Are BPA Substitutes as Obesogenic as BPA?
Abstract
1. Introduction
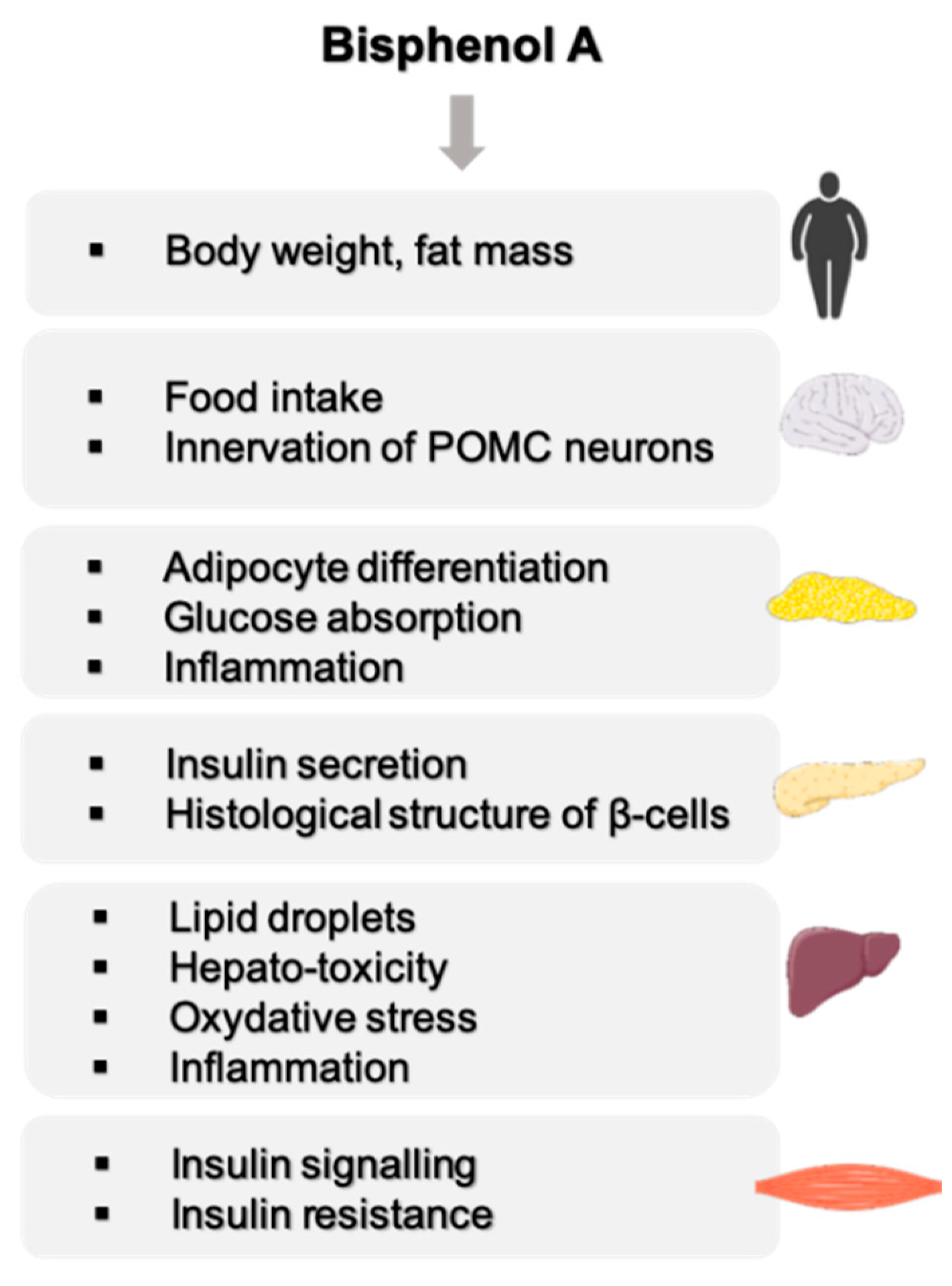
2. A Strong Link between BPA and Metabolic Disorders
2.1. Effect of BPA on Body Weight
2.2. Effect of BPA on the Central Nervous Functions Related to Energy Homeostasis
2.3. BPA, a Disruptor of Carbohydrate Homeostasis
2.4. BPA, a Disruptor of Lipid Metabolism
2.4.1. Effect of BPA on Adipose Tissue
2.4.2. Effect of BPA on the Liver
2.4.3. Effect of BPA on the Muscle
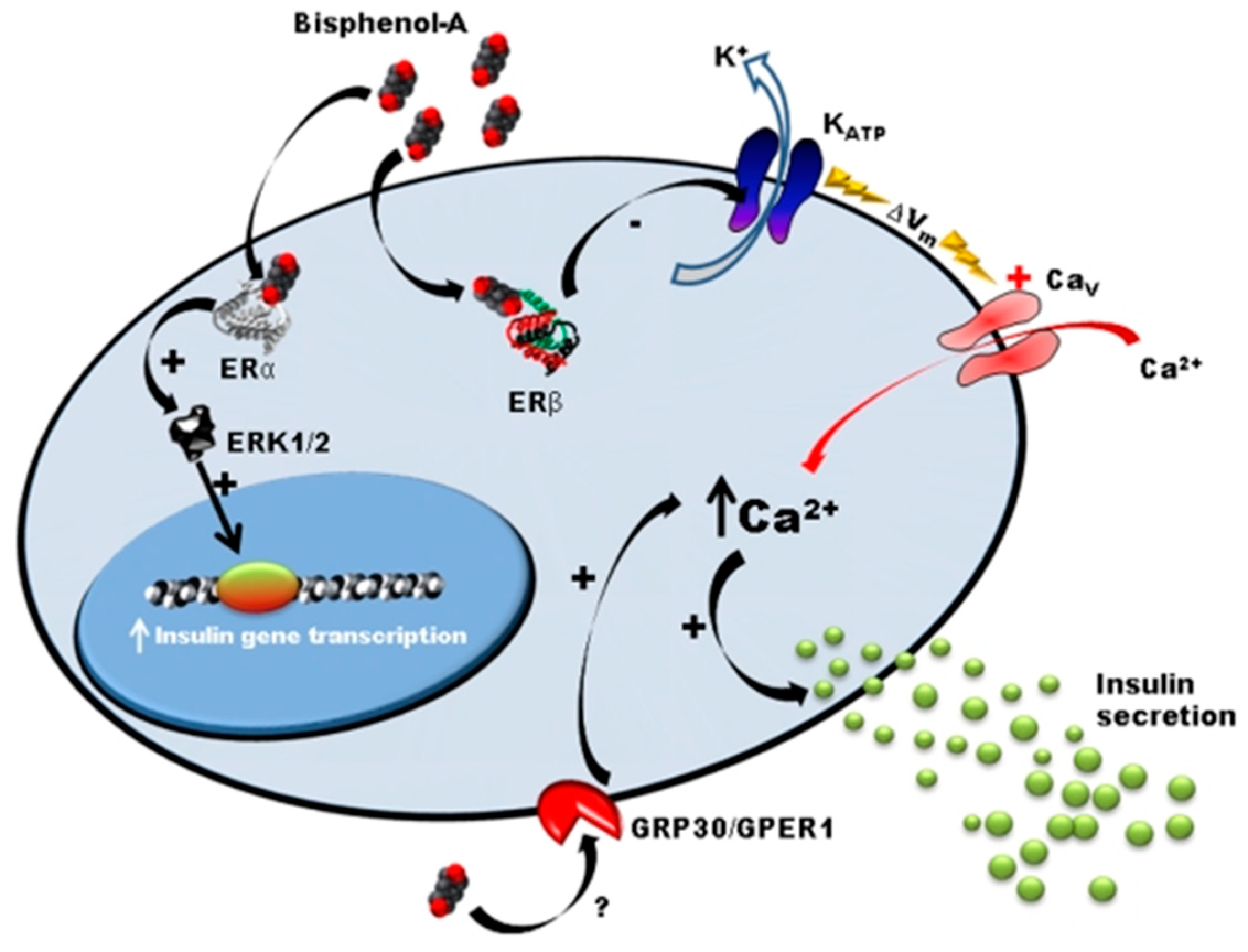
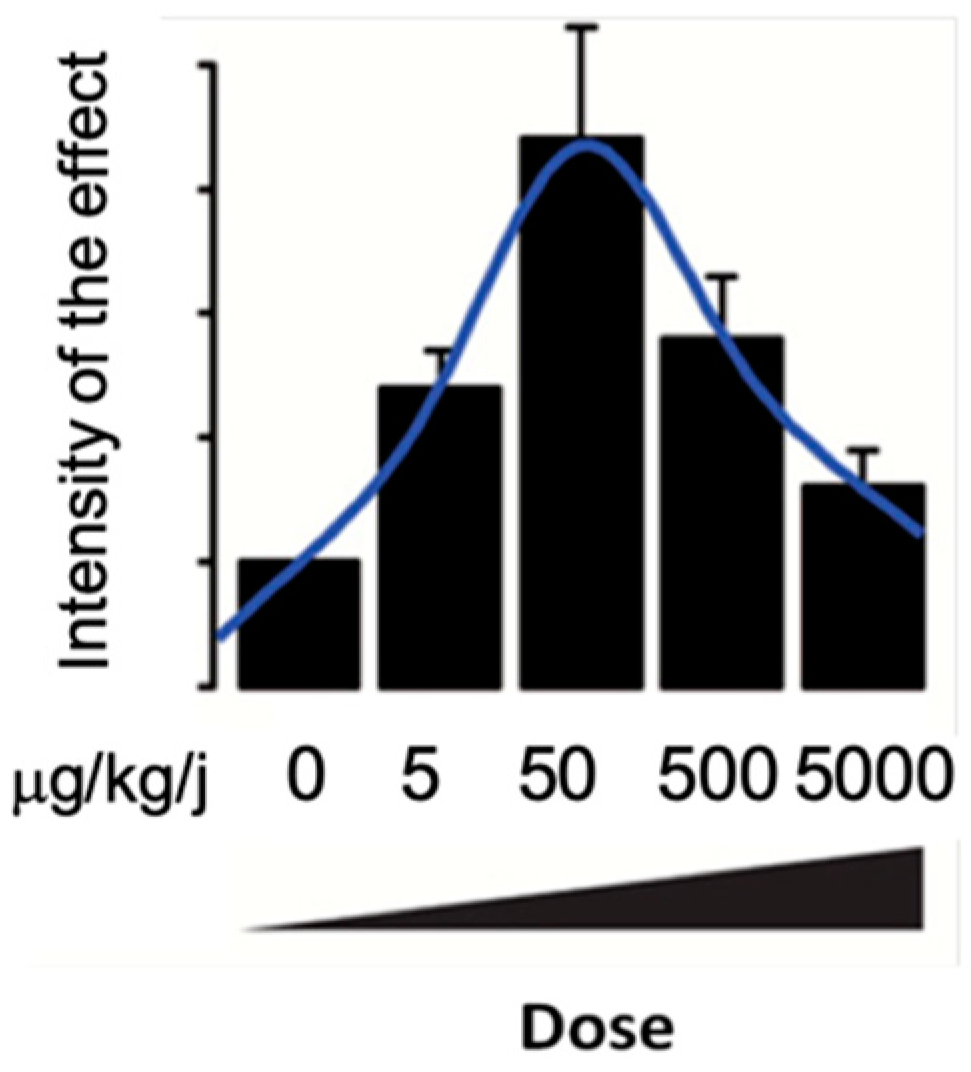
2.5. Mechanism of BPA Action
3. Are BPA Substitutes as Obesogenic as BPA?
3.1. Bisphenol S Effects
3.1.1. BPS Presents the Same Adipogenic Effect as BPA
3.1.2. Effect of BPS on Carbohydrate and Lipid Metabolism
3.1.3. Effect of BPS on Central Nervous System
3.2. Other Bisphenols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Shahnazaryan, U.; Wójcik, M.; Bednarczuk, T.; Kuryłowicz, A. Role of Obesogens in the Pathogenesis of Obesity. Medicina 2019, 55, 515. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Brucker-Davis, F.; Chevalier, N. The history of Distilbène® (Diethylstilbestrol) told to grandchildren—The transgenerational effect. In Annales d’Endocrinologie; Elsevier Masson: Paris, France, 2015; Volume 76, pp. 253–259. [Google Scholar]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A physiologically based approach to the study of bisphenol A and other estro-genic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health 1998, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.-E.; Choi, Y.; Jee, S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: A meta-analysis. BMC Endocr. Disord. 2018, 18, 81. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, e114–e122. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303. [Google Scholar] [CrossRef]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of Urinary Bisphenol A Concentration with Heart Disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Relationship between Urinary Bisphenol A Levels and Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Low-Dose Effects of Hormones and Endocrine Disruptors. Vitam. Horm. 2014, 94, 129–165. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and Postnatal Exposure to Bisphenol A Increases Adipose Tissue Mass and Serum Cholesterol Level in Mice. J. Atheroscler. Thromb. 2007, 14, 245–252. [Google Scholar] [CrossRef]
- Rubin, B.S.; Murray, M.K.; Damassa, D.A.; King, J.C.; Soto, A.M. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001, 109, 675–680. [Google Scholar] [CrossRef]
- Ryan, K.; Haller, A.M.; Sorrell, J.E.; Woods, S.C.; Jandacek, R.J.; Seeley, R.J. Perinatal Exposure to Bisphenol-A and the Development of Metabolic Syndrome in CD-1 Mice. Endocrinology 2010, 151, 2603–2612. [Google Scholar] [CrossRef]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M. Bisphenol A Exposure Disrupts Metabolic Health across Multiple Generations in the Mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal Exposure to Bisphenol A Alters Early Adipogenesis in the Rat. Environ. Health Perspect. 2009, 117, 1549–1555. [Google Scholar] [CrossRef]
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X. Perinatal Exposure to Bisphenol A at Reference Dose Predisposes Offspring to Metabol-ic Syndrome in Adult Rats on a High-Fat Diet. Endocrinology 2011, 152, 3049–3061. [Google Scholar] [CrossRef]
- Magdalena, P.A.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A Exposure during Pregnancy Disrupts Glucose Homeostasis in Mothers and Adult Male Offspring. Environ. Health Perspect. 2010, 118, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Ishido, M.; Masuo, Y.; Kunimoto, M.; Oka, S.; Morita, M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 2004, 76, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kabuto, H.; Amakawa, M.; Shishibori, T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004, 74, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.C.; Vandenbergh, J.G. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 2006, 50, 85–93. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Richter, C.A.; Ruhlen, R.R.; Nagel, S.C.; Timms, B.G.; Welshons, W.V. The importance of appropriate controls, animal feed, and animal models in inter-preting results from low-dose studies of bisphenol A. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 140–145. [Google Scholar] [CrossRef]
- Mackay, H.; Patterson, Z.R.; Khazall, R.; Patel, S.; Tsirlin, D.; Abizaid, A. Organizational Effects of Perinatal Exposure to Bisphenol-A and Diethylstilbestrol on Arcuate Nucleus Circuitry Controlling Food Intake and Energy Expenditure in Male and Female CD-1 Mice. Endocrinology 2013, 154, 1465–1475. [Google Scholar] [CrossRef]
- Mackay, H.; Patterson, Z.R.; Abizaid, A. Perinatal Exposure to Low-Dose Bisphenol-A Disrupts the Structural and Functional Development of the Hypothalamic Feeding Circuitry. Endocrinology 2017, 158, 768–777. [Google Scholar] [CrossRef]
- Salehi, A.; Loganathan, N.; Belsham, D.D. Bisphenol A induces Pomc gene expression through neuroinflammatory and PPARγ nuclear receptor-mediated mechanisms in POMC-expressing hypo-thalamic neuronal models. Mol. Cell. Endocrinol. 2019, 479, 12–19. [Google Scholar] [CrossRef]
- Martinez-Pinna, J.; Marroqui, L.; Hmadcha, A.; Lopez-Beas, J.; Soriano, S.; Villar-Pazos, S.; Alonso-Magdalena, P.; Dos Santos, R.S.; Quesada, I.; Martin, F.; et al. Oestrogen receptor β mediates the actions of bisphenol-A onion channel expression in mouse pancreatic beta cells. Diabetologia 2019, 62, 1667–1680. [Google Scholar] [CrossRef]
- Villar-Pazos, S.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Magdalena, P.A.; Marroqui, L.; Quesada, I.; Gustafsson, J.-A.; Nadal, A. Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on Ca2+ entry in mouse pancreatic β-cells. Sci. Rep. 2017, 7, 11770. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Magdalena, P.A.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009, 304, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Pang, Y.; Alonso-Magdalena, P.; Thomas, P.; Nadal, Á. Role of ERβ and GPR30 in the endocrine pancreas: A matter of estrogen dose. Steroids 2012, 77, 951–958. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquie, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008, 3, e2069. [Google Scholar] [CrossRef]
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.-A.; Quesada, I.; Nadal, A. Rapid Insulinotropic Action of Low Doses of Bisphenol-A on Mouse and Human Islets of Langerhans: Role of Estrogen Receptor β. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef]
- Makaji, E.; Raha, S.; Wade, M.G.; Holloway, A.C. Effect of Environmental Contaminants on Beta Cell Function. Int. J. Toxicol. 2011, 30, 410–418. [Google Scholar] [CrossRef]
- Dahlman-Wright, K.; Cavailles, V.; Fuqua, S.A.; Jordan, V.C.; Katzenellenbogen, J.A.; Korach, K.; Maggi, A.; Muramatsu, M.; Parker, M.G.; Gustafsson, J. International Union of Pharmacology. LXIV. Estrogen Receptors. Pharmacol. Rev. 2006, 58, 773–781. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Sharma, G.; Prossnitz, E.R. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology 2011, 152, 3030–3039. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Laribi, O.; Ropero, A.B.; Fuentes, E.; Ripoll, C.; Soria, B.; Nadal, A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langer-hans. Environ. Health Perspect. 2005, 113, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tsutsumi, O.; Ikezuki, Y.; Takai, Y.; Taketani, Y. Positive Relation-ship between Androgen and the Endocrine Disruptor, Bisphenol A, in Normal Women and Women with Ovarian Dysfunction. Endocr. J. 2004, 51, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Arrebola, J.P.; Taoufiki, J.; Navalón, A.; Ballesteros, O.; Pulgar, R.; Vilchez, J.; Olea, N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, A.; Shidfar, F.; Hedayati, M.; Tehrani, A.N.; Farshad, A.A.; Mohammadi, S. Bisphenol A enhances adipogenic signaling pathways in human mesenchymal stem cells. Genes Environ. 2020, 42, 13. [Google Scholar] [CrossRef]
- Choi, S.-I.; Kwon, H.-Y.; Han, X.; Men, X.; Choi, Y.-E.; Jang, G.-W.; Park, K.-T.; Han, J.; Lee, O.-H. Environmental obesogens (bisphenols, phthalates and parabens) and their impacts on adipogenic transcription factors in the absence of dexamethasone in 3T3-L1 cells. J. Steroid Biochem. Mol. Biol. 2021, 214, 105994. [Google Scholar] [CrossRef]
- Sargis, R.M.; Johnson, D.N.; Choudhury, R.A.; Brady, M.J. Environmental Endocrine Disruptors Promote Adipogenesis in the 3T3-L1 Cell Line through Glucocorticoid Receptor Activation. Obesity 2010, 18, 1283–1288. [Google Scholar] [CrossRef]
- Masuno, H.; Kidani, T.; Sekiya, K.; Sakayama, K.; Shiosaka, T.; Yamamoto, H.; Honda, K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 2002, 43, 676–684. [Google Scholar] [CrossRef]
- Wada, K.; Sakamoto, H.; Nishikawa, K.; Sakuma, S.; Nakajima, A. Forum Minireview Life Style-Related Diseases of the Digestive System: Endocrine Disruptors Stimulate Lipid Accumulation in Target Cells Related to Metabolic Syndrome. J. Pharmacol. Sci. 2007, 137, 133–137. [Google Scholar] [CrossRef]
- Sakurai, K.; Kawazuma, M.; Adachi, T.; Harigaya, T.; Saito, Y.; Hashimoto, N.; Mori, C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. J. Cereb. Blood Flow Metab. 2004, 141, 209–214. [Google Scholar] [CrossRef]
- Linehan, C.; Gupta, S.; Samali, A.; O’Connor, L. Bisphenol A-Mediated Suppression of LPL Gene Expression Inhibits Triglyceride Accumulation during Adipogenic Differentiation of Human Adult Stem Cells. PLoS ONE 2012, 7, e36109. [Google Scholar] [CrossRef]
- Kidani, T.; Kamei, S.; Miyawaki, J.; Aizawa, J.; Sakayama, K.; Masuno, H. Bisphenol A Downregulates Akt Signaling and Inhibits Adiponectin Production and Secretion in 3T3-L1 Adipocytes. J. Atheroscler. Thromb. 2010, 17, 834–843. [Google Scholar] [CrossRef]
- Hugo, E.R.; Brandebourg, T.D.; Woo, J.G.; Loftus, J.; Alexander, J.W.; Ben-Jonathan, N. Bisphenol A at Environmentally Relevant Doses Inhibits Adiponectin Release from Human Adipose Tissue Explants and Adipocytes. Environ. Health Perspect. 2008, 116, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Ben-jonathan, N.; Hugo, E.R.; Brandebourg, T.D. Tissue: Implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009, 304, 49–54. [Google Scholar] [CrossRef]
- An, S.J.; Yang, E.-J.; Oh, S.; Park, K.J.; Kim, T.; Hong, Y.-P. The association between urinary bisphenol A levels and nonalcoholic fatty liver disease in Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ. Health Prev. Med. 2021, 26, 91. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Gravina, A.G.; Diano, N.; Errico, S.; Masarone, M.; Romeo, M.; Tuccillo, C.; Stiuso, P.; Morisco, F.; et al. The Bisphenol A Induced Oxidative Stress in Non-Alcoholic Fatty Liver Disease Male Patients: A Clinical Strategy to Antagonize the Progression of the Disease. Int. J. Environ. Res. Public Health 2020, 17, 3369. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, S.G.; Wojcicki, J.M.; Perito, E.R.; Rosenthal, P. Bisphenol a increases risk for pre-sumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003–2010. Environ. Health 2018, 17, 12. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, E.R.; Li, A.A.; Cholankeril, G.; Tighe, S.; Kim, W.; Harrison, S.A.; Ahmed, A. Elevated urinary bisphenol A levels are associated with non-alcoholic fatty liver disease among adults in the United States. Liver Int. 2019, 39, 1335–1342. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.-X.; Lezmi, S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef]
- Diamante, G.; Cely, I.; Zamora, Z.; Ding, J.; Blencowe, M.; Lang, J.; Bline, A.; Singh, M.; Lusis, A.J.; Yang, X. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ. Int. 2021, 146, 106260. [Google Scholar] [CrossRef]
- Marmugi, A.; Ducheix, S.; Lasserre, F.; Polizzi, A.; Paris, A.; Priymenko, N.; Bertrand-Michel, J.; Pineau, T.; Guillou, H.; Martin, P.G.P.; et al. Low doses of bisphenol a induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 2012, 55, 395–407. [Google Scholar] [CrossRef]
- Huc, L.; Lemarié, A.; Guéraud, F.; Héliès-toussaint, C. Toxicology in Vitro Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. In Vitro 2012, 26, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Li, L.; Eguchi, A.; Kannan, K.; Kim, E.-Y.; Iwata, H. Effects on the liver lipidome of rat offspring prenatally exposed to bisphenol A. Sci. Total Environ. 2021, 759, 143466. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Segatto, M.; Bertoli, S.; Leone, A.; Mazzoli, A.; Cigliano, L.; Barberio, L.; Mandalà, M.; Pallottini, V. Prenatal Exposure to BPA: The Effects on Hepatic Lipid Metabolism in Male and Female Rat Fetuses. Nutrients 2021, 13, 1970. [Google Scholar] [CrossRef]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A Induces Hepatotoxicity through Oxidative Stress in Rat Model. Oxidative Med. Cell. Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef]
- Moon, M.K.; Kim, M.J.; Jung, I.K.; Koo, Y.D.; Ann, H.Y.; Lee, K.J.; Kim, S.H.; Yoon, Y.C.; Cho, B.-J.; Park, K.S.; et al. Bisphenol A Impairs Mitochondrial Function in the Liver at Doses below the No Observed Adverse Effect Level. J. Korean Med. Sci. 2012, 27, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Asahi, J.; Kamo, H.; Baba, R.; Doi, Y.; Yamashita, A.; Murakami, D.; Hanada, A.; Hirano, T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010, 87, 431–438. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W. Aberrant Lipid Metabolism Disrupts Calcium Homeostasis Causing Liver Endoplasmic Reticulum Stress in Obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef]
- Batista, T.; Magdalena, P.A.; Vieira, E.; Amaral, M.E.C.D.; Cederroth, C.R.; Nef, S.; Quesada, I.; Carneiro, E.M.; Nadal, A. Short-Term Treatment with Bisphenol-A Leads to Metabolic Abnormalities in Adult Male Mice. PLoS ONE 2012, 7, e33814. [Google Scholar] [CrossRef]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large Effects from Small Exposures. III. Endocrine Mechanisms Mediating Effects of Bisphenol A at Levels of Human Exposure. Endocrinology 2006, 147 (Suppl. 6), s56–s69. [Google Scholar] [CrossRef]
- Paris, F.; Balaguer, P.; Lacoste, C.; Nicolas, J.; Sultan, C. Phenylphenols, bisphenols, bisphenol-A and 4-tert-octylphenol exhibit a and b estrogen activities and antiandrogen activity in reporter cell lines. Mol. Cell. Endocrinol. 2002, 193, 43–49. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Brieño-Enriquez, M.A.; Reig-Viader, R.; Cabero, L.; Toran, N.; Martinez, F.; Roig, I.; Caldes, M.G. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol. Hum. Reprod. 2011, 18, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tokunaga, T.; Liu, X.; Takayanagi, S.; Matsushima, A.; Shimohigashi, Y. Direct Evidence Revealing Structural Elements Essential for the High Binding Ability of Bisphenol A to Human Estrogen-Related Receptor-γ. Environ. Health Perspect. 2008, 116, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related re-ceptor g ( ERR g ) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Vanacker, J.M.; Pettersson, K.; Gustafsson, J.Å.; Laudet, V. Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) α, but not by ER β. EMBO J. 1999, 18, 4270–4279. [Google Scholar] [CrossRef]
- Kubo, M.; Ijichi, N.; Ikeda, K.; Horie-inoue, K. Biochimica et Biophysica Acta Modulation of adi-pogenesis-related gene expression by estrogen-related receptor γ during adipocytic differentiation. BBA—Gene Regul. Mech. 2009, 1789, 71–77. [Google Scholar]
- Kim, D.-K.; Ryu, D.; Koh, M.; Lee, M.-W.; Lim, D.; Kim, M.-J.; Kim, Y.-H.; Cho, W.-J.; Lee, C.-H.; Park, S.B. Orphan Nuclear Receptor Estrogen-Related Receptor γ (ERRγ) Is Key Regulator of Hepatic Gluconeogenesis. J. Biol. Chem. 2012, 287, 21628–21639. [Google Scholar] [CrossRef]
- Watson, C.S.; Jeng, Y.-J.; Kochukov, M.Y. Nongenomic Signaling Pathways of Estrogen Toxicity. Toxicol. Sci. 2009, 115, 1–11. [Google Scholar] [CrossRef]
- Watson, C.S.; Jeng, Y.-J.; Guptarak, J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. J. Steroid Biochem. Mol. Biol. 2011, 127, 44–50. [Google Scholar] [CrossRef]
- Marino, M.; Ascenzi, P.; Acconcia, F. S-palmitoylation modulates estrogen receptor α localization and functions. Steroids 2006, 71, 298–303. [Google Scholar] [CrossRef]
- Chevalier, N.; Vega, A.; Bouskine, A.; Siddeek, B.; Michiels, J.-F.; Chevallier, D.; Fénichel, P. GPR30, the Non-Classical Membrane G Protein Related Estrogen Receptor, Is Overexpressed in Human Seminoma and Promotes Seminoma Cell Proliferation. PLoS ONE 2012, 7, e34672. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Liu, M.; Clegg, D.J.; Portincasa, P.; Wang, D.Q.-H. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2009, 1791, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- DeKeyser, J.G.; Laurenzana, E.M.; Peterson, E.C.; Chen, T.; Omiecinski, C.J. Selective Phthalate Activation of Naturally Occurring Human Constitutive Androstane Receptor Splice Variants and the Pregnane X Receptor. Toxicol. Sci. 2011, 120, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Ai, N.; Park, S.-H.; Rios-Pilier, J.; Perkins, J.T.; Welsh, W.J.; Zhou, C. Bisphenol A and Its Analogues Activate Human Pregnane X Receptor. Environ. Health Perspect. 2012, 120, 399–405. [Google Scholar] [CrossRef]
- Tabb, M.M.; Kholodovych, V.; Grün, F.; Zhou, C.; Welsh, W.J.; Blumberg, B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR). Environ. Health Perspect. 2004, 112, 163–169. [Google Scholar] [CrossRef]
- Zhou, X.; Kramer, J.P.; Calafat, A.M.; Ye, X. Automated on-line column-switching high perfor-mance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatog-Raphy B Anal. Technol. Biomed. Life Sci. 2014, 944, 152–156. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Woodward, M.; Bao, W.; Liu, B.; Trasande, L. Urinary Bisphenols and Obesity Prevalence among U.S. Children and Adolescents. J. Endocr. Soc. 2019, 3, 1715–1726. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef]
- Duan, Y.; Yao, Y.; Wang, B.; Han, L.; Wang, L.; Sun, H.; Chen, L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ. Pollut. 2018, 243, 1719–1726. [Google Scholar] [CrossRef]
- Song, S.; Duan, Y.; Zhang, T.; Zhang, B.; Zhao, Z.; Bai, X.; Xie, L.; He, Y.; Ouyang, J.-P.; Huang, X.; et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose. Ecotoxicol. Environ. Saf. 2018, 169, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Levine, D.; Wertenteil, S.; Vento, S.; Xue, J.; Rajendiran, K.; Kannan, K.; Thurman, J.M.; Morrison, D.; Brody, R.; et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res. 2017, 81, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Andújar, N.; Gálvez-Ontiveros, Y.; Zafra-Gómez, A.; Rodrigo, L.; Álvarez-Cubero, M.J.; Aguilera, M.; Monteagudo, C.; Rivas, A. Bisphenol A analogues in food and their hormonal and obesogenic effects: A review. Nutrients 2019, 11, 2136. [Google Scholar] [CrossRef] [PubMed]
- Le Fol, V.; Aït-Aïssa, S.; Cabaton, N.; Dolo, L.; Grimaldi, M.; Balaguer, P.; Perdu, E.; Debrauwer, L.; Brion, F.; Zalko, D. Cell-specific biotransformation of benzophenone-2 and bisphenol-s in zebrafish and human in vitro models used for toxicity and estrogenicity screening. Environ. Sci. Technol. 2015, 49, 3860–3868. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.; Grandin, F.C.; Collet, S.H.; Mila, H.; Viguié, C.; Gély, C.A.; Rabozzi, B.; Bouchard, M.; Léandri, R.; et al. Oral Systemic Bioavailability of Bisphenol A and Bisphenol S in Pigs. Environ. Health Perspect. 2019, 127, 077005. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.Z.; Gély, C.A.; Grandin, F.C.; Léandri, R.; Bouchard, M.; Roques, B.; Toutain, P.-L.; Picard-Hagen, N. Toxicokinetics of bisphenol S in rats for predicting human bisphenol S clearance from allometric scaling. Toxicol. Appl. Pharmacol. 2020, 386, 114845. [Google Scholar] [CrossRef]
- Khmiri, I.; Côté, J.; Mantha, M.; Khemiri, R.; Lacroix, M.; Gely, C.; Toutain, P.-L.; Picard-Hagen, N.; Gayrard, V.; Bouchard, M. Toxicokinetics of bisphenol-S and its glucuronide in plasma and urine following oral and dermal exposure in volunteers for the interpretation of biomonitoring data. Environ. Int. 2020, 138, 105644. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Ahn, Y.-A.; Baek, H.; Choi, M.; Park, J.; Son, S.J.; Seo, H.J.; Jung, J.; Seong, J.K.; Lee, J.; Kim, S. Adipogenic effects of prenatal exposure to bisphenol S (BPS) in adult F1 male mice. Sci. Total Environ. 2020, 728, 138759. [Google Scholar] [CrossRef]
- Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Ruiz-Ojeda, F.J.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem. Toxicol. 2021, 152, 112216. [Google Scholar] [CrossRef]
- Schaffert, A.; Krieg, L.; Weiner, J.; Schlichting, R.; Ueberham, E.; Karkossa, I.; Bauer, M.; Landgraf, K.; Junge, K.M.; Wabitsch, M.; et al. Alternatives for the worse: Molecular insights into adverse effects of bisphenol a and substitutes during human adipocyte differentiation. Environ. Int. 2021, 156, 106730. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.G.; Ahmed, S.; Atlas, E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 2016, 157, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Gingrich, J.D.; Steibel, J.P.; Veiga-Lopez, A. Sex-Specific Modulation of Fetal Adipogenesis by Gestational Bisphenol A and Bisphenol S Exposure. Endocrinology 2017, 158, 3844–3858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brulport, A.; Vaiman, D.; Chagnon, M.-C.; Le Corre, L. Obesogen effect of bisphenol S alters mRNA expression and DNA methylation profiling in male mouse liver. Chemosphere 2020, 241, 125092. [Google Scholar] [CrossRef]
- Del Moral, L.I.; Le Corre, L.; Poirier, H.; Niot, I.; Truntzer, T.; Merlin, J.-F.; Rouimi, P.; Besnard, P.; Rahmani, R.; Chagnon, M.-C. Obesogen effects after perinatal exposure of 4,4′-sulfonyldiphenol (Bisphenol S) in C57BL/6 mice. Toxicology 2016, 357–358, 11–20. [Google Scholar] [CrossRef]
- Gao, P.; Wand, L.; Yang, N.; Wen, J.; Zhao, M.; Su, G.; Zhang, J.; Weng, D. Peroxisome proliferator-activated receptor gamma (PPARγ) activation and metabolism disturbance induced by bisphenol A and its replacement analog bisphenol S using in vitro macrophages and in vivo mouse models. Environ. Int. 2020, 134, 105328. [Google Scholar] [CrossRef]
- Rezg, R.; Abot, A.; Mornagui, B.; Knauf, C. Bisphenol S exposure affects gene expression related to intestinal glucose absorption and glucose metabolism in mice. Environ. Sci. Pollut. Res. 2019, 26, 3636–3642. [Google Scholar] [CrossRef]
- Rezg, R.; Abot, A.; Mornagui, B.; Aydi, S.; Knauf, C. Effects of Bisphenol Son hypothalamic neuropeptides regulating feeding behavior and apelin/APJ system in mice. Ecotoxicol. Environ. Saf. 2018, 161, 459–466. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Muñoz, M.; dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.-A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, H.; Wei, P.; Jiang, G.; Wang, W.; Zhang, X.; Ru, S. Impairment of bisphenol F on the glucose metabolism of zebrafish larvae. Ecotoxicol. Environ. Saf. 2018, 165, 386–392. [Google Scholar] [CrossRef]
- Charisiadis, P.; Andrianou, X.D.; Van Der Meer, T.P.; Dunnen, W.F.A.D.; Swaab, D.F.; Wolffenbuttel, B.H.R.; Makris, K.C.; Van Vliet-Ostaptchouk, J.V. Possible Obesogenic Effects of Bisphenols Accumulation in the Human Brain. Sci. Rep. 2018, 8, 8186. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Wang, H.; Cayer, J.; Leroux, M.; Salvail, D.; Das, B.; Wrobel, J.E.; Mani, S. In Vivo and In Vitro Characterization of a First-in-Class Novel Azole Analog That Targets Pregnane X Receptor Activation. Mol. Pharmacol. 2011, 80, 124–135. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliviero, F.; Marmugi, A.; Viguié, C.; Gayrard, V.; Picard-Hagen, N.; Mselli-Lakhal, L. Are BPA Substitutes as Obesogenic as BPA? Int. J. Mol. Sci. 2022, 23, 4238. https://doi.org/10.3390/ijms23084238
Oliviero F, Marmugi A, Viguié C, Gayrard V, Picard-Hagen N, Mselli-Lakhal L. Are BPA Substitutes as Obesogenic as BPA? International Journal of Molecular Sciences. 2022; 23(8):4238. https://doi.org/10.3390/ijms23084238
Chicago/Turabian StyleOliviero, Fabiana, Alice Marmugi, Catherine Viguié, Véronique Gayrard, Nicole Picard-Hagen, and Laila Mselli-Lakhal. 2022. "Are BPA Substitutes as Obesogenic as BPA?" International Journal of Molecular Sciences 23, no. 8: 4238. https://doi.org/10.3390/ijms23084238
APA StyleOliviero, F., Marmugi, A., Viguié, C., Gayrard, V., Picard-Hagen, N., & Mselli-Lakhal, L. (2022). Are BPA Substitutes as Obesogenic as BPA? International Journal of Molecular Sciences, 23(8), 4238. https://doi.org/10.3390/ijms23084238





