Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of PS and PDI of VZ-Cub
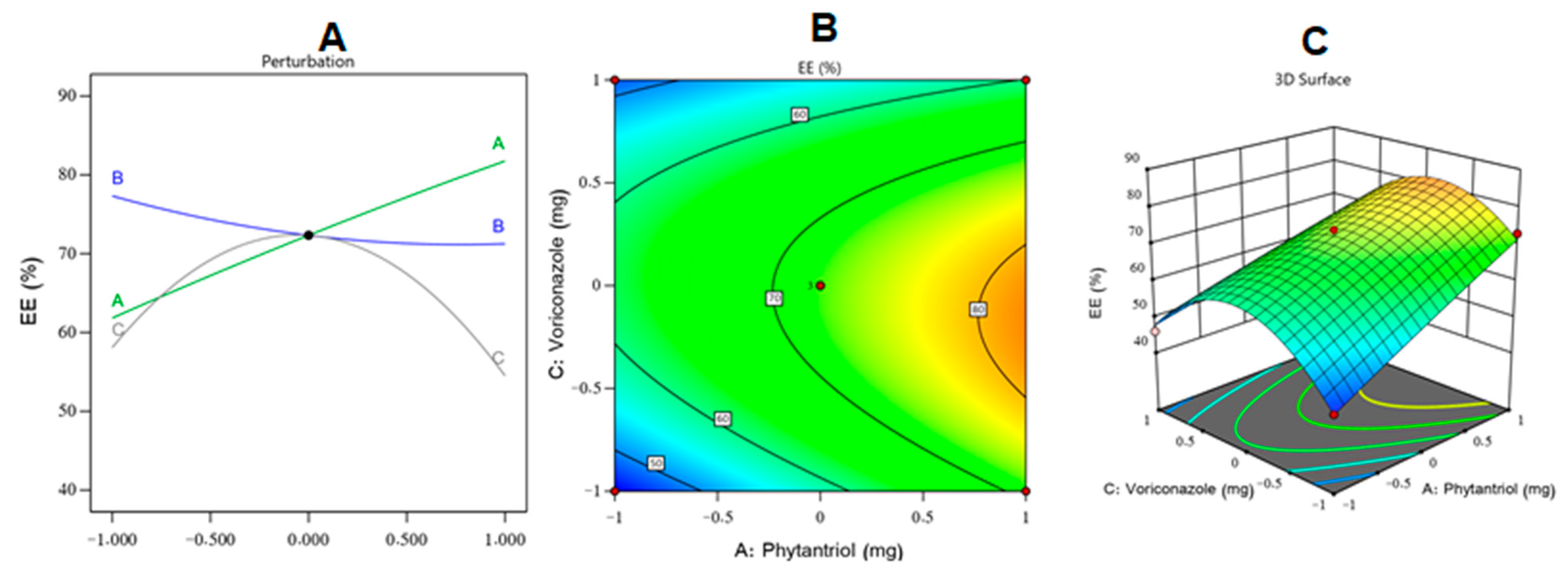
2.2. EE% Assessment
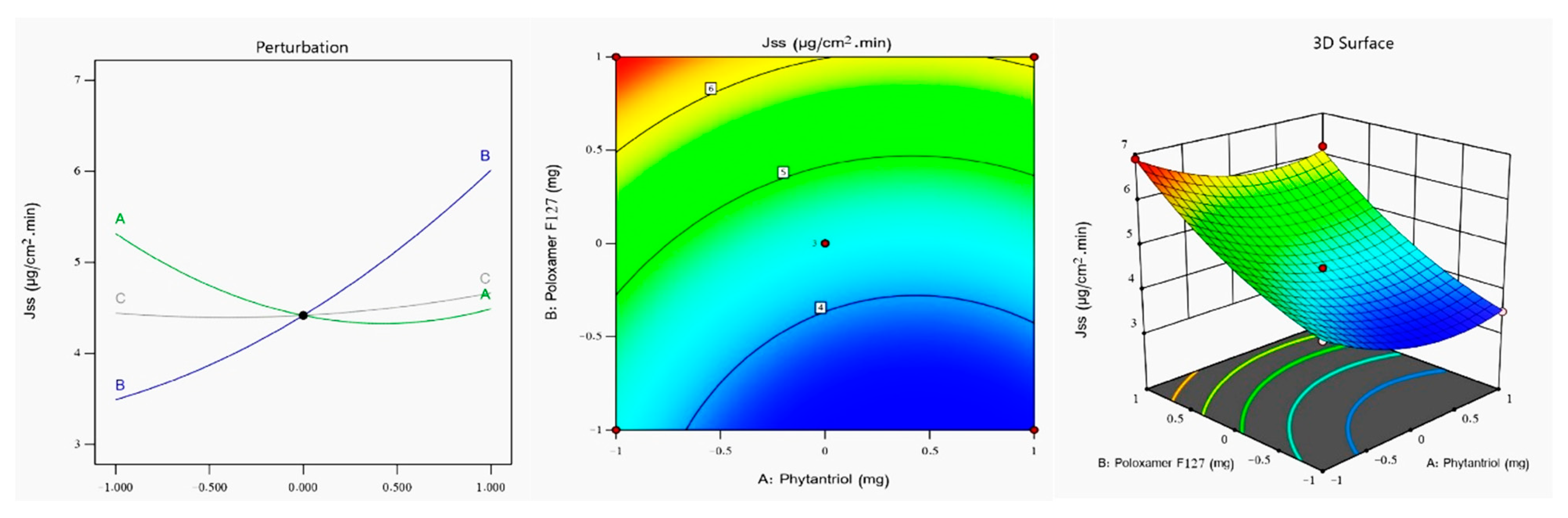
2.3. Jss Determination
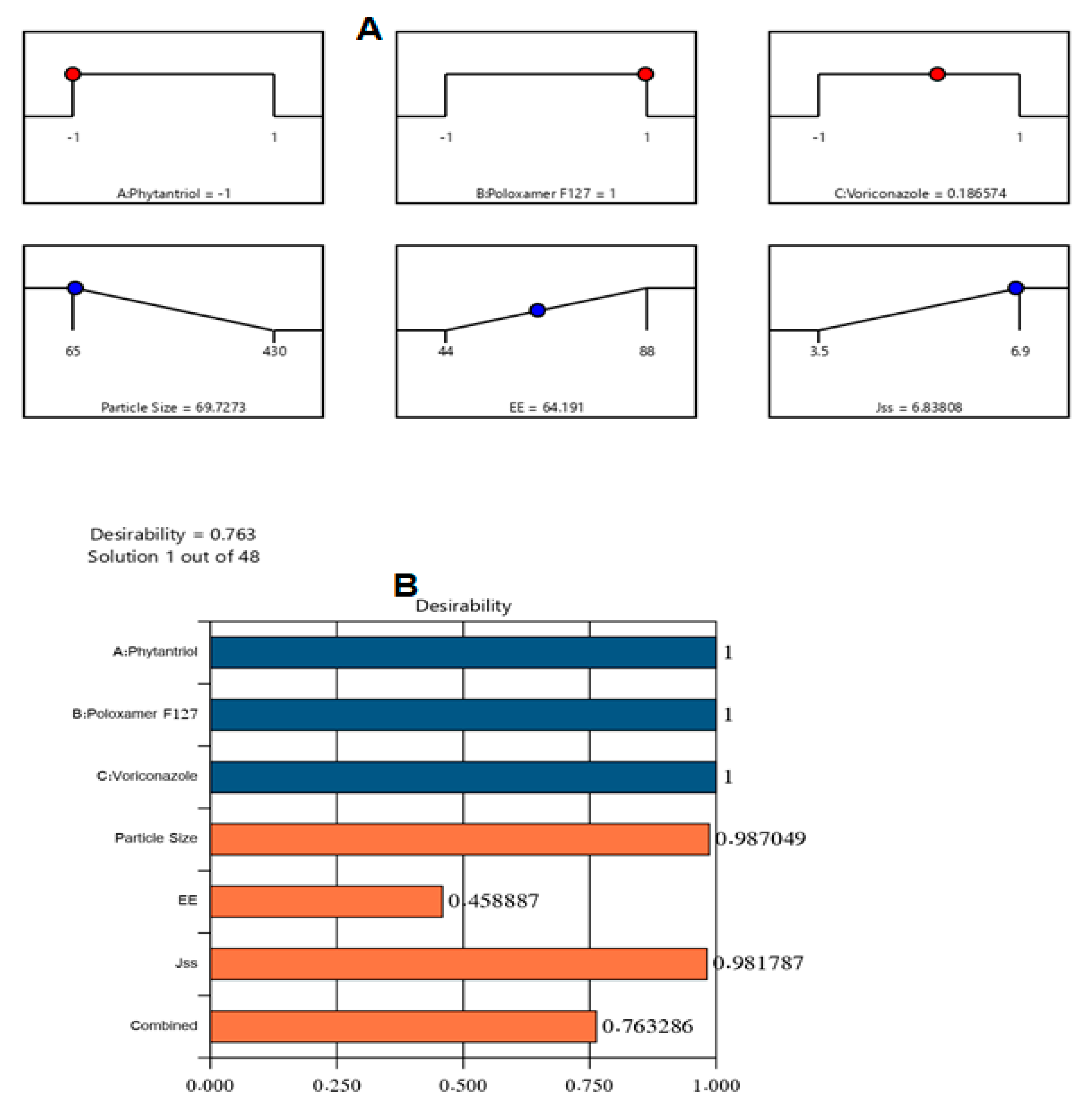
2.4. Optimization of VZ-Cub
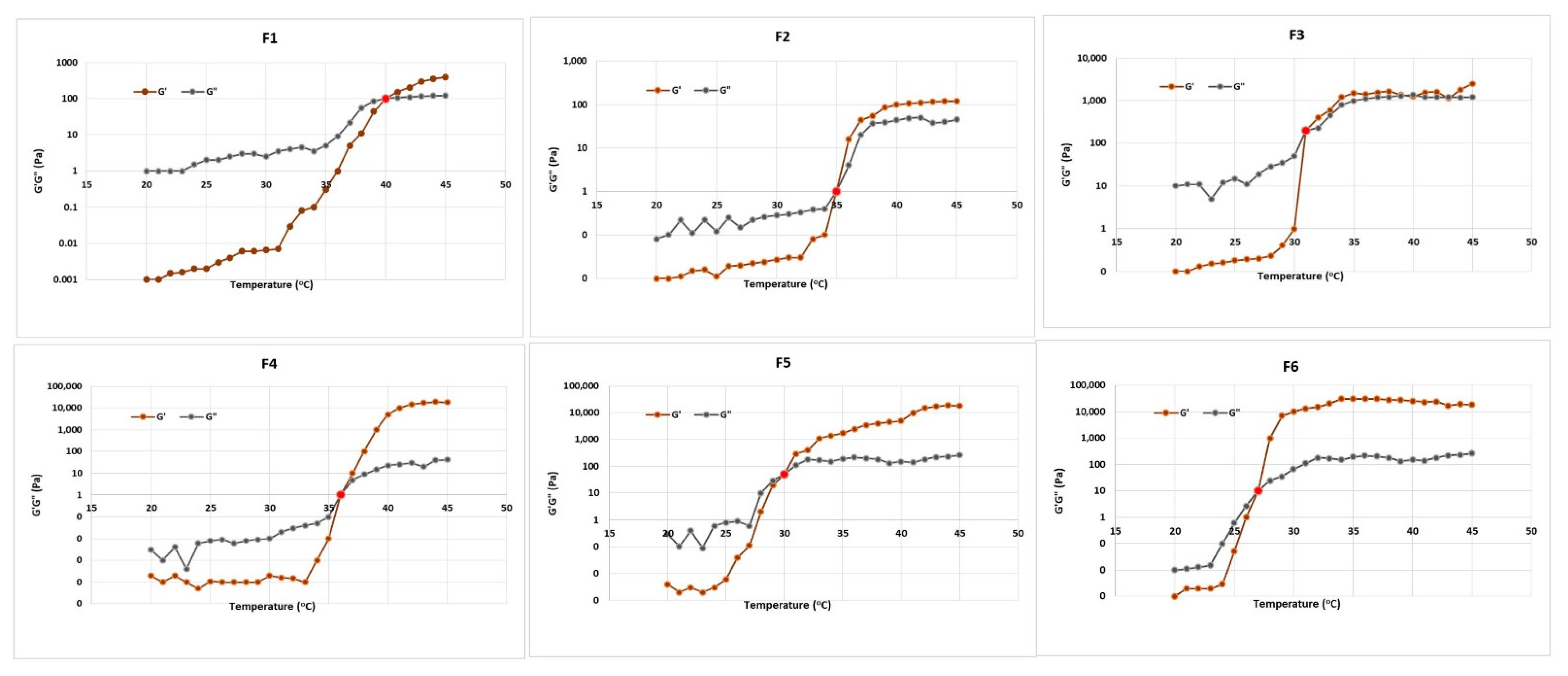
2.5. Rheological Characterization of Optimal VZ-Cub-Loaded In Situ Gel
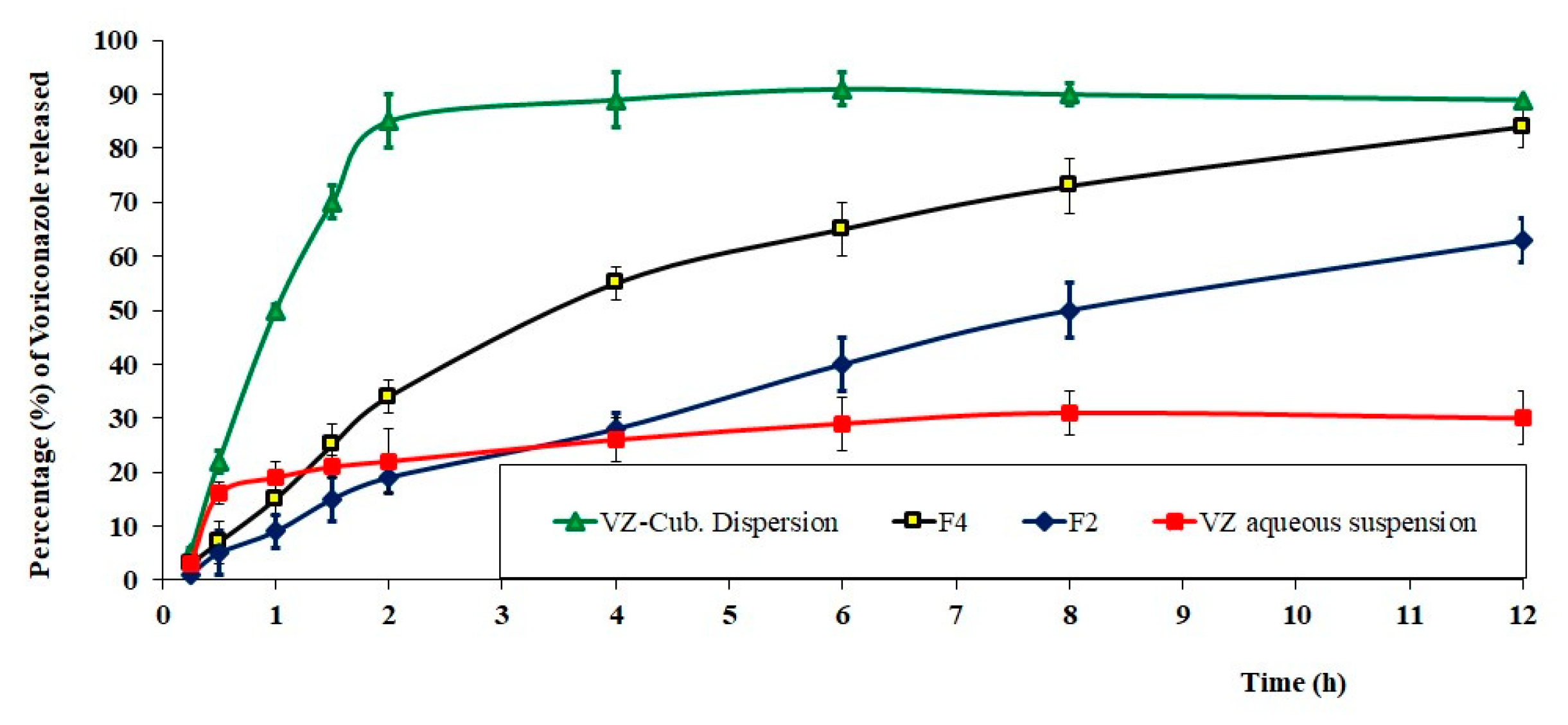
2.6. In Vitro Release Studies
2.7. Ex Vivo Transcorneal Permeation Studies
2.8. Assessment of Antifungal Activity of VZ-Cub-Loaded In Situ Gel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Experimental Design
4.2.2. VZ-Cub Preparation
4.2.3. Determination of Particle Size and Polydispersity Index of VZ-Cub
4.2.4. Determination of VZ EE%
4.2.5. Ex Vivo Permeation Study (Jss Measurement)
4.2.6. Statistical Analysis of Box-Behnken Design
4.2.7. Preparation and Characterization of the Optimized Formulation
4.2.8. In Situ Gel Preparation
4.2.9. Evaluation of VZ-Cub-Loaded In Situ Gels
Rheological Characterization
In Vitro Release Studies
Ex Vivo Transcorneal Permeation Studies
Antifungal Activity Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, E49–E57. [Google Scholar] [CrossRef]
- Acharya, Y.; Acharya, B.; Karki, P. Fungal keratitis: Study of increasing trend and common determinants. Nepal J. Epidemiol. 2017, 7, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowik, M.; Biernat, M.M.; Urbaniak-Kujda, D.; Kapelko-Slowik, K.; Misiuk-Hojlo, M. Mycotic Infections of the Eye. Adv. Clin. Exp. Med. 2015, 24, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Chandra, A.; Prakash, P.; Banerjee, T.; Maurya, O.P.; Tilak, R. Fungal keratitis in north India; Spectrum and diagnosis by Calcofluor white stain. Indian J. Med. Microbiol. 2015, 33, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Morand, K.; Bartoletti, A.C.; Bochot, A.; Barratt, G.; Brandely, M.L.; Chast, F. Liposomal amphotericin B eye drops to treat fungal keratitis: Physico-chemical and formulation stability. Int. J. Pharm. 2007, 344, 150–153. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, G.Q.; Lin, J.; Wang, X.; Hu, L.T.; Du, Z.D.; Wang, Q.; Zhu, C.C. Natamycin in the treatment of fungal keratitis: A systematic review and meta-analysis. Int. J. Ophthalmol. 2015, 8, 597–602. [Google Scholar]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718. [Google Scholar]
- Luttrull, J.K.; Wan, W.L.; Kubak, B.M.; Smith, M.D.; Oster, H.A. Treatment of ocular fungal infections with oral fluconazole. Am. J. Ophthalmol. 1995, 119, 477–481. [Google Scholar]
- Scott, L.J.; Simpson, D. Voriconazole: A review of its use in the management of invasive fungal infections. Drugs 2007, 67, 269–298. [Google Scholar] [CrossRef]
- Herbrecht, R. Voriconazole: Therapeutic review of a new azole antifungal. Expert Rev. Anti-Infect. Ther. 2004, 2, 485–497. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 20, 760–769. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011, 2011, 863734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, K.S.; Nema, R.K. Review on ocular inserts. Int. J. PharmTech. Res. 2009, 1, 164–169. [Google Scholar]
- Okore, V.C.; Attama, A.A.; Ofokansi, K.C.; Esimone, C.O.; Onuigbo, E.B. Formulation and evaluation of niosomes. Indian J. Pharm. Sci. 2011, 73, 323–328. [Google Scholar]
- Sahoo, R.K.; Biswas, N.; Guha, A.; Sahoo, N.; Kuotsu, K. Nonionic surfactant vesicles in ocular delivery: Innovative approaches and perspectives. BioMed Res. Int. 2014, 2014, 263604. [Google Scholar] [CrossRef] [Green Version]
- Abdelbary, A.; Salem, H.F.; Khallaf, R.A.; Ali, A.M. Mucoadhesive niosomal in situ gel for ocular tissue targeting: In vitro and in vivo evaluation of lomefloxacin hydrochloride. Pharm. Dev. Technol. 2017, 22, 409–417. [Google Scholar] [CrossRef]
- Abdelbary, A.; Salem, H.F.; Khallaf, R.A.; Ali, A.M.A. Modeling, optimization and in-vitro corneal permeation of chitosan-lomefloxacin HCl nanosuspension intended for ophthalmic delivery. J. Pharm. Innov. 2015, 10, 254–268. [Google Scholar]
- Attama, A.A.; Reichl, S.; Müller-Goymann, C.C. Sustained release and permeation of timolol from surface modified solid lipid nanoparticles through bioengineered human cornea. Curr. Eye Res. 2009, 34, 698–706. [Google Scholar] [CrossRef]
- Attama, A.A.; Reichl, S.; Müller-Goymann, C.C. Diclofenac sodium delivery to the eye: In-vitro evaluation of novel solid lipid nanoparticles formulation using human cornea construct. Int. J. Pharm. 2008, 355, 307–313. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. Engl. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box-Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2022, 32, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.; El Garhy, O.; Abdelkader, H. Cubosomes: Composition, preparation, and drug delivery applications. J. Adv. Biomed. Pharm. Sci. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Hosny, K.M.; Rizg, W.Y.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; Alghaith, A.F.; Alshehri, S.; El Sisi, A.M. Nanocubosomal based in situ gel loaded with natamycin for ocular fungal diseases: Development, optimization, in-vitro, and in-vivo assessment. Drug Deliv. 2021, 28, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Firestone, M.A. Electron density mapping of triblock copolymers associated with model biomembranes: Insights into conformational states and effect on bilayer structure. Biomacromolecules 2008, 9, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric micelles for delivery of poorly soluble drugs: Preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly (ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target 2005, 13, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakam, M.; Bell, L.N.; Banga, A.K. Effect of surfactants on the physical stability of recombinant human growth hormone. J. Pharm. Sci. 1995, 84, 713–771. [Google Scholar] [CrossRef]
- Newa, M.; Bhandari, K.H.; Li, D.X.; Kwon, T.H.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Lyoo, W.S.; Yong, C.S.; Choi, H.G. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int. J. Pharm. 2007, 343, 228–237. [Google Scholar] [CrossRef]
- Nirmal, H.B.; Bakliwal, S.R.; Pawar, S.P. In-Situ gel: New trends in controlled and sustained drug delivery system. Int. J. PharmTech. Res. 2010, 2, 1398–1408. [Google Scholar]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. As. J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 thermosensitive hydrogel as an injectable dexamethasone delivery carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: Optimization study by factorial design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Qi, H.; Chen, W.; Huang, C.; Li, L.; Chen, C.; Li, W.; Wu, C. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int. J. Pharm. 2007, 1, 178–187. [Google Scholar] [CrossRef]
- Lin, H.R.; Sung, K.C.; Vong, W.J. In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules 2004, 6, 2358–2365. [Google Scholar] [CrossRef]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer- based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 4, 328–335. [Google Scholar] [CrossRef]
- Liao, Y.H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 6, 327–342. [Google Scholar] [CrossRef]
- Huynh, A.; Priefer, R. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef]
- Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel artificial tears containing cross-linked hyaluronic acid: An in vitro re-epithelialization study. Molecules 2017, 22, 2104. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y.; et al. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Rizg, W.Y.; Hosny, K.M.; Elgebaly, S.S.; Alamoudi, A.J.; Felimban, R.I.; Tayeb, H.H.; Alharbi, M.; Bukhary, H.A.; Abualsunun, W.A.; Almehmady, A.M.; et al. Preparation and Optimization of garlic oil/apple cider vinegar nanoemulsion loaded with minoxidil to treat alopecia. Pharmaceutics 2021, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Hosny, K.M. Nanosized cubosomal thermogelling dispersion loaded with saquinavir mesylate to improve its bioavailability: Preparation, optimization, in vitro and in vivo evaluation. Int. J. Nanomed. 2020, 15, 5113–5129. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Ther. 2015, 3, 4209–4218. [Google Scholar] [CrossRef] [Green Version]
- El-Nabarawi, M.A.; Bendas, E.R.; El Rehem, R.T.A.; Abary, M.Y.S. Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int. J. Pharm. 2013, 443, 307–317. [Google Scholar] [CrossRef]
- Perugini, P.; Pavanetto, F. Liposomes containing boronophenylalanine for boron neutron capture therapy. J. Microencapsul. 1998, 15, 473–483. [Google Scholar] [CrossRef]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Hady, M.A.; Awad, G.A. Phospholipid based colloidal poloxamer–nanocubic vesicles for brain targeting via the nasal route. Colloids Surf. B Biointerfaces 2012, 100, 146–154. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Assmus, D.; Boehnke, A.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur. J. Pharm. Biopharm. 2011, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Shen, J.Q.; Gan, Y.; Geng, H.M.; Zhang, X.X.; Zhu, C.L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Cho, C.W.; Oh, I.J. Effects of non-ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int. J. Pharm. 2001, 222, 199–203. [Google Scholar] [CrossRef]
- Ghosh, S.; Samarasinghe, A.E.; Hoselton, S.A.; Dorsam, G.P.; Schuh, J.M. Hyaluronan deposition and co-localization with inflammatory cells and collagen in a murine model of fungal allergic asthma. Inflamm. Res. 2014, 63, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, S.P.; Ribeiro, I.R.; Boyd, B.J.; Loh, W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127 cubosomes. Colloids Surf. B Biointerfaces 2016, 145, 845–853. [Google Scholar] [CrossRef]
- Hosny, K.M.; Khallaf, R.A.; Asfour, H.Z.; Rizg, W.Y.; Alhakamy, N.A.; Sindi, A.M.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; et al. Development and optimization of cinnamon oil Nanoemulgel for enhancement of solubility and evaluation of antibacterial, antifungal and analgesic effects against oral microbiota. Pharmaceutics 2021, 13, 1008. [Google Scholar] [CrossRef]
- Ali, S.A.; Sindi, A.M.; Mair, Y.M.; Khallaf, R.A. Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: Preparation, characterization, and assessment of antifungal activity. J. Drug Deliv. Sci. Tech. 2021, 66, 102841. [Google Scholar] [CrossRef]
- Van Der Bijl, P.; Engelbrecht, A.H.; Van Eyk, A.D.; Meyer, D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J. Ocul. Pharmacol. Ther. 2002, 18, 419–427. [Google Scholar] [CrossRef]
- Nordin, M.A.; Wan Harun, W.H.; Abdul Razak, F. Antifungal susceptibility and growth inhibitory response of oral Candida species to Brucea javanica Linn. extract. BMC Complement. Altern. Med. 2013, 13, 342. [Google Scholar] [CrossRef] [Green Version]


| A | B | C | Y1 | Y2 | Y3 | ||
|---|---|---|---|---|---|---|---|
| Run | Phytantriol | Poloxamer F127 | Voriconazole | Particle Size | EE | Jss | PDI |
| (mg) | (mg) | (mg) | (nm) | (%) | µg/(cm2·min) | ||
| 1 | 200 | 20 | 20 | 430 ± 6.0 | 88 ± 5.3 | 3.5 ± 0.50 | 0.17 |
| 2 | 150 | 20 | 15 | 350 ± 6.5 | 69 ± 3.1 | 3.5 ± 0.22 | 0.22 |
| 3 | 100 | 40 | 15 | 140 ± 5.8 | 44 ± 2.0 | 5.8 ± 0.61 | 0.19 |
| 4 | 150 | 20 | 25 | 260 ± 8.0 | 55 ± 1.9 | 4.1 ± 0.44 | 0.15 |
| 5 | 100 | 20 | 20 | 290 ± 6.6 | 67 ± 3.8 | 4.4 ± 0.31 | 0.34 |
| 6 | 150 | 60 | 25 | 180 ± 2.9 | 57 ± 4.1 | 5.9 ± 0.24 | 0.40 |
| 7 | 100 | 40 | 25 | 150 ± 5.2 | 46 ± 2.2 | 5.4 ± 9.62 | 0.39 |
| 8 | 200 | 40 | 25 | 350 ± 9.9 | 60 ± 5.1 | 4.7 ± 0.41 | 0.38 |
| 9 | 100 | 60 | 20 | 65 ± 2.5 | 65 ± 5.4 | 6.9 ± 0.45 | 0.33 |
| 10 | 150 | 60 | 15 | 165 ± 3.5 | 52 ±2.7 | 6.1± 0.33 | 0.35 |
| 11 | 200 | 40 | 15 | 400 ± 10 | 73 ± 6.6 | 4.3 ± 0.18 | 0.40 |
| 12 | 150 | 40 | 20 | 240 ± 7.2 | 72 ± 5.9 | 4.3 ± 0.27 | 0.29 |
| 13 | 150 | 40 | 20 | 255 ± 6.5 | 71 ± 7.0 | 4.5 ± 0.41 | 0.19 |
| 14 | 150 | 40 | 20 | 220 ± 4.5 | 74 ± 6.9 | 4.4 ± 0.42 | 0.26 |
| 15 | 200 | 60 | 20 | 265 ± 8.1 | 75 ± 5.7 | 6.2 ± 0.39 | 0.38 |
| 16 | 200 | 60 | 25 | 290 ± 5.9 | 64 ± 3.9 | 6.5 ± 0.51 | 0.37 |
| 17 | 100 | 20 | 15 | 270 ± 7.1 | 48 ± 3.3 | 4.1 ± 0.22 | 0.25 |
| R2 | Adjusted R2 | Predicted R2 | SD | CV% | Adeq. Precision | |
|---|---|---|---|---|---|---|
| Response Y1 | 0.915 | 0.8954 | 0.844 | 31.1 | 12.24 | 24.1108 |
| Response Y2 | 0.9774 | 0.9484 | 0.8404 | 2.72 | 4.28 | 21.9001 |
| Response Y3 | 0.9789 | 0.9517 | 0.8851 | 0.2349 | 4.72 | 19.9592 |
| Phytantriol (mg) | Poloxamer F127 (mg) | VZ (mg) | Particle Size (nm) | EE (%) | Jss (µg/cm2·h) | Desirability | |
|---|---|---|---|---|---|---|---|
| Predicated value | 100 | 60 | 21 | 69.7 | 64.19 | 6.83 | 0.763 |
| Experimental value | 100 | 60 | 21 | 71 | 60 | 6.5 | 0.763 |
| Formulation | Poloxamer Conc. (% w/w) | Hyaluronic Acid Conc. (% w/w) | Tgel |
|---|---|---|---|
| F1 | 10% | 0.2% | 40 ± 0.5 °C |
| F2 | 15% | 0.2% | 35 ± 0.2 °C |
| F3 | 20% | 0.2% | 31 ± 0.3 °C |
| F4 | 10% | 0.4% | 36 ± 0.2 °C |
| F5 | 15% | 0.4% | 30 ± 0.1 °C |
| F6 | 20% | 0.4% | 27 ± 0.4 °C |
| Parameters of Permeation | F4 | VZ-Cub Aqueous Dispersion | VZ Aqueous Dispersion |
|---|---|---|---|
| Cumulative amount permeated (μg/cm2) | 1741 ± 201 | 939 ± 113 | 379 ± 52 |
| Steady-state flux, Jss, (μg/cm2·min) | 13.21 ± 1.1 | 6.5 ± 0.3 | 1.7 ± 0.2 |
| Permeability coefficient, Pc, (cm/min) | 12.3 × 10−4 | 7.6 × 10−4 | 3.2 × 10−4 |
| Diffusion coefficient, D, (cm2/min) | 33.2 × 10−5 | 18.4 × 10−5 | 7.1 × 10−5 |
| Enhancement factor (EF) | 4.59 | 2.477 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Hosny, K.M.; Rizg, W.Y.; Eshmawi, B.A.; Badr, M.Y.; Safhi, A.Y.; Murshid, S.S.A. Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection. Gels 2022, 8, 241. https://doi.org/10.3390/gels8040241
Alhakamy NA, Hosny KM, Rizg WY, Eshmawi BA, Badr MY, Safhi AY, Murshid SSA. Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection. Gels. 2022; 8(4):241. https://doi.org/10.3390/gels8040241
Chicago/Turabian StyleAlhakamy, Nabil A., Khaled M. Hosny, Waleed Y. Rizg, Bayan A. Eshmawi, Moutaz Y. Badr, Awaji Y. Safhi, and Samar S. A. Murshid. 2022. "Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection" Gels 8, no. 4: 241. https://doi.org/10.3390/gels8040241
APA StyleAlhakamy, N. A., Hosny, K. M., Rizg, W. Y., Eshmawi, B. A., Badr, M. Y., Safhi, A. Y., & Murshid, S. S. A. (2022). Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection. Gels, 8(4), 241. https://doi.org/10.3390/gels8040241






