Calcined Hydroxyapatite with Collagen I Foam Promotes Human MSC Osteogenic Differentiation
Abstract
:1. Introduction
2. Results
2.1. Scaffold Structure and Morphology
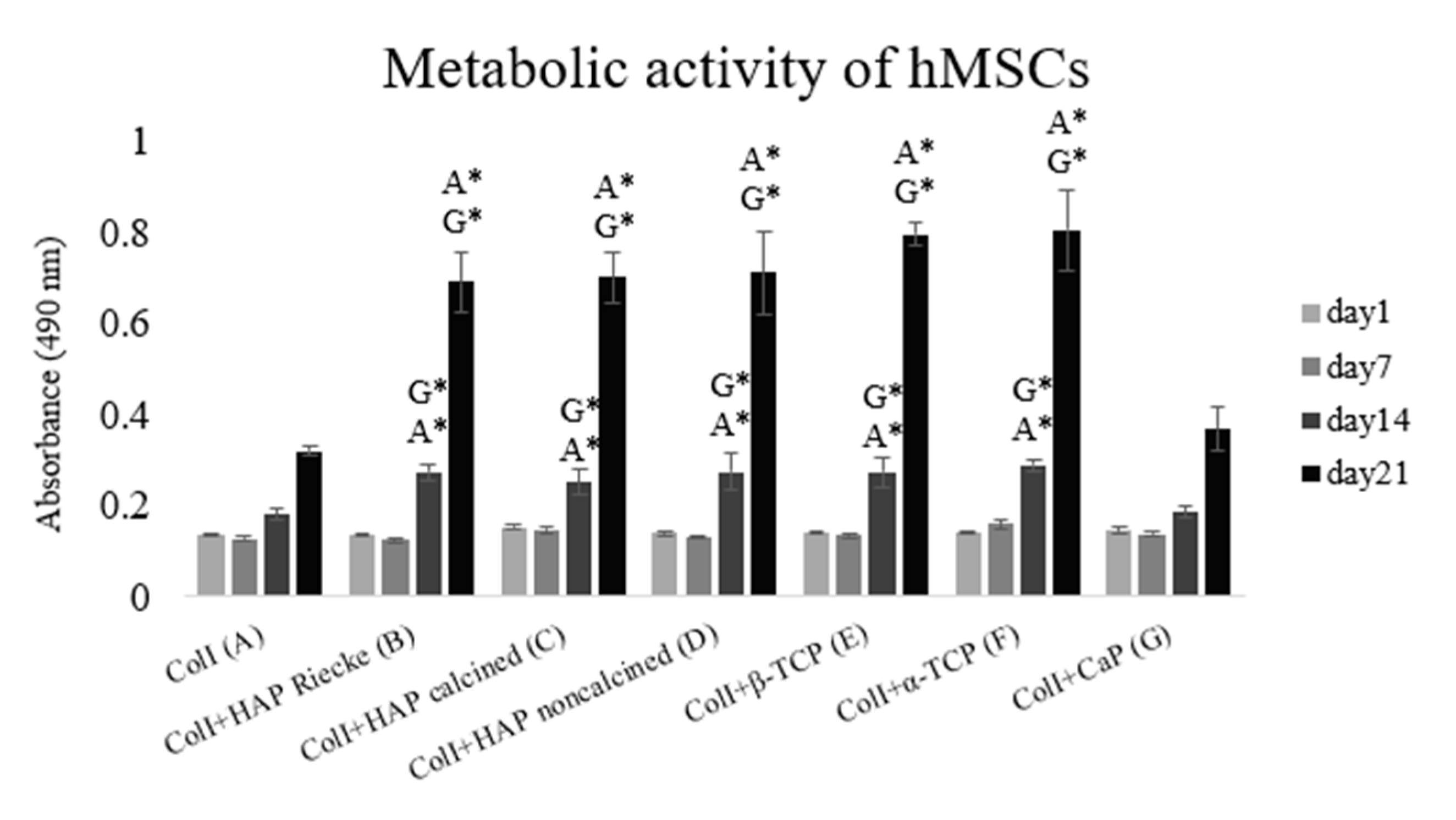
2.2. A Significantly Higher Level of Metabolic Activity Was Observed in Samples with the Combination of Coli and Hap or Tcp Scaffolds
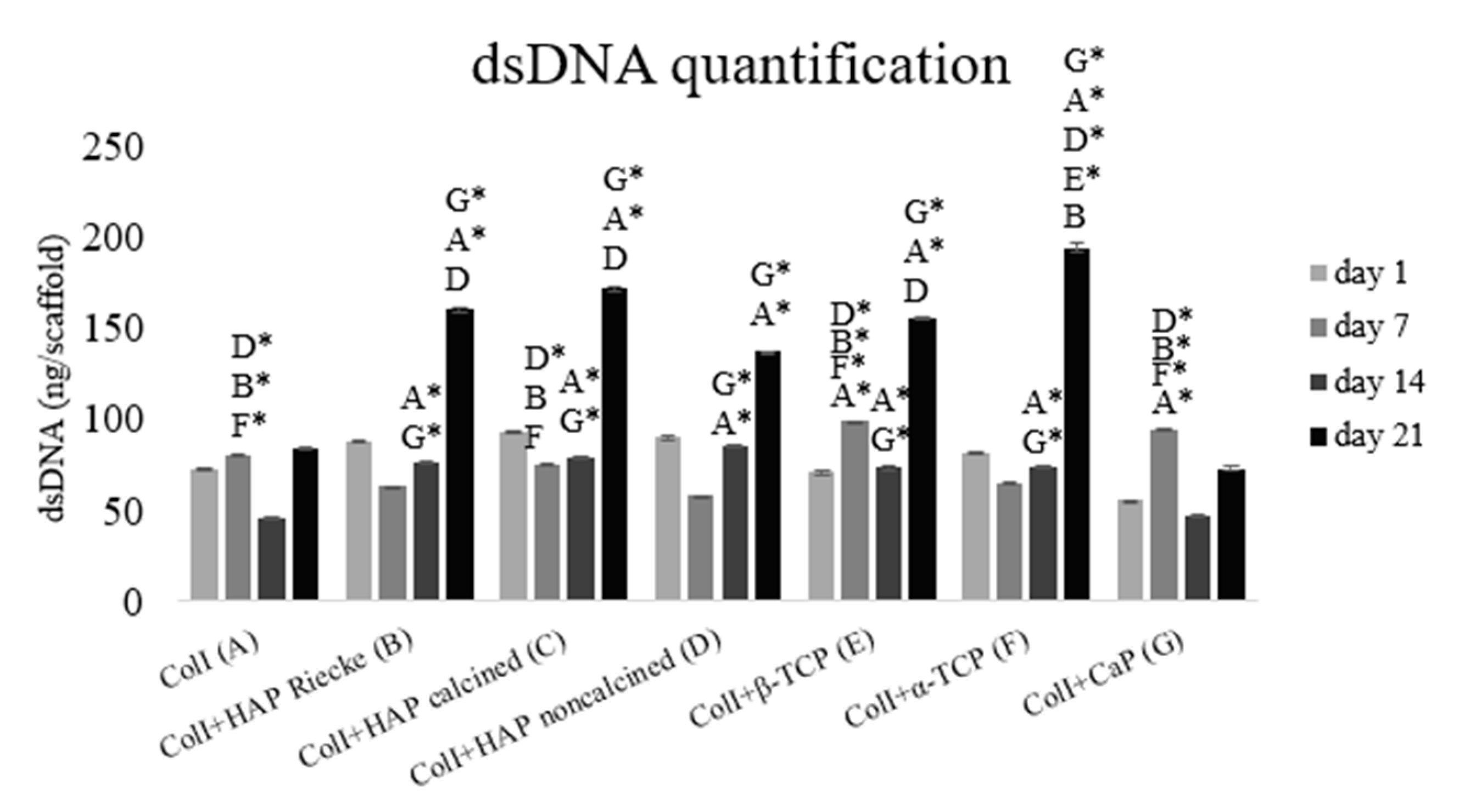
2.3. A Significantly Higher Amount of dsDNA Was Detected in Samples with the Combination of Coli and Hap or Tcp Scaffolds
2.4. RunX2 and ColI mRNA Expression Were Significantly Higher in Pure ColI Scaffold or in Combination with Calcined HAP
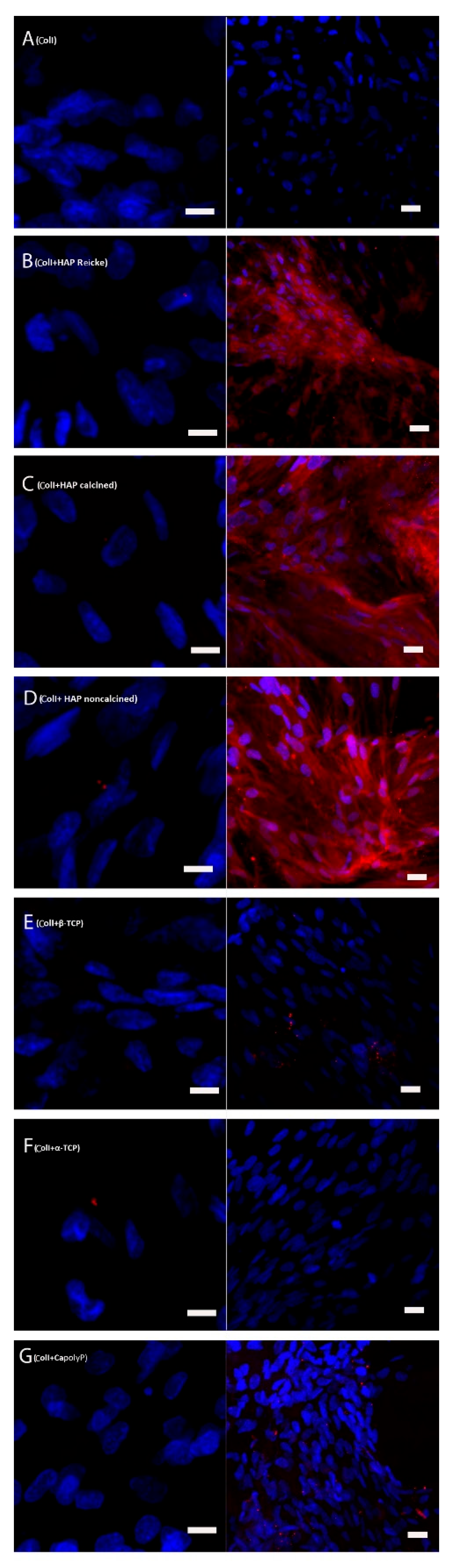
2.5. Bone Extracellular Protein Osteocalcin Was Present in High Amounts in the Samples with ColI and HAP Scaffolds
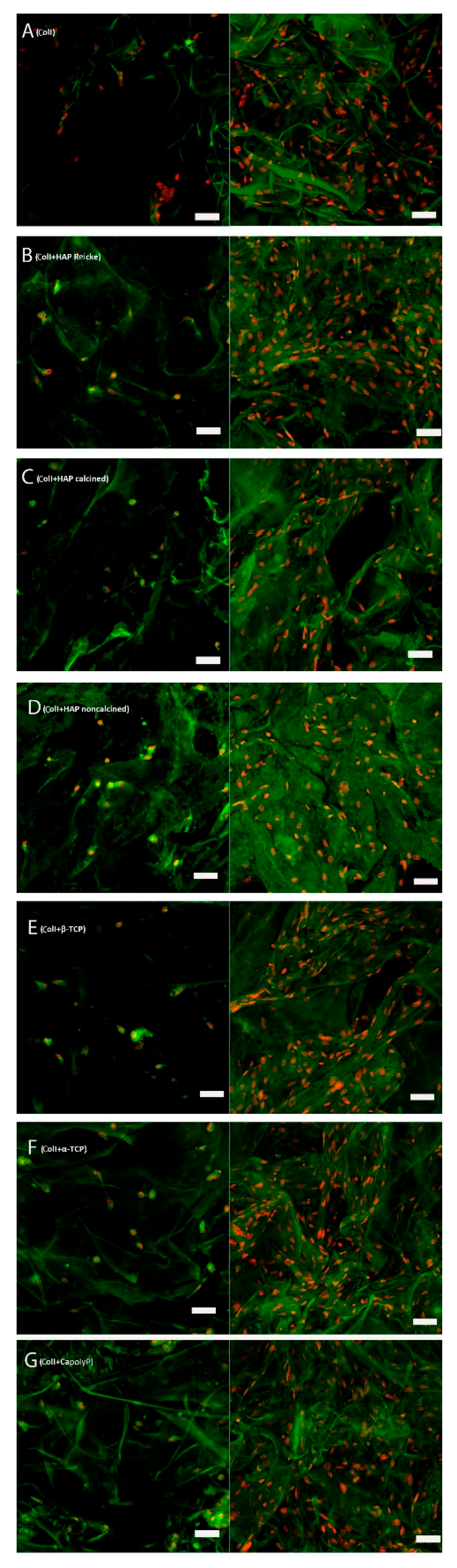
2.6. The Number of Cells on Scaffolds Increased in All Samples
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation
4.2. Scaffold Morphology
4.3. Cells and Culture Conditions
4.4. Viability Test
4.5. dsDNA Quantification
4.6. Immunohistochemical Staining of Osteocalcin
4.7. Staining of Nuclei and Cytoplasmic Membrane
4.8. qPCR
4.9. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schemitsch, E.H. Size matters: Defining critical in bone defect size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Rh Owen, G.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. B. Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiz, E.; Zimmermann, E.A.; Lee, J.S.; Wegst, U.G.K.; Tomsia, A.P. Perspectives on the role of nanotechnology in bone tissue engineering. Dent. Mater. 2013, 29, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically inspired collagen/apatite composite biomaterials for potential use in bone tissue regeneration-A review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.H.; Lin, Y.W.; Sun, J.S.; Lin, F.H. The chitosan/tri-calcium phosphate bio-composite bone cement promotes better osteo-integration: An in vitro and in vivo study. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Zeng, J.; Lin, J.; Yao, G.; Kong, K.; Wang, X. Effect of modified compound calcium phosphate cement on the differentiation and osteogenesis of bone mesenchymal stem cells. J. Orthop. Surg. Res. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fernández, T.; Olave, G.; Valencia, C.H.; Arce, S.; Quinn, J.M.; Thouas, G.A.; Chen, Q.Z. Effects of calcium phosphate/chitosan composite on bone healing in rats: Calcium phosphate induces osteon formation. Tissue Eng. Part A 2014, 20, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Prosecká, E.; Rampichová, M.; Litvinec, A.; Tonar, Z.; Králíčková, M.; Vojtová, L.; Kochová, P.; Plencner, M.; Buzgo, M.; Míčková, A.; et al. Collagen/hydroxyapatite scaffold enriched with polycaprolactone nanofibers, thrombocyte-rich solution and mesenchymal stem cells promotes regeneration in large bone defect in vivo. J. Biomed. Mater. Res. Part. A 2015, 103, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Prosecka, E.; Rampichova, M.; Vojtová, L.; Tvrdik, D.; Melčáková, Š.; Juhasova, J.; Plencner, M.; Jakubová, R.; Jančář, J.; Kochová, P.; et al. Optimized conditions for mesenchymal stem cells to differentiate into osteoblasts on a collagen/hydroxyapatite matrix. J. Biomed. Mater. Res. Part A 2011, 99, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C. Collagen-hydroxyapatite composite scaffolds for tissue engineering. Hydroxyapatite Biomed. Appl. 2015, 211–234. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Manfrini, M.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Trevisiol, L.; Patergnani, S.; et al. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. Biochem. Collagens, Laminins Elastin Struct. Funct. Biomarkers 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Che Hashim, N.; Nordin, D. Nano-Hydroxyapatite Powder for Biomedical Implant Coating. Mater. Today Proc. 2019, 19, 1562–1571. [Google Scholar] [CrossRef]
- Agbabiaka, O.G.; Oladele, I.O.; Akinwekomi, A.D.; Adediran, A.A.; Balogun, A.O.; Olasunkanm, O.G.; Olayanju, T.M.A. Effect of calcination temperature on hydroxyapatite developed from waste poultry eggshell. Sci. African 2020, 8, e00452. [Google Scholar] [CrossRef]
- Bočková, J.; Vojtová, L.; Přikryl, R.; Čechal, J.; Jančář, J. Collagen-grafted ultra-high molecular weight polyethylene for biomedical applications. Chem. Pap. 2008, 62, 580–588. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. α-Tricalcium phosphate hydrolysis to hydroxyapatite at and near physiological temperature. J. Mater. Sci. Mater. Med. 2000, 11, 365–371. [Google Scholar] [CrossRef]
- Zeng, S.; Shi, H.; Yu, T.; Zhou, C. Enhanced hydrated properties of α-tricalcium phosphate bone cement mediated by loading magnesium substituted octacalcium phosphate. Adv. Powder Technol. 2017, 28, 3288–3295. [Google Scholar] [CrossRef]
- Eskandari, N.; Shafiei, S.S. Fabrication and Evaluation of Layered Double Hydroxide-Enriched ß-Tricalcium Phosphate Nanocomposite Granules for Bone Regeneration: In Vitro Study. Mol. Biotechnol. 2021, 63, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jyoti, M.A.; Youn, M.H.; Youn, H.S.; Seo, H.S.; Lee, B.T.; Song, H.Y. In vitro and in vivo evaluation of a macro porous β-TCP granule-shaped bone substitute fabricated by the fibrous monolithic process. Biomed. Mater. 2010, 5, 035007. [Google Scholar] [CrossRef]
- Wang, X.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering. J. Mater. Chem. B 2018, 6, 2385–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, W.E.; Wang, X.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Hong-Shum, L. Sodium Polyphosphate. Food Addit. Data B. 2007, 852–853. [Google Scholar] [CrossRef]
- Foley, B.; Greiner, M.; McGlynn, G.; Schmahl, W.W. Anatomical variation of human bone bioapatite crystallography. Crystals 2020, 10, 859. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials 2001, 22, 1327–1333. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef] [Green Version]
- Fritton, S.P.; Weinbaum, S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef] [Green Version]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. Am. 2001, 83, S105–S115. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Jakubcová, B.; Danilová, I.; Kostakova, E.K.; Jarosikova, T.; Chernyavskiy, O.; Hejda, J.; Handl, M.; Beznoska, J.; Nečas, A.; et al. Polycaprolactone Foam Functionalized With Chitosan Microparticles-a Suitable Scaffold for Cartilage Regeneration. Physiol. Res. 2016, 65, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780–3787. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Pamula, E.; Bacakova, L.; Filova, E.; Buczynska, J.; Dobrzynski, P.; Noskova, L.; Grausova, L. The influence of pore size on colonization of poly(L-lactide-glycolide) scaffolds with human osteoblast-like MG 63 cells in vitro. J. Mater. Sci. Mater. Med. 2008, 19, 425–435. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Dušková-Smrčková, M.; Zavřel, J.; Bartoš, M.; Kaberova, Z.; Filová, E.; Zárubová, J.; Šlouf, M.; Michálek, J.; Vampola, T.; Kubies, D. Communicating macropores in PHEMA-based hydrogels for cell seeding: Probabilistic open pore simulation and direct micro-CT proof. Mater. Des. 2021, 198, 109312. [Google Scholar] [CrossRef]
- Higashi, T.; Okamoto, H. Influence of particle size of hydroxyapatite as a capping agent on cell proliferation of cultured fibroblasts. J. Endod. 1996, 22, 236–239. [Google Scholar] [CrossRef]
- Khashaba, R.M.; Lockwood, P.E.; Lewis, J.B.; Messer, R.L.; Chutkan, N.B.; Borke, J.L. Cytotoxicity, calcium release, and pH changes generated by novel calcium phosphate cement formulations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 297–303. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Weir, M.D.; Sun, L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent. Mater. 2009, 25, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witek, L.; Shi, Y.; Smay, J. Controlling calcium and phosphate ion release of 3D printed bioactive ceramic scaffolds: An in vitro study. J. Adv. Ceram. 2017, 6, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Brazda, L.; Rohanova, D.; Helebrant, A. Kinetics of dissolution of calcium phosphate (Ca-P) bioceramics. Process. Appl. Ceram. 2008, 2, 57–62. [Google Scholar] [CrossRef]
- Irawan, R.M.; Margono, A.; Djauhari, N. The comparison of calcium ion release and pH changes from modified MTA and bioceramics in regeneration. J. Phys. Conf. Ser. 2017, 884, 012110. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.C.X.; Kihara, A.H.; Goulart, V.A.; Tonelli, F.M.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015, 27, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef]
- Penido, M.G.M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediat. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. ‘false’ cytotoxicity of ions-adsorbing hydroxyapatite - Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef]
- Šupová, M.; Suchý, T.; Sucharda, Z.; Filová, E.; der Kinderen, J.N.; Steinerová, M.; Martynková, G.S. The comprehensive in vitro evaluation of eight different calcium phosphates: Significant parameters for cell behavior. J. Am. Ceram. Soc. 2018, 102, 2882–2904. [Google Scholar] [CrossRef]
- Chow, L.C. Solubility of calcium phosphates. Monogr. Oral Sci. 2001, 18, 94–111. [Google Scholar]
- Shih, Y.-R.V.; Chen, C.-N.; Tsai, S.-W.; Wang, Y.J.; Lee, O.K. Growth of Mesenchymal Stem Cells on Electrospun Type I Collagen Nanofibers. Stem Cells 2006, 24, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Huri, P.Y.; Ozilgen, B.A.; Hutton, D.L.; Grayson, W.L. Scaffold pore size modulates in vitro osteogenesis of human adipose-derived stem/stromal cells. Biomed. Mater. 2014, 9, 045003. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4 v Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef] [PubMed]
- Viti, F.; Landini, M.; Mezzelani, A.; Petecchia, L.; Milanesi, L.; Scaglione, S. Osteogenic differentiation of MSC through calcium signaling activation: Transcriptomics and functional analysis. PLoS ONE 2016, 11, e0148173. [Google Scholar] [CrossRef] [Green Version]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Carlier, A.; Chai, Y.C.; Moesen, M.; Theys, T.; Schrooten, J.; Van Oosterwyck, H.; Geris, L. Designing optimal calcium phosphate scaffold-cell combinations using an integrative model-based approach. Acta Biomater. 2011, 7, 3573–3585. [Google Scholar] [CrossRef]
- Eyckmans, J.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. A clinically relevant model of osteoinduction: A process requiring calcium phosphate and BMP/Wnt signalling. J. Cell. Mol. Med. 2010, 14, 1845–1856. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef]
- Akahane, M.; Ueha, T.; Shimizu, T.; Inagaki, Y.; Kido, A.; Imamura, T.; Kawate, K.; Tanaka, Y. Increased osteogenesis with hydroxyapatite constructs combined with serially-passaged bone marrow-derived mesenchymal stem cells. Stem Cell Discov. 2012, 02, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, W.K.; Kim, S.; Choi, M.; Kim, C.; Jeong, Y.S.; Cho, B.R.; Hahn, J.S.; Jang, J. Cellular uptake, cytotoxicity, and innate immune response of silica-Titania hollow nanoparticles based on size and surface functionality. ACS Nano 2010, 4, 5301–5313. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, D.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Sloviková, A.; Vojtová, L.; Jančař, J. Preparation and modification of collagen-based porous scaffold for tissue engineering. Chem. Pap. 2008, 62, 417–422. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
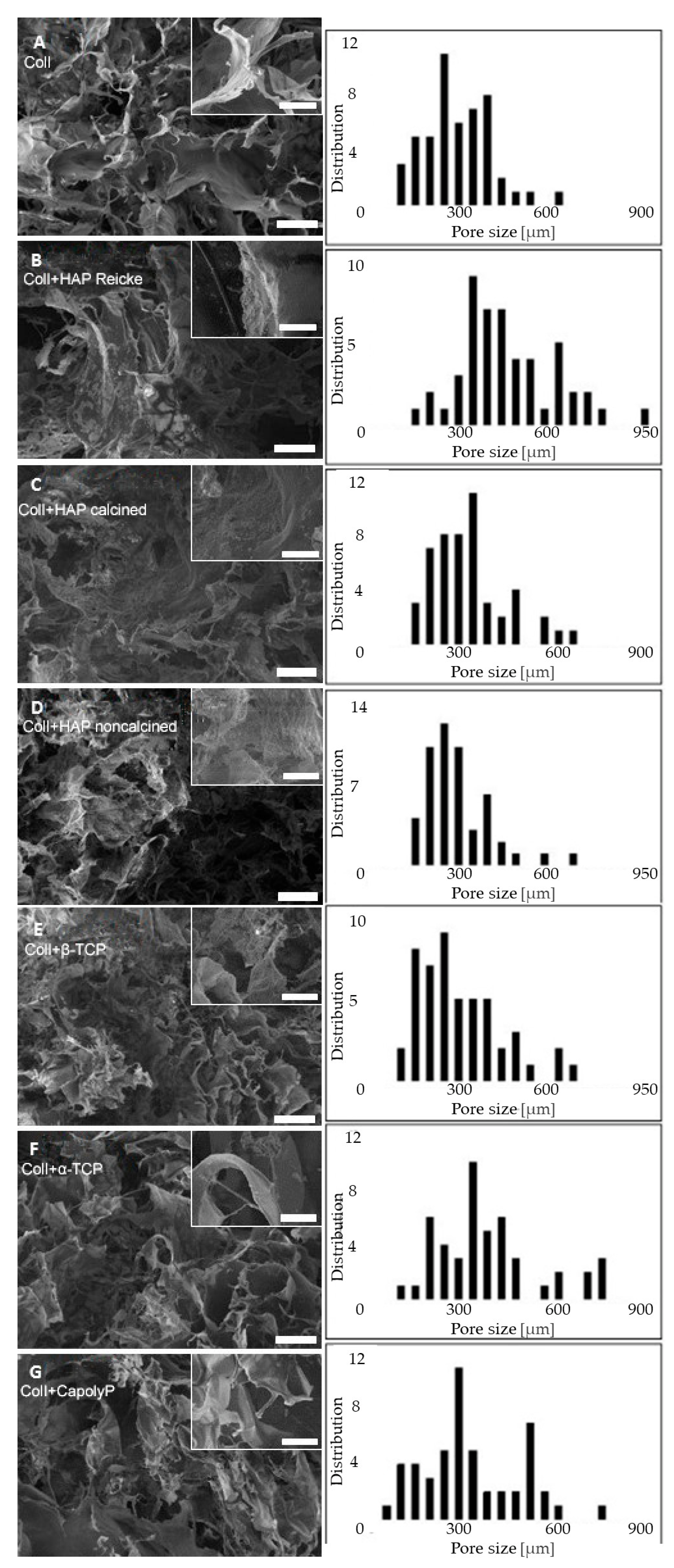


| Sample | Pore Size [µm] |
|---|---|
| ColI (A) | 320 ± 120 |
| ColI+HAP Reicke (B) | 490 ± 170 |
| ColI+HAP calcined (C) | 360 ± 130 |
| ColI+HAP noncalcined (D) | 320 ± 110 |
| ColI+β-TCP (E) | 330 ± 150 |
| ColI+α-TCP (F) | 460 ± 230 |
| ColI+CapolyP (G) | 370 ± 170 |
| Sample | Type of Material | Mean Particle Size [µm] | Formula |
|---|---|---|---|
| ColI (A) | Bovine Collagen Type I pure | - | |
| ColI+HAP Reicke (B) | Hydroxyapatite commercial | 30 | Ca10(PO4)6(OH)2 |
| ColI+HAP calcined (C) | Hydroxyapatite calcined at 1000 °C | 0.130 | Ca10(PO4)6(OH)2 |
| ColI+HAP noncalcined (D) | Hydroxyapatite noncalcined | 0.029 | Ca10(PO4)6(OH)2 |
| ColI+β-TCP (E) | β-tricalcium phosphate | 4.21 | 89 wt% Ca3(PO4)2 11 wt% Ca2P2O7 |
| ColI+α-TCP (F) | α-tricalcium phosphate | 11.01 | 92 wt% Ca3(PO4)2 8 wt% Ca10(PO4)6(OH)2 |
| ColI+CapolyP (G) | Sodium polyphosphate transferred by CaCl2 | 8.26 | Ca(PO3)n n = 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefka Blahnová, V.; Vojtová, L.; Pavliňáková, V.; Muchová, J.; Filová, E. Calcined Hydroxyapatite with Collagen I Foam Promotes Human MSC Osteogenic Differentiation. Int. J. Mol. Sci. 2022, 23, 4236. https://doi.org/10.3390/ijms23084236
Hefka Blahnová V, Vojtová L, Pavliňáková V, Muchová J, Filová E. Calcined Hydroxyapatite with Collagen I Foam Promotes Human MSC Osteogenic Differentiation. International Journal of Molecular Sciences. 2022; 23(8):4236. https://doi.org/10.3390/ijms23084236
Chicago/Turabian StyleHefka Blahnová, Veronika, Lucy Vojtová, Veronika Pavliňáková, Johana Muchová, and Eva Filová. 2022. "Calcined Hydroxyapatite with Collagen I Foam Promotes Human MSC Osteogenic Differentiation" International Journal of Molecular Sciences 23, no. 8: 4236. https://doi.org/10.3390/ijms23084236






