PEC Cryogels
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pellá, M.; Lima-Tenório, M.K.; Tenório-Neto, E.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2018, 207, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2019, 146, 104372. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-specific drug delivery of clar-ithromycin using a semi-interpenetrating polymeric network hydrogel made of montmorillonite and chitosan: Synthesis, characterization and in vitro drug release study. Adv. Pharm. Bull. 2019, 9, 159–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, K.; Wang, F.; Wang, H.; Wang, J.; Jiang, Z.; Diao, S. Three dimensional macroporous hydroxyapatite/chitosan foam-supported polymer micelles for enhanced oral delivery of poorly soluble drugs. Colloids Surf. B Biointerfaces 2018, 170, 497–504. [Google Scholar] [CrossRef]
- Kang, B.; Vales, T.P.; Cho, B.-K.; Kim, J.-K.; Kim, H.-J. Development of Gallic Acid-Modified Hydrogels Using Interpenetrating Chitosan Network and Evaluation of Their Antioxidant Activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, E.S.; Cocarta, A.I.; Gierszewska, M. Designing novel macroporous composite hydrogels based on methacrylic acid copolymers and chitosan and in vitro assessment of lysozyme controlled delivery. Colloids Surf. B Biointerfaces 2016, 139, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Xie, Y.-Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2018, 122, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, Y.; Zhang, X.; Li, J.; Sun, L.; Gui, Z.; Du, B.; Chen, S. Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohydr. Polym. 2018, 202, 246–257. [Google Scholar] [CrossRef]
- Bourganis, V.; Karamanidou, T.; Kammona, O.; Kiparissides, C. Polyelectrolyte complexes as prospective carriers for the oral delivery of protein therapeutics. Eur. J. Pharm. Biopharm. 2017, 111, 44–60. [Google Scholar] [CrossRef]
- Insua, I.; Wilkinson, A.; Fernandez-Trillo, F. Polyion complex (PIC) particles: Preparation and biomedical applications. Eur. Polym. J. 2016, 81, 198–215. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-X.; Wang, D.-D.; Su, T.; Cheng, X.-D.; Xu, X.; Chen, Y. Self-assembly of polyelectrolyte complexes microcapsules with natural polysaccharides for sustained drug release. Cellulose 2017, 24, 4949–4962. [Google Scholar] [CrossRef]
- Michaels, A.S.; Miekka, R.G. Polycation-polyanion complexes: Preparation and properties of poly- (vinylbenzyltrime-thylammonium) poly-(styrenesulfonate). J. Phys. Chem. 1961, 65, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Philipp, B.; Dautzenberg, H.; Linow, K.-J.; Kötz, J.; Dawydoff, W. Polyelectrolyte complexes—Recent developments and open problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Dragan, S.; Cristea, M.; Luca, C.; Simionescu, B.C. Polyeledrolyte complexes. I. Synthesis and characterization of some insoluble polyanion-polycation complexes. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3485–3494. [Google Scholar] [CrossRef]
- Dragan, S.; Dragan, D.; Cristea, M.; Airinei, A.; Ghimici, L. Polyelectrolyte complexes. II. Specific aspects of the formation of polycation/dye/polyanion complexes. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 409–418. [Google Scholar] [CrossRef]
- Drogoz, A.; David, L.; Rochas, C.; Domard, A.A.; Delair, T. Polyelectrolyte Complexes from Polysaccharides: Formation and Stoichiometry Monitoring. Langmuir 2007, 23, 10950–10958. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.; Dragan, E.S. Chitosan based nonstoichiometric polyelectrolyte complexes as specialized flocculants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 39–46. [Google Scholar] [CrossRef]
- Fu, J.; Fares, H.M.; Schlenoff, J.B. Ion-Pairing Strength in Polyelectrolyte Complexes. Macromolecules 2017, 50, 1066–1074. [Google Scholar] [CrossRef]
- Dragan, E.S.; Schwarz, S. Polyelectrolyte Complexes. VI. Polycation Structure, Polyanion Molar Mass, and Polyion Concen-tration Effects on Complex Nanoparticles Based on Poly(sodium 2- acrylamido-2-methylpropanesulfonate). J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2495–2505. [Google Scholar] [CrossRef]
- Lalevée, G.; David, L.; Montembault, A.; Blanchard, K.; Meadows, J.; Malaise, S.; Crépet, A.; Grillo, I.; Morfin, I.; Delair, T.; et al. Highly stretchable hydrogels from complex coacervation of natural polyelectrolytes. Soft Matter 2017, 13, 6594–6605. [Google Scholar] [CrossRef]
- Dragan, S.; Cristea, M. Influence of low-molecular-weight salts on the formation of polyelectrolyte complexes based on poly-cations with quaternary ammonium salt groups in the main chain and poly(sodium acrylate). Eur. Polym. J. 2001, 37, 1571–1575. [Google Scholar] [CrossRef]
- Kalinov, K.; Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Momekova, D. N,N,N-trimethylchitosan iodide complexes with a weak or a strong polyacid and nanoparticles thereof. Colloid Polym. Sci. 2014, 292, 2899–2912. [Google Scholar] [CrossRef]
- Baig, M.I.; Durmaz, E.N.; Villott, J.D.; de Vos, W.M. Sustainable membrane production through polyelectrolyte complexation induced aqueous phase separation. Adv. Funct. Mater. 2020, 30, 1907344. [Google Scholar] [CrossRef]
- Carvalho, S.G.; dos Santos, A.M.; Silvestre, A.L.P.; Meneguin, A.B.; Ferreira, L.M.B.; Chorilli, M.; Gremião, M.P.D. New insights into physicochemical aspects involved in the formation of polyelectrolyte complexes based on chitosan and dextran sulfate. Carbohydr. Polym. 2021, 271, 118436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Su, T.; Zhao, J.; Wu, Z.; Wang, D.; Zhang, W.-N.; Wu, Q.-X.; Chen, Y. Fabrication of polysaccharides-based hydrogel films for transdermal sustained delivery of Ibuprofen. Cellulose 2020, 27, 10277–10292. [Google Scholar] [CrossRef]
- Ćirić, A.; Medarević, D.; Čalija, B.; Dobričić, V.; Mitrić, M.; Djekic, L. Study of chitosan/xanthan gum polyelectrolyte complexes formation, solid state and influence on ibuprofen release kinetics. Int. J. Biol. Macromol. 2020, 148, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2019, 306, 125632. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin, C. Carbohydr. Polym. 2020, 234, 115920. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Parolin, C.E.; Vitali, B.; Bigucci, F.; Gallucci, M.C.; Nicoletta, F.P.; Luppi, B. Microparticles based on chitosan/carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin. Carbohydr. Polym. 2016, 143, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, J.P.; Nareddy, P.K.; Moreno-Villoslada, I.; Moerschbacher, B.M.; Swamy, M.J.; Pan, S.; Ostermeier, M.; Goycoolea, F.M. On the role of alginate structure in complexing with lysozyme and application for enzyme delivery. Food Hydrocoll. 2016, 53, 239–248. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef]
- Rahmani, V.; Sheardown, H. Protein-alginate complexes as pH-/ion-sensitive carriers of proteins. Int. J. Pharm. 2018, 535, 452–461. [Google Scholar] [CrossRef]
- Shu, M.; Long, S.; Huang, Y.; Li, D.; Li, H.; Li, X. High strength and antibacterial polyelectrolyte complex CS/HS hydrogel films for wound healing. Soft Matter 2019, 15, 7686–7694. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Bui, Q.A.; Nguyen, H.H.N.; Nguyen, T.T.; Ly, K.L.; Tran, H.L.B.; Doan, V.N.; Nhi, T.Y.; Nguyen, N.H.; Tran, N.Q.; et al. Curcuminoid co-loading platinum heparin-poloxamer P403 nanogel increasing ef-fectiveness in antitumor activity. Gels 2022, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Pi, C.; Yang, H.; Zheng, X.; Zhao, L.; Wei, Y. Review of Curcumin Physicochemical Targeting Delivery System. Int. J. Nanomed. 2020, 15, 9799–9821. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Panaro, M.; la Torre, M.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.K.; Akhbari, K. Biocompatible MIL-101(Fe) as a Smart Carrier with High Loading Potential and Sustained Release of Curcumin. Inorg. Chem. 2020, 59, 3570–3578. [Google Scholar] [CrossRef]
- Jardim, K.V.; Garfias, A.F.P.; Andrade, B.Y.G.; Chaker, J.A.; Báo, S.; Márquez-Beltrán, C.; Moya, S.; Parize, A.L.; Sousa, M.H. Novel magneto-responsive nanoplatforms based on MnFe2O4 nanoparticles layer-by-layer functionalized with chitosan and sodium alginate for magnetic controlled release of curcumin. Mater. Sci. Eng. C 2018, 92, 184–195. [Google Scholar] [CrossRef]
- Omer, A.; Ziora, Z.; Tamer, T.; Khalifa, R.; Hassan, M.; Mohy-Eldin, M.; Blaskovich, M. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Gao, N.; Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Feng, C.; Liu, M. Injectable shell-crosslinked F127 micelle/hydrogel composites with pH and redox sensitivity for combined release of anticancer drugs. Chem. Eng. J. 2016, 287, 20–29. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Liou, N.S. Influence of a nonionic sur-factant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, J.; Wang, Y.; Jia, J.; Liu, F.; Liu, X. Polysaccharide-based delivery system for curcumin: Fabrication and charac-terization of carboxymethylated corn fiber gum/chitosan biopolymer particles. Food Hydrocol. 2022, 125, 107367. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applica-tions of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Szczęsna, W.; Tsirigotis-Maniecka, M.; Lamch, L.; Szyk-Warszyńska, L.; Zboińska, E.; Warszyński, P.; Wilk, K.A. Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules 2022, 27, 1415. [Google Scholar] [CrossRef] [PubMed]
- Raschip, I.E.; Fifere, N.; Dinu, M.V. A comparative Analysis on the effect of a variety of grape pomace exctracts on the ice-templated 3D cryogel features. Gels 2021, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; He, B.; Wang, S.; Kong, F. Synthesis of cellulose aerogels as promising carriers for drug delivery: A review. Cellulose 2021, 28, 2697–2714. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2019, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of Chitosan Aerogels: Three-Dimensional Pore Control for Tailored Applications. Angew. Chem. Int. Ed. 2020, 60, 9828–9851. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.; Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2018, 204, 223–231. [Google Scholar] [CrossRef]
- Wang, R.; Shou, D.; Lv, Q.; Kong, Y.; Deng, L.; Shen, J. pH-Controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int. J. Biol. Macromol. 2017, 103, 248–253. [Google Scholar] [CrossRef]
- Gorshkova, N.; Brovko, O.; Palamarchuk, I.; Bogolitsyn, K.; Ivakhnov, A. Preparation of bioactive aerogel material based on sodium alginate and chitosan for controlled release of levomycetin. Polym. Adv. Technol. 2021, 32, 3474–3482. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, A.; Jatmiko, A.; Ananda, M.B.; Rachmawati, S.A.; Ardy, H.; Aimon, A.H.; Iskandar, F. Facile fabrication of poly-electrolyte complex nanoparticles based on chitosan—poly-2-acrylamido-2-methylpropane sulfonic acid as a potential drug carrier material. Int. J. Technol. 2021, 12, 561–570. [Google Scholar] [CrossRef]
- Varguez-Catzim, P.; Rodríguez-Fuentes, N.; Borges-Argáez, R.; Cáceres-Farfán, M.; González-Díaz, A.; Alonzo-Garcia, A.; Duarte, S.; Aguilar-Vega, M.; González-Díaz, M.O. Bilayer asymmetric PVA/PAMPS membranes with efficient antimicrobial surface and enhanced biocompatibility. Appl. Surf. Sci. 2021, 565, 150544. [Google Scholar] [CrossRef]
- Dinu, M.V.; Přádný, M.; Drăgan, E.S.; Michálek, J. Ice-templated hydrogels based on chitosan with tailored porous morphology. Carbohydr. Polym. 2013, 94, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective view on the more than 40 years of studies performed in the A.N. Nesmeyanov institute of organoelement compounds with respect of the cryostructuring processes in polymeric sys-tems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Q.; Sun, J.; Zhou, Q. Preparation of poly(ethylene glycol) aligned porous cryogels using a unidirectional freezing technique. Soft Matter 2012, 8, 3620–3626. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Chen, L.; Dai, B. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Bocourt, M.; Arguelles-Monal, W.; Cauich-Rodríguez, J.V.; May, A.; Bada, N.; Peniche, C. Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies. Mater. Sci. Appl. 2011, 2, 509–520. [Google Scholar]
- Suneetha, M.; Rao, K.M.; Han, S.S. Mechanically improved porous hydrogels with polysaccharides via polyelectrolyte com-plexation for bone tissue engineering. Int. J. Biol. Macromol. 2020, 144, 160–169. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yu, H.-Y.; Song, M.-L.; Yang, R.-T.; Yao, J.-M. Superfast Adsorption–Disinfection Cryogels Decorated with Cellulose Nanocrystal/Zinc Oxide Nanorod Clusters for Water-Purifying Microdevices. ACS Sustain. Chem. Eng. 2017, 5, 6776–6785. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, J.; Wu, X.; Lin, Y. Macroporous double-network cryogels: Formation mechanism, enhanced mechanical strength and temperature/pH dual sensitivity. Soft Matter 2011, 7, 4284–4293. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2020, 251, 117023. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gamzazade, A.I.; Shimac, V.M.; Skljar, A.M.; Stykova, E.V.; Pavlova, S.A.; Rogozin, S.V. Investigation of the hydrodynamic properties of chitosan solutions. Acta Polym. 1985, 36, 420–424. [Google Scholar] [CrossRef]
- Dragan, S.; Mihai, M.; Ghimici, L. Viscometric study of poly(sodium 2-acrylamido-2-methylpropanesulfonate) and two ran-dom copolymers. Eur. Polym. J. 2003, 39, 1847–1854. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V.; Olariu, R.I. Kinetics, equilibrium modeling, and thermodynamics on removal of Cr(VI) ions from aqueous solution using novel composites with strong base anion exchanger microspheres embedded into chitosan/poly(vinyl amine) cryogels. Chem. Eng. J. 2017, 330, 675–691. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei Loghin, D.F. Fabrication and characterization of composite cryobeads based on chitosan and starch-es-g-PAN as efficient and reusable biosorbents for removal of Cu2+, Ni2+, and Co2+ ions. Int. J. Biol. Macromol. 2018, 120, 1872–1883. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Yao, Y.; Tong, Z.R.; Li, F.; Yang, Z.; Jin, S. Preparation of the polyelectrolyte complex hydrogel of biopoly-mers via a semi-dissolution acidification sol-gel transition method and its application in solid-state supercapacitors. J. Power Sources 2018, 378, 603–609. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A.; Lazar, M.M.; Doroftei, F. Preparation and characterization of Semi-IPN cryogels based on polyacrylamide and poly(N,N-dimethylaminoethyl methacrylate); Functionalization of carrier with monochlorotri-azinyl-β-cyclodextrin and release kinetics of curcumin. Molecules 2021, 26, 6975. [Google Scholar] [CrossRef]

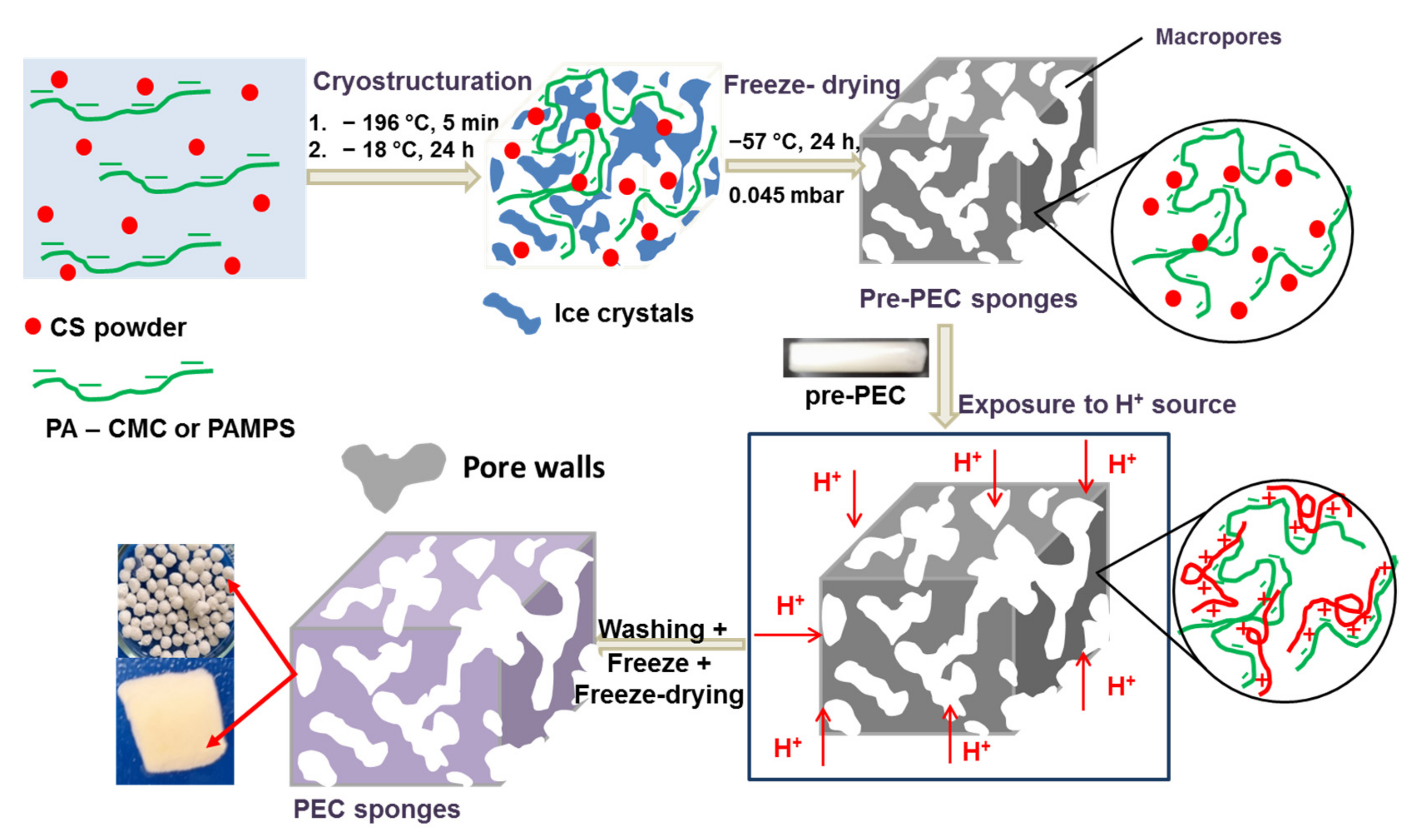

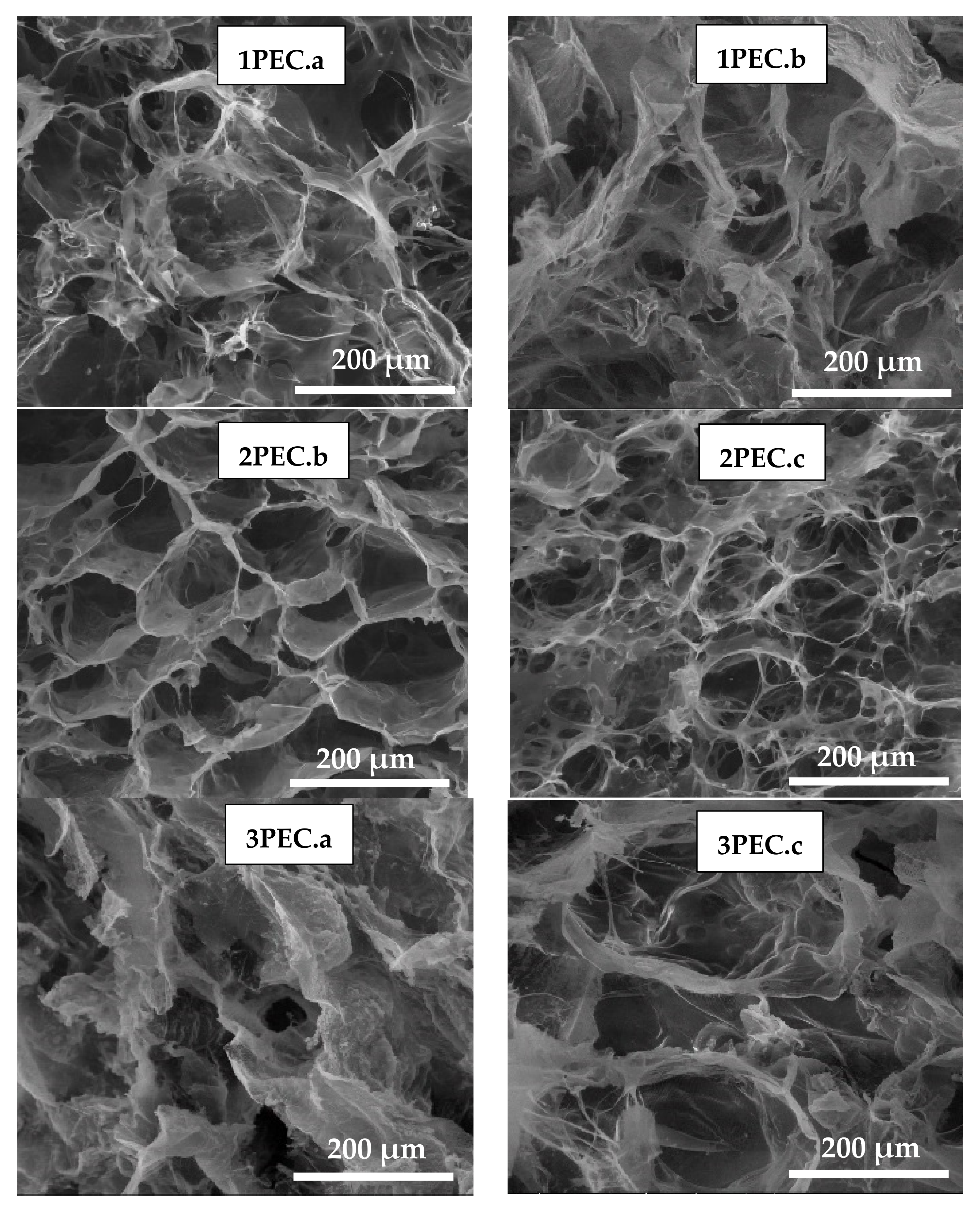
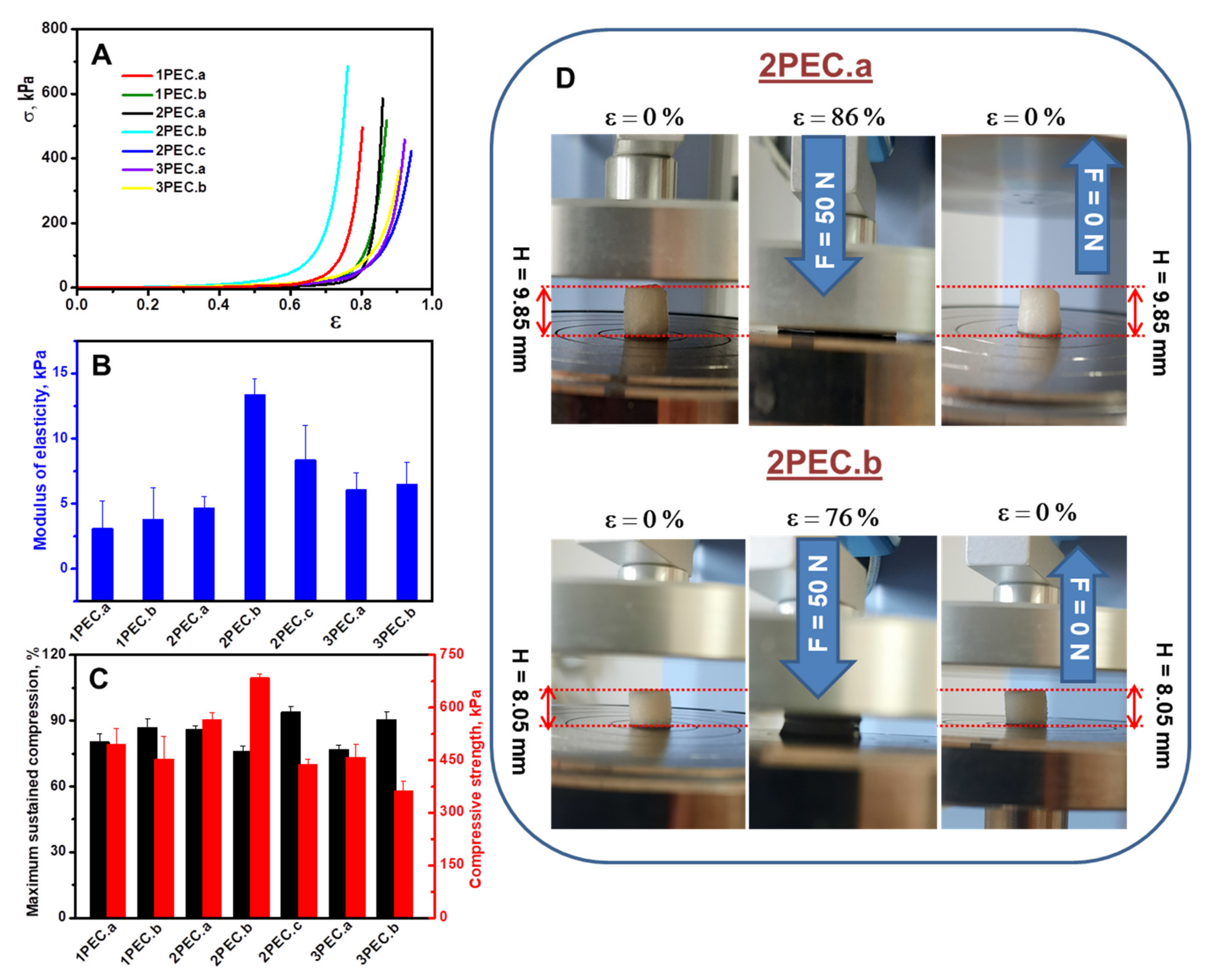


| Sample ID | Polyanion Solution | Chitosan | PEC Shape * | ||||
|---|---|---|---|---|---|---|---|
| Name | Mv, kDa | Conc., wt.% | Name | Mv, kDa | wt.% | ||
| 1PEC.a | CMC1 | 90 | 3 | CS1 | 207 | 3 | UF |
| 1PEC.b | CMC1 | 90 | 4 | CS1 | 207 | 4 | UF |
| 1PEC.c | CMC1 | 90 | 4 | CS1 | 207 | 4 | CB |
| 2PEC.a | CMC2 | 250 | 3 | CS1 | 207 | 3 | CG |
| 2PEC.b | CMC2 | 250 | 3 | CS1 | 207 | 3 | UF |
| 2PEC.c | CMC2 | 250 | 3 | CS2 | 305 | 3 | UF |
| 3PEC.a | PAMPS | 1400 | 3 | CS1 | 207 | 3 | UF |
| 3PEC.b | PAMPS | 1400 | 4 | CS1 | 207 | 4 | UF |
| 3PEC.c | PAMPS | 1400 | 4 | CS1 | 207 | 4 | CB |
| Sample Code | 1PEC.b | 1PEC.c | 2PEC.b | 2PEC.c | 3PEC.a | 3PEC.b |
|---|---|---|---|---|---|---|
| Pore diameter, µm | 126.5 ± 11 | 109.5 ± 38 | 107.9 ± 21.5 | 51.7 ± 11 | 117.8 ± 18 | 107.8 ± 28 |
| Drug loading, % | 6.67 | 5.62 | 8.9 | 9.73 | 8 | 7.7 |
| Model Name | Parameters | PEC Samples | |||||
|---|---|---|---|---|---|---|---|
| 1PEC.b | 1PEC.c | 2PEC.b | 2PEC.c | 3PEC.a | 3PECb | ||
| First order | k1 | −0.0869 | −0.0803 | −0.0504 | −0.0386 | −0.0606 | −0.0332 |
| R2 | 0.9549 | 0.9634 | 0.9584 | 0.9379 | 0.9679 | 0.9150 | |
| Higuchi | kH | 28.6841 | 26.0885 | 21.8286 | 19.8796 | 25.1502 | 17.8917 |
| R2 | 0.9644 | 0.9742 | 0.9826 | 0.9753 | 0.9848 | 0.9657 | |
| Korsmeyer–Peppas | nr | 0.4663 | 0.3934 | 0.4146 | 0.4788 | 0.4974 | 0.4327 |
| kKP (min−nr) | 7.0075 | 7.2855 | 6.5836 | 5.8426 | 6.0586 | 6.1079 | |
| R2 | 09563 | 0.9824 | 0.9911 | 0.9886 | 0.9858 | 0.9859 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. https://doi.org/10.3390/gels8040240
Dragan ES, Dinu MV, Ghiorghita CA. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels. 2022; 8(4):240. https://doi.org/10.3390/gels8040240
Chicago/Turabian StyleDragan, Ecaterina Stela, Maria Valentina Dinu, and Claudiu Augustin Ghiorghita. 2022. "Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps" Gels 8, no. 4: 240. https://doi.org/10.3390/gels8040240







