Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging
Abstract
:1. Introduction
2. Results
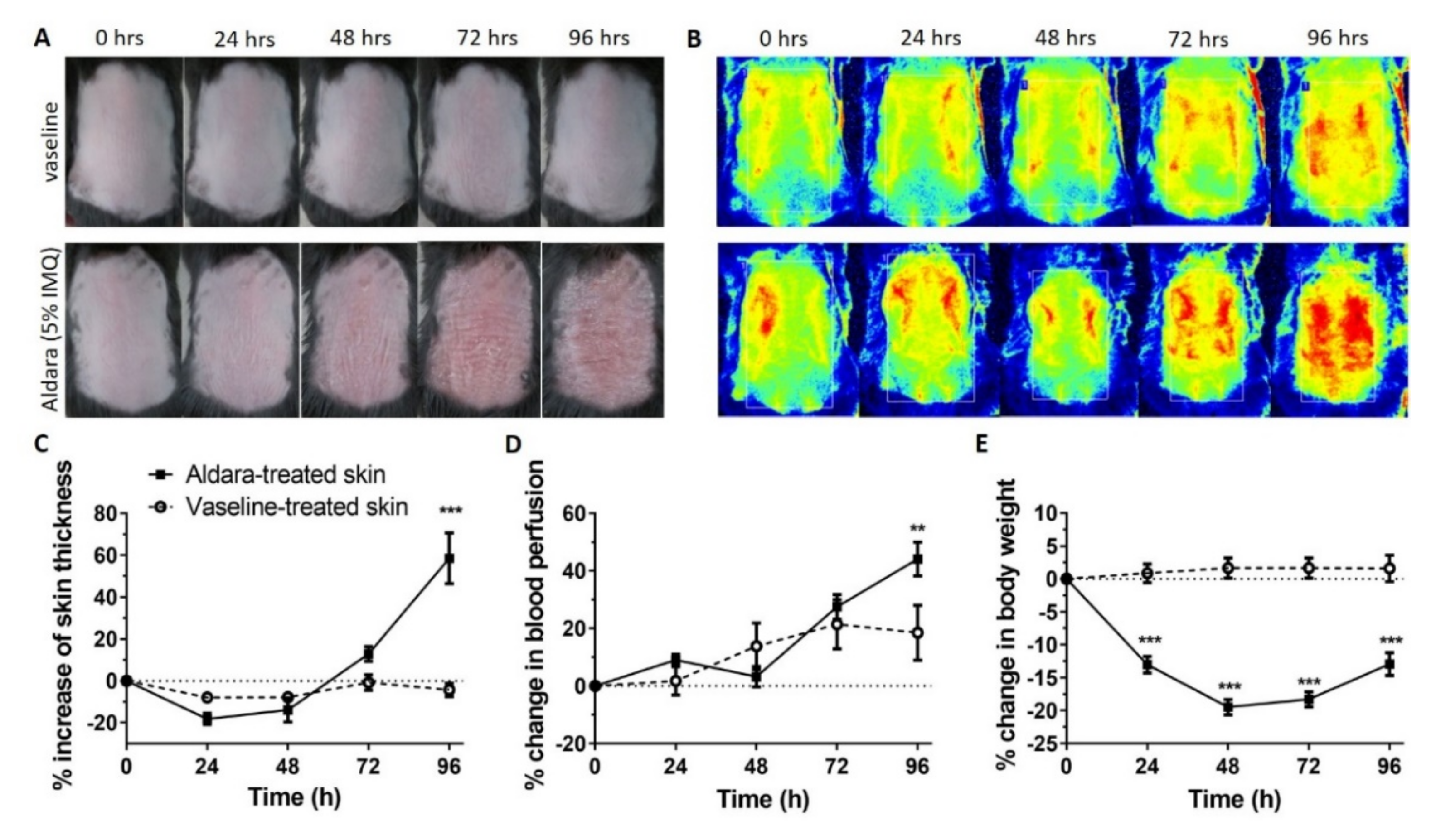
2.1. In Vivo Induction of Psoriasiform Dermatitis
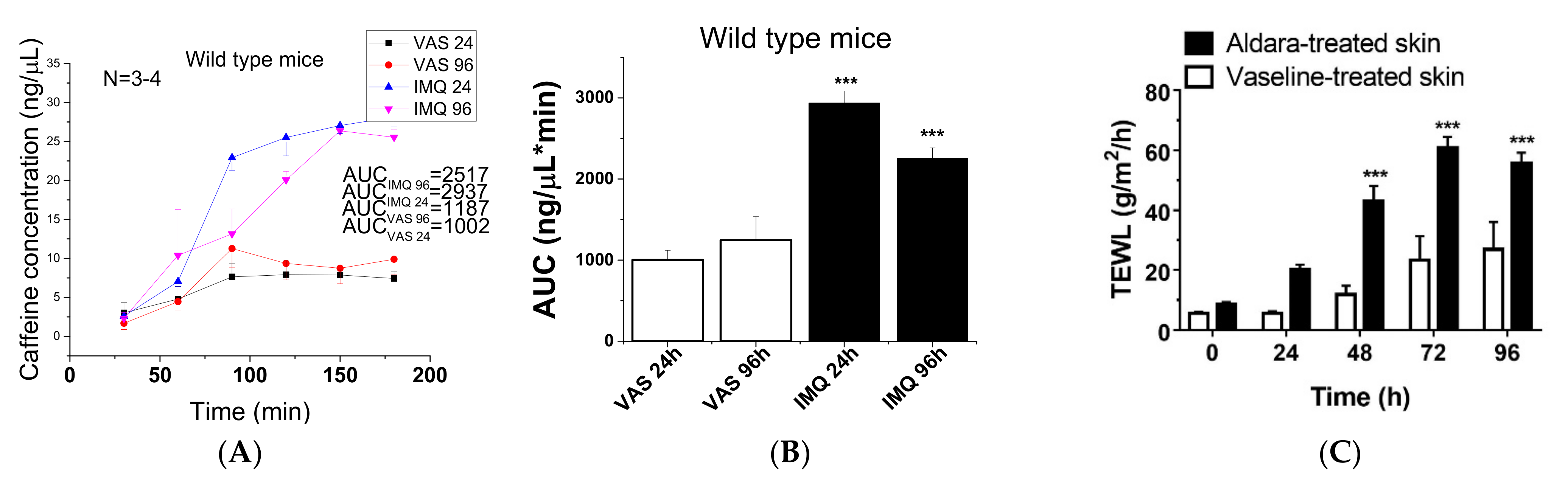
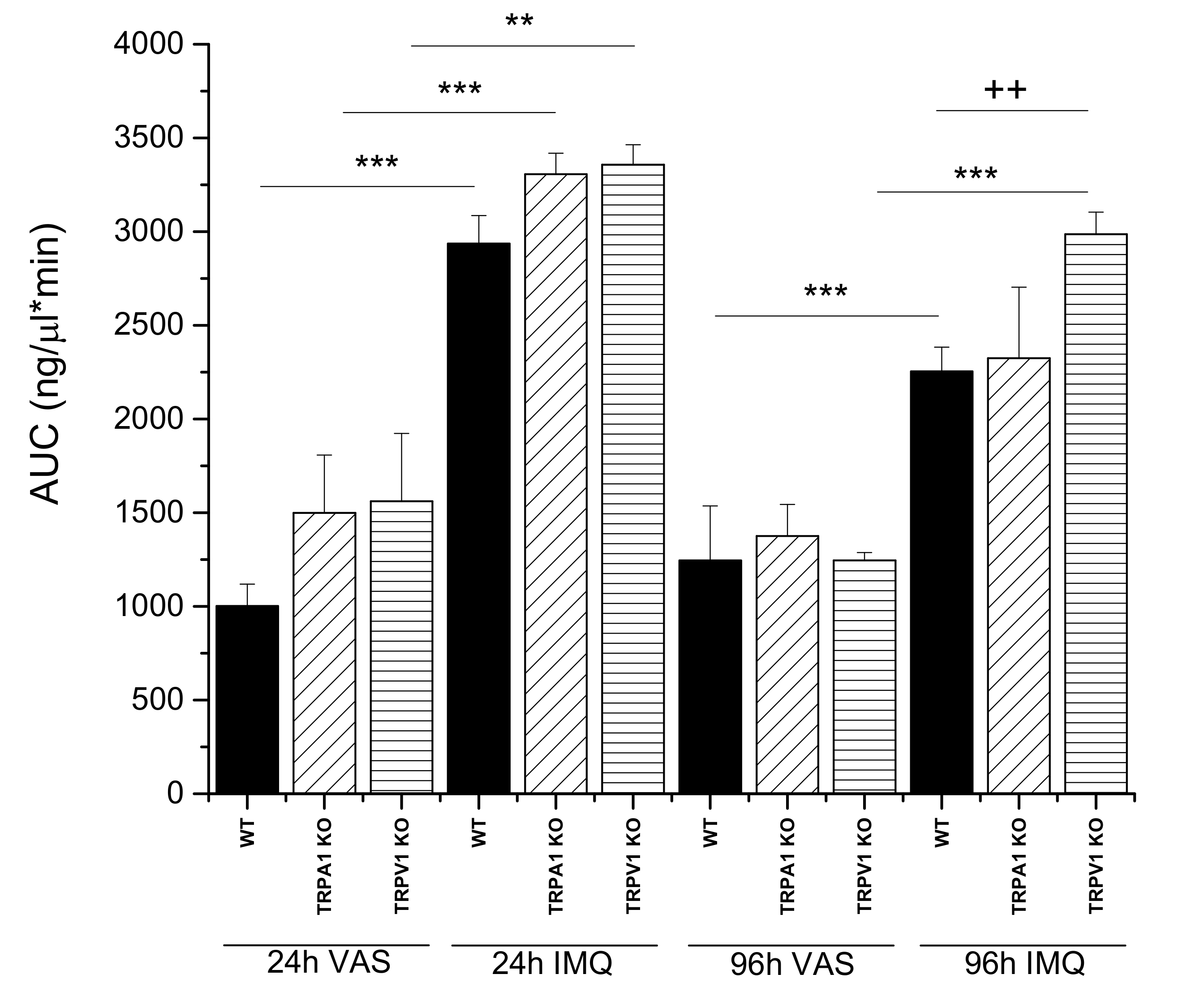
2.2. Diffusion Studies
2.2.1. Barrier Desintegration during Progression of Psoriasis
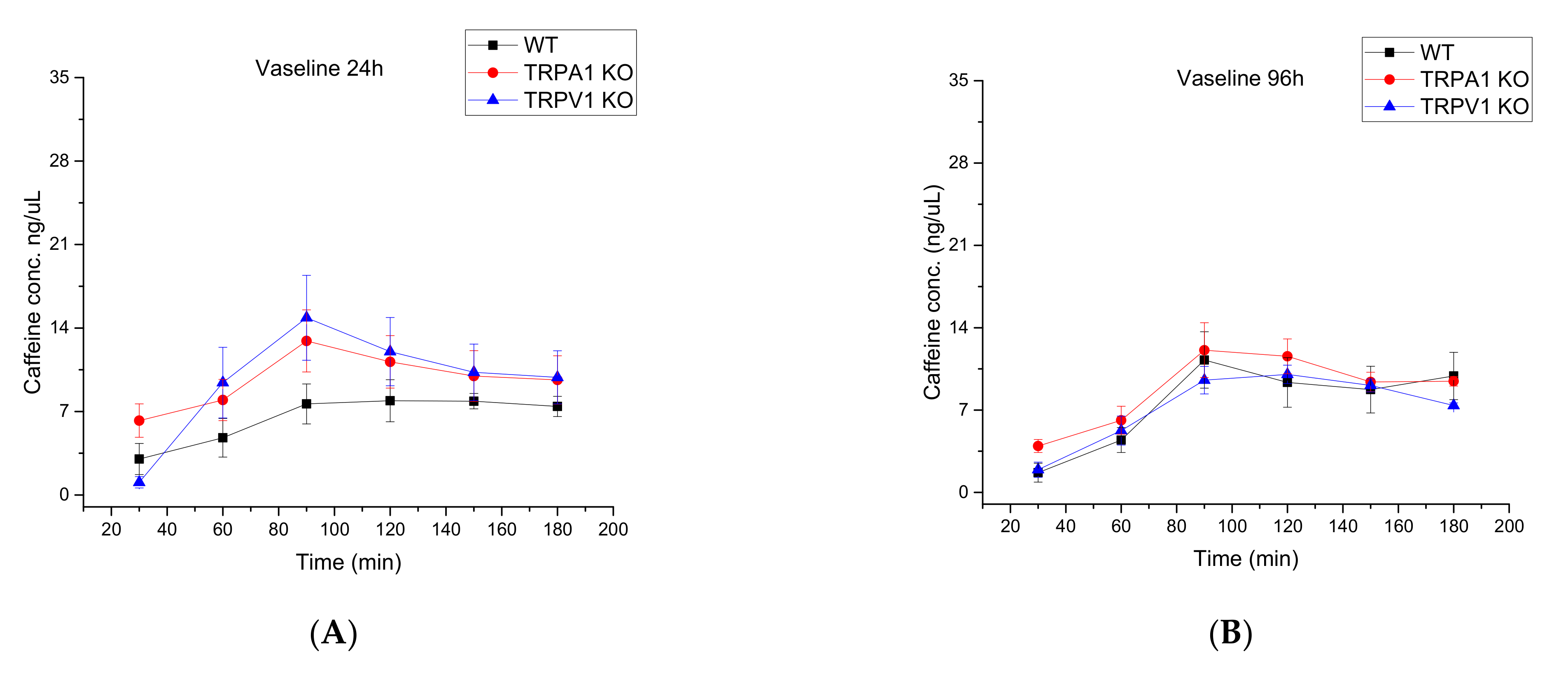
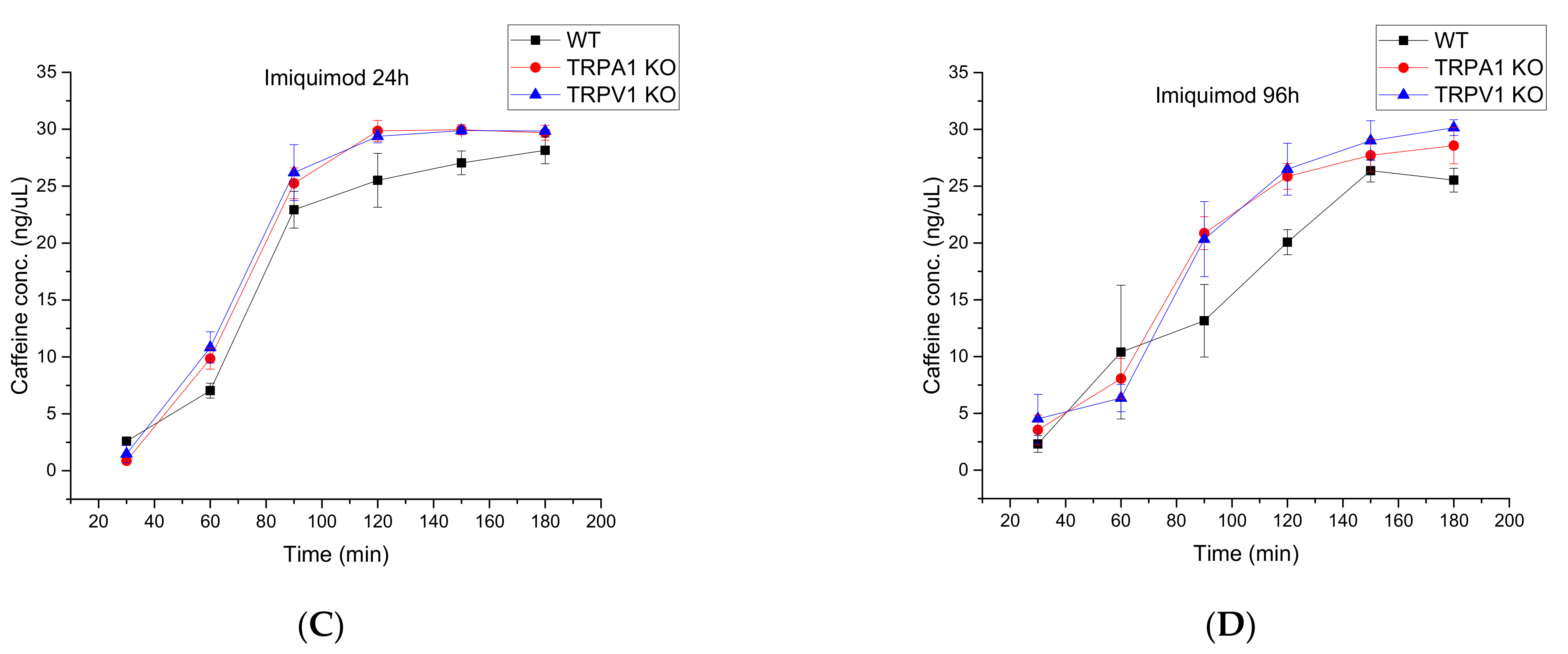
2.2.2. Comparison of Wild Type, TRPA1 KO and TRPV1 KO Knock Out Mouse Strains by Testing Drug Permeability during Progression of Psoriasis
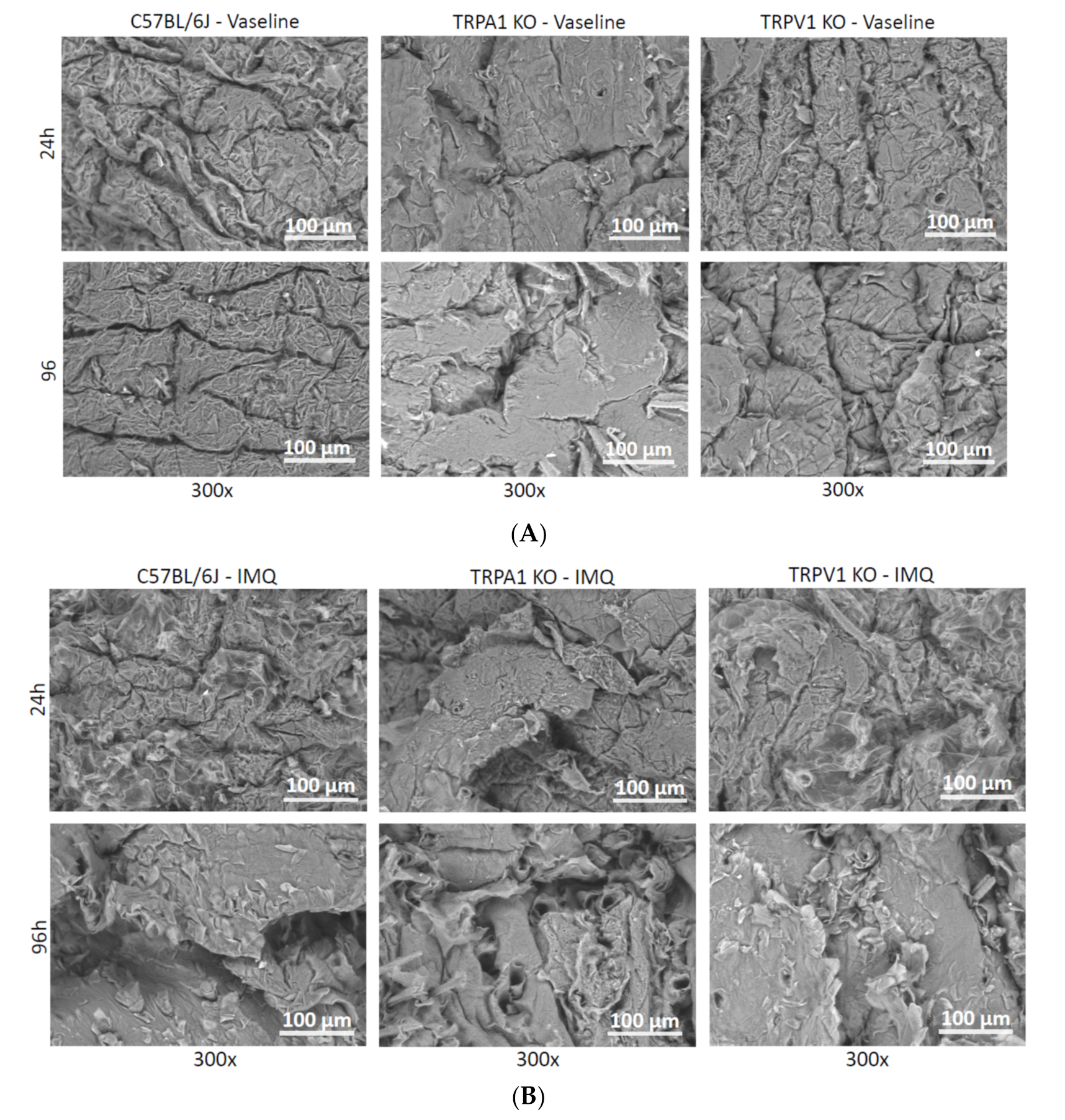
2.3. Scanning Electron Microscopy (SEM)
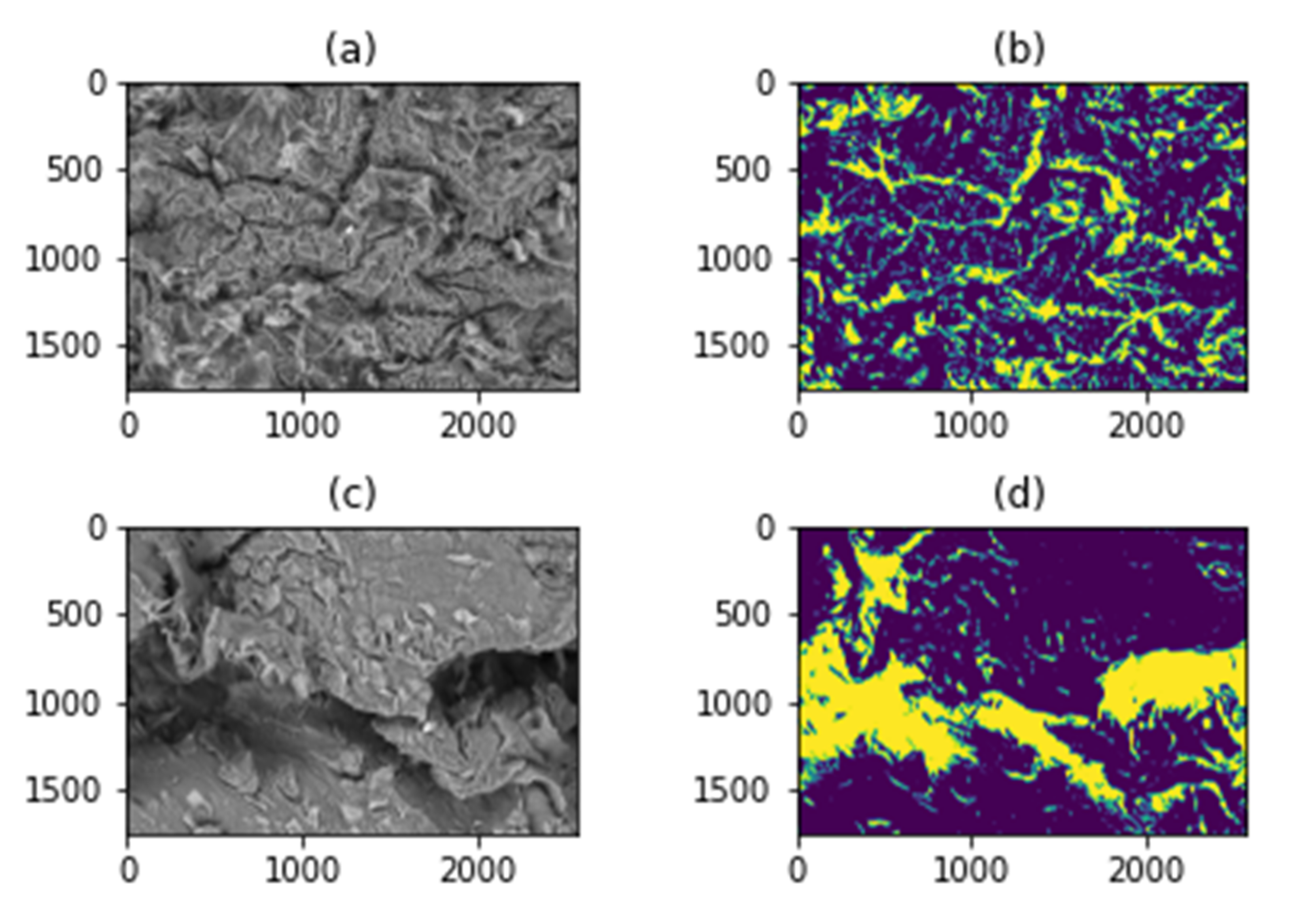
2.4. SEM Image Analysis by Classical Image Processing
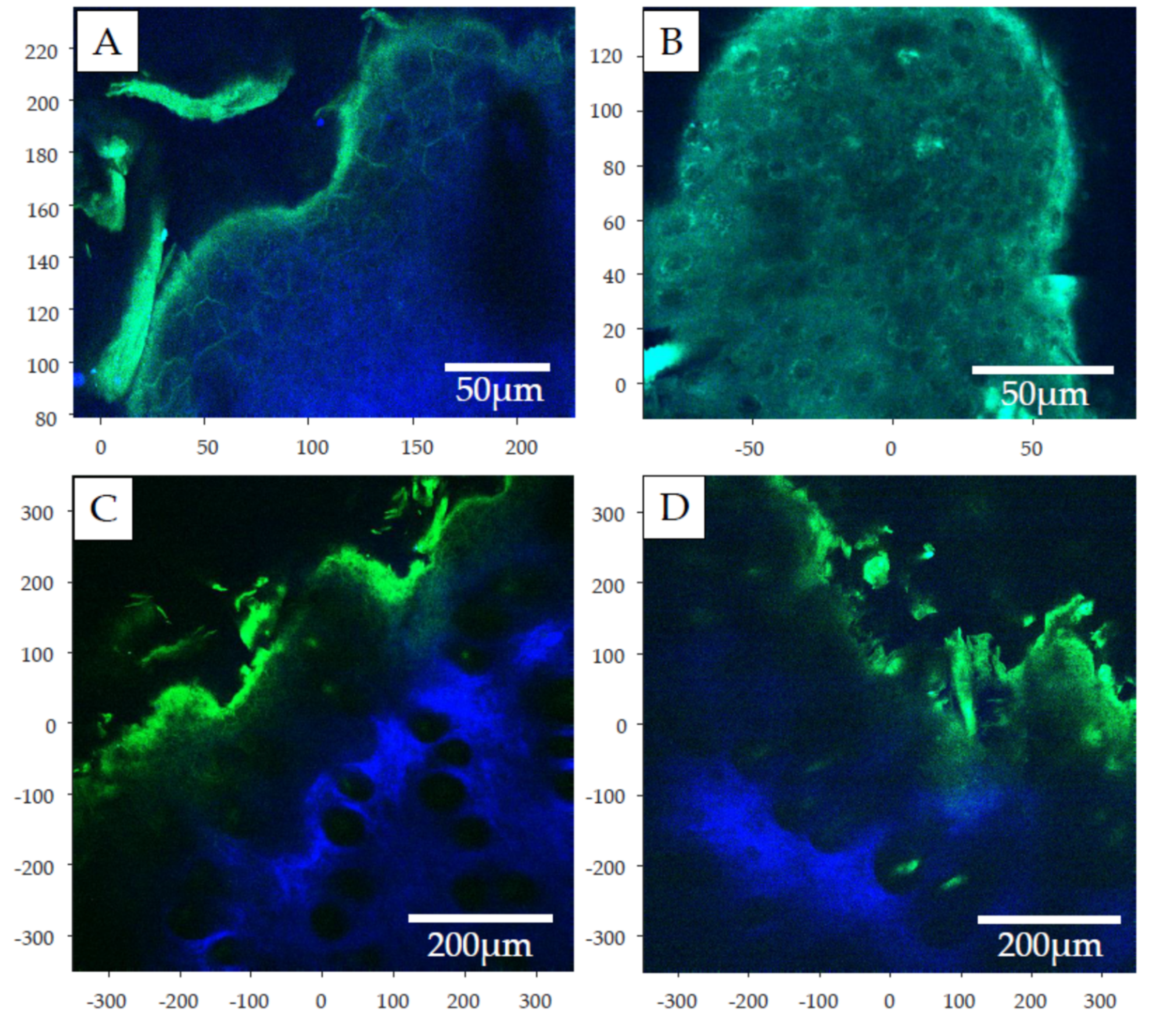
2.5. Two-Photon Microscopy
3. Discussion and Conclusions
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Drug Formulations
4.3. Induction of Psoriasiform Dermatitis in Mice
4.4. Measurement of Functional Parameters of Psoriasiform Skin Inflammation
4.5. Ex Vivo Drug Diffusion Study on “Skin-On-A-Chip”
4.6. Microscopic Evaluation of Ex Vivo Skin Samples
4.6.1. Scanning Electron Microscopy (SEM)
4.6.2. Classical Image Processing for SEM Image Analysis
4.6.3. Two-Photon Microscopy
4.7. Statistical Evaluation of the Data
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.K.; Chand Yadav, T.; Khera, H.K.; Mishra, P.; Raghuwanshi, N.; Pruthi, V.; Prasad, R. Insights into Interplay of Immunopathophysiological Events and Molecular Mechanistic Cascades in Psoriasis and Its Associated Comorbidities. J. Autoimmun. 2021, 118, 102614. [Google Scholar] [CrossRef] [PubMed]
- Grozdev, I.; Korman, N.; Tsankov, N. Psoriasis as a Systemic Disease. Clin. Dermatol. 2014, 32, 343–350. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156518-9. [Google Scholar]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal Models of Psoriasis-Highlights and Drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Talero, E.; Ávila-Román, J.; Romero, A.M.F.; Rabasco, A.M.; Motilva, V.; González-Rodríguez, M.L. Preparation and In Vivo Evaluation of Rosmarinic Acid-Loaded Transethosomes After Percutaneous Application on a Psoriasis Animal Model. AAPS Pharm. Sci. Tech. 2021, 22, 103. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Lin, C.-F.; Hsiao, C.-Y.; Chen, E.-L.; Lin, C.-Y.; Lien, W.-C.; Fang, J.-Y. Development of Flavanone and Its Derivatives as Topical Agents against Psoriasis: The Prediction of Therapeutic Efficiency through Skin Permeation Evaluation and Cell-Based Assay. Int. J. Pharm. 2020, 581, 119256. [Google Scholar] [CrossRef]
- Horváth, S.; Komlódi, R.; Perkecz, A.; Pintér, E.; Gyulai, R.; Kemény, Á. Methodological Refinement of Aldara-Induced Psoriasiform Dermatitis Model in Mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef] [Green Version]
- Horváth, S.; Kemény, Á.; Pintér, E.; Gyulai, R. A Localized Aldara (5% Imiquimod)-Induced Psoriasiform Dermatitis Model in Mice Using Finn Chambers. Curr. Protoc. Pharmacol. 2020, 90, e78. [Google Scholar] [CrossRef] [PubMed]
- Kemény, Á.; Kodji, X.; Horváth, S.; Komlódi, R.; Szőke, É.; Sándor, Z.; Perkecz, A.; Gyömörei, C.; Sétáló, G.; Kelemen, B.; et al. TRPA1 Acts in a Protective Manner in Imiquimod-Induced Psoriasiform Dermatitis in Mice. J. Investig. Dermatol. 2018, 138, 1774–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, J.E.; Gudjonsson, J.E.; Ward, N.L. The Snowballing Literature on Imiquimod-Induced Skin Inflammation in Mice: A Critical Appraisal. J. Investig. Dermatol. 2017, 137, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Sumida, H.; Yanagida, K.; Kita, Y.; Abe, J.; Matsushima, K.; Nakamura, M.; Ishii, S.; Sato, S.; Shimizu, T. Interplay between CXCR2 and BLT1 Facilitates Neutrophil Infiltration and Resultant Keratinocyte Activation in a Murine Model of Imiquimod-Induced Psoriasis. J. Immunol. 2014, 192, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N. Regulation of Nociceptive Transmission at the Periphery via TRPA1-TRPV1 Interactions. Curr. Pharm. Biotechnol. 2011, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct Expression of TRPM8, TRPA1, and TRPV1 MRNAs in Rat Primary Afferent Neurons with Adelta/c-Fibers and Colocalization with Trk Receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in Cutaneous Neurogenic and Chronic Inflammation: Pro-Inflammatory Response Induced by Their Activation and Their Sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [Green Version]
- Valdes-Rodriguez, R.; Kaushik, S.B.; Yosipovitch, G. Transient Receptor Potential Channels and Dermatological Disorders. Curr. Top. Med. Chem. 2013, 13, 335–343. [Google Scholar] [CrossRef]
- Ho, J.-C.; Lee, C.-H. TRP Channels in Skin: From Physiological Implications to Clinical Significances. Biophysics 2015, 11, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.S.; Vong, C.T.; Quek, S.; Cheong, J.; Awal, S.; Gentry, C.; Aubdool, A.A.; Liang, L.; Bodkin, J.V.; Bevan, S.; et al. Superoxide Generation and Leukocyte Accumulation: Key Elements in the Mediation of Leukotriene B4-Induced Itch by Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1. FASEB J. 2013, 27, 1664–1673. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.-H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-Dependent Pruritus in IL-13-Induced Chronic Atopic Dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Follansbee, T.; Wu, X.; Han, D.; Yu, S.; Domocos, D.T.; Shi, Z.; Carstens, M.; Carstens, E.; Hwang, S.T. TRPV1 Mediates Inflammation and Hyperplasia in Imiquimod (IMQ)-Induced Psoriasiform Dermatitis (PsD) in Mice. J. Dermatol. Sci. 2018, 92, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.M.; Proksch, E. The Skin’s Barrier. G Ital. Dermatol. Venereol. 2009, 144, 689–700. [Google Scholar] [PubMed]
- Elsholz, F.; Harteneck, C.; Muller, W.; Friedland, K. Calcium--a Central Regulator of Keratinocyte Differentiation in Health and Disease. Eur. J. Dermatol. 2014, 24, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids Modulate Human Epidermal Keratinocyte Proliferation and Survival via the Sequential Engagement of Cannabinoid Receptor-1 and Transient Receptor Potential Vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of Skin Surface Temperature on Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.-W.; Seo, J.A.; Jang, W.-H.; Koh, H.J.; Bae, I.-H.; Park, Y.-H.; Lim, K.-M. Antipruritic Effects of TRPV1 Antagonist in Murine Atopic Dermatitis and Itching Models. J. Investig. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef] [Green Version]
- Denda, M.; Tsutsumi, M.; Goto, M.; Ikeyama, K.; Denda, S. Topical Application of TRPA1 Agonists and Brief Cold Exposure Accelerate Skin Permeability Barrier Recovery. J. Investig. Dermatol. 2010, 130, 1942–1945. [Google Scholar] [CrossRef] [Green Version]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs-Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef] [Green Version]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Varga-Medveczky, Z.; Kocsis, D.; Naszlady, M.B.; Fónagy, K.; Erdő, F. Skin-on-a-Chip Technology for Testing Transdermal Drug Delivery—Starting Points and Recent Developments. Pharmaceutics 2021, 13, 1852. [Google Scholar] [CrossRef]
- Suzuki, S.; Be, K. Topological Structural Analysis of Digitized Binary Images by Border Following. Comput. Vis. Graph. Image Processing 1985, 30, 32–46. [Google Scholar] [CrossRef]
- Dancik, Y.; Favre, A.; Loy, C.J.; Zvyagin, A.V.; Roberts, M.S. Use of Multiphoton Tomography and Fluorescence Lifetime Imaging to Investigate Skin Pigmentation in Vivo. J. Biomed. Opt. 2013, 18, 26022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-G.; König, K.; Halbhuber, K.-J. Two-Photon Microscopy of Deep Intravital Tissues and Its Merits in Clinical Research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patapoutian, A.; Peier, A.M.; Story, G.M.; Viswanath, V. ThermoTRP Channels and beyond: Mechanisms of Temperature Sensation. Nat. Rev. Neurosci. 2003, 4, 529–539. [Google Scholar] [CrossRef]
- Kuehl, B.; Shear, N.H. The Evolution of Topical Formulations in Psoriasis. Skin Ther. Lett. 2018, 23, 5–9. [Google Scholar]
- Weng, H.J.; Tsai, T.F. ABCB1 in Dermatology: Roles in Skin Diseases and Their Treatment. J. Mol. Med. 2021, 99, 1527–1538. [Google Scholar] [CrossRef]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin Barrier Dysregulation in Psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, W.; Henrot, L.; Schnebert, S.; Dumas, M.; Heusèle, C.; Yang, J. Modelling Epidermis Homoeostasis and Psoriasis Pathogenesis. J. R. Soc. Interface 2015, 12, 20141071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.A.; Ryu, Y.W.; Kwon, J.I.; Choe, M.S.; Jung, J.W.; Cho, J.W. Differential Expression of Cyclin D1, Ki-67, PRb, and P53 in Psoriatic Skin Lesions and Normal Skin. Mol. Med. Rep. 2018, 17, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.-J.; Na, J.-I.; Byun, S.-Y.; Kwon, S.-H.; Yang, S.-H.; Lee, H.-S.; Choi, H.-R.; Cho, S.; Youn, S.W.; Park, K.-C. Histone Deacetylase 1 and Sirtuin 1 Expression in Psoriatic Skin: A Comparison between Guttate and Plaque Psoriasis. Life 2020, 10, 157. [Google Scholar] [CrossRef]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic Acid Induces Keratinocyte Proliferation in a Skin Equivalent Model via TRPV1 Activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef] [PubMed]
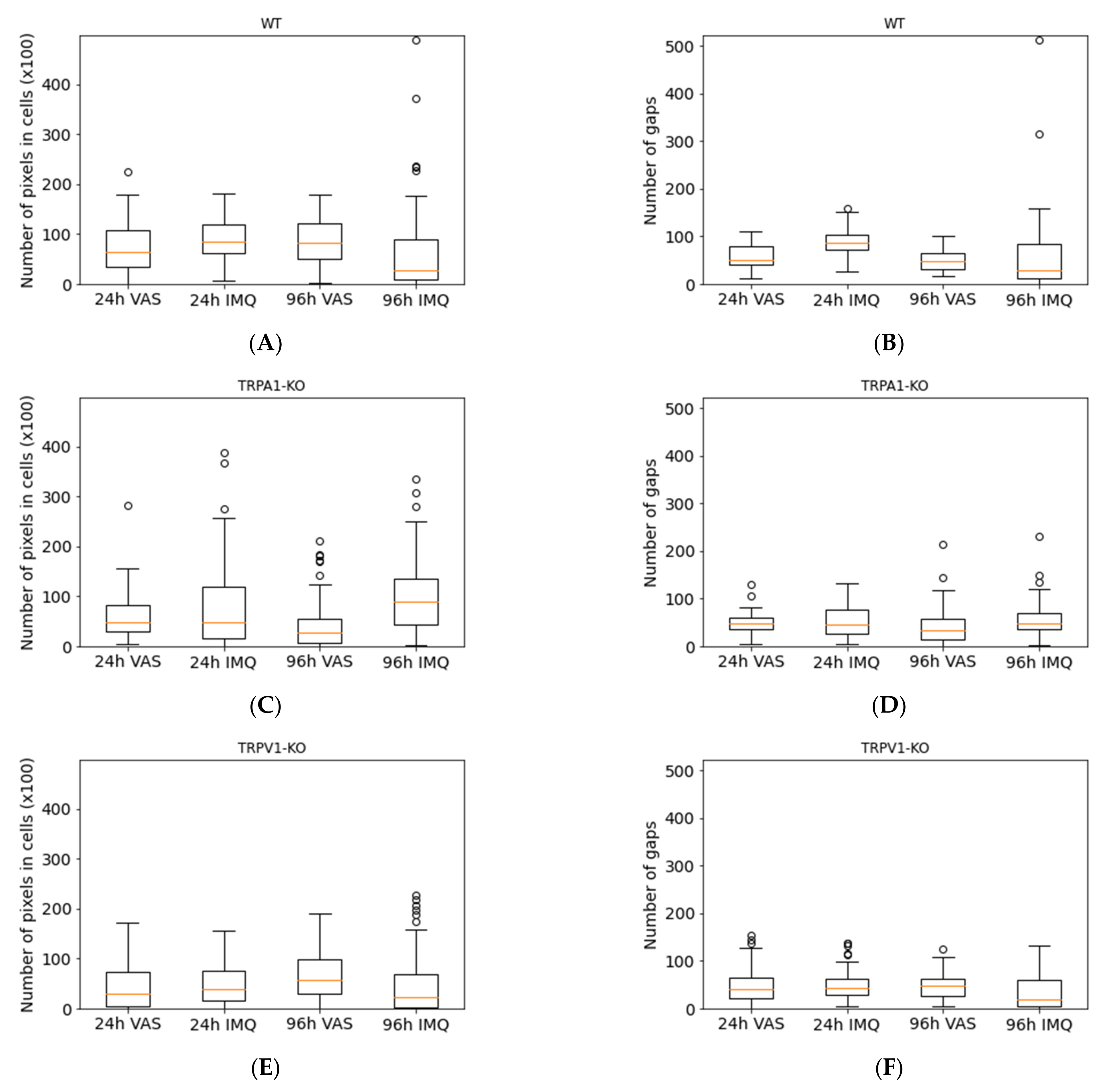
| Ples (VAS vs. IMQ) | NG p-Value | NPC p-Value |
|---|---|---|
| 24 h WT | 6.14 × 10−7 | 0.0299 |
| 96 h WT | 0.037 | 0.0001 |
| 24 h TRPA1 | 0.486 | 0.48 |
| 96 h TRPA1 | 0.00167 | 8.58 × 10−7 |
| 24 h TRPV1 | 0.28 | 0.129 |
| 96 h TRPV1 | 0.00047 | 0.0022 |
| Fluorophore | Emission Wavelength [nm] | Blue/Green |
|---|---|---|
| FADs | 490–650 | Blue and Green |
| Keratin | 450–550 | Blue and Green |
| Flavoprotein | 460–480 | Blue |
| Lipoamide dehydrogenase | 510–550 | Green |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, D.; Horváth, S.; Kemény, Á.; Varga-Medveczky, Z.; Pongor, C.; Molnár, R.; Mihály, A.; Farkas, D.; Naszlady, B.M.; Fülöp, A.; et al. Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging. Int. J. Mol. Sci. 2022, 23, 4237. https://doi.org/10.3390/ijms23084237
Kocsis D, Horváth S, Kemény Á, Varga-Medveczky Z, Pongor C, Molnár R, Mihály A, Farkas D, Naszlady BM, Fülöp A, et al. Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging. International Journal of Molecular Sciences. 2022; 23(8):4237. https://doi.org/10.3390/ijms23084237
Chicago/Turabian StyleKocsis, Dorottya, Szabina Horváth, Ágnes Kemény, Zsófia Varga-Medveczky, Csaba Pongor, Rózsa Molnár, Anna Mihály, Dániel Farkas, Bese Márton Naszlady, András Fülöp, and et al. 2022. "Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging" International Journal of Molecular Sciences 23, no. 8: 4237. https://doi.org/10.3390/ijms23084237
APA StyleKocsis, D., Horváth, S., Kemény, Á., Varga-Medveczky, Z., Pongor, C., Molnár, R., Mihály, A., Farkas, D., Naszlady, B. M., Fülöp, A., Horváth, A., Rózsa, B., Pintér, E., Gyulai, R., & Erdő, F. (2022). Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging. International Journal of Molecular Sciences, 23(8), 4237. https://doi.org/10.3390/ijms23084237










