Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology
Abstract
:1. Introduction
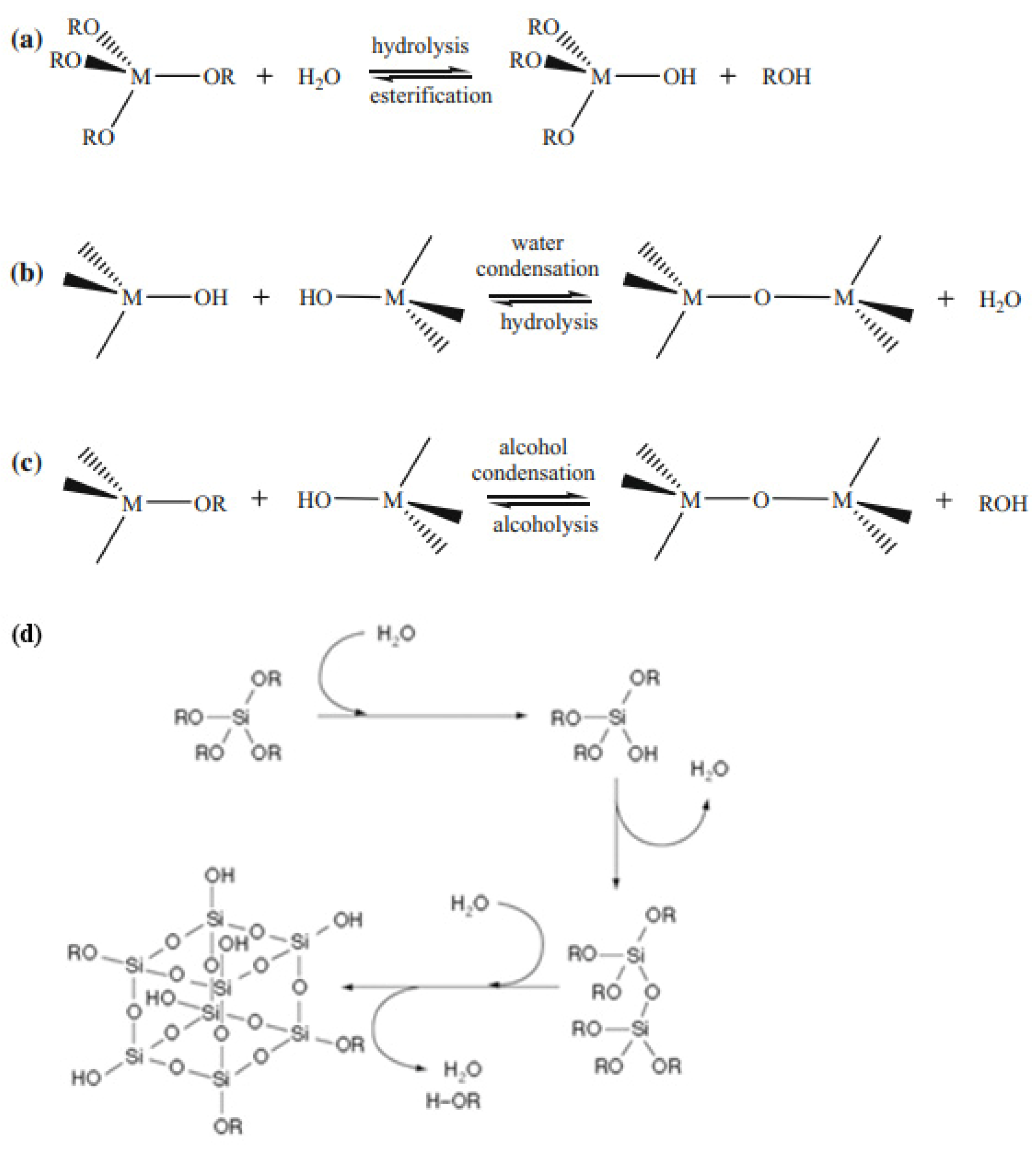
2. The Classification of Hybrid Materials According to the Type of Immobilized Cells
2.1. Material Formation Procedure Optimization
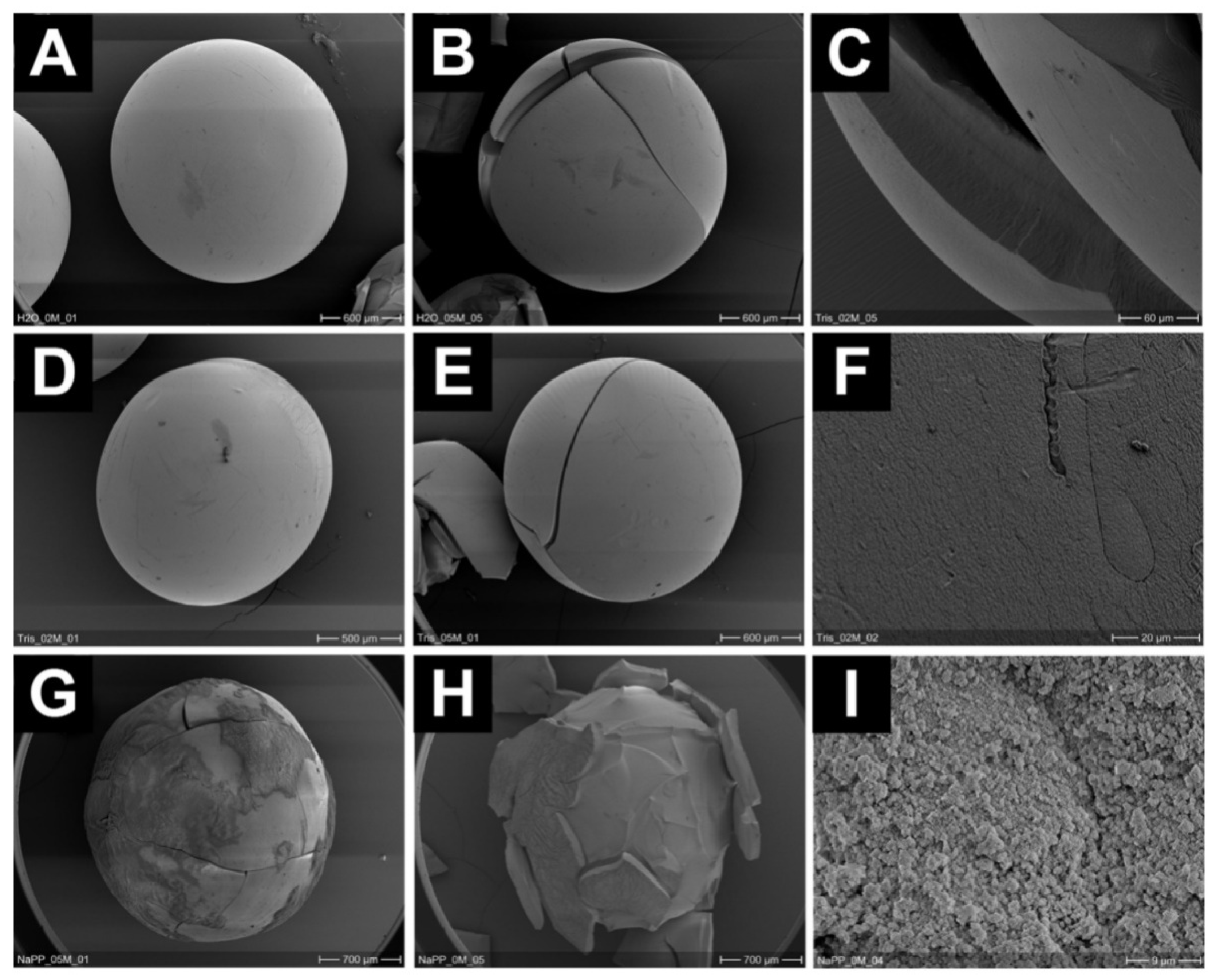
2.2. Immobilization of Bacteria by the Sol-Gel Method
2.3. Immobilization of Yeast Cells by the Sol-Gel Method
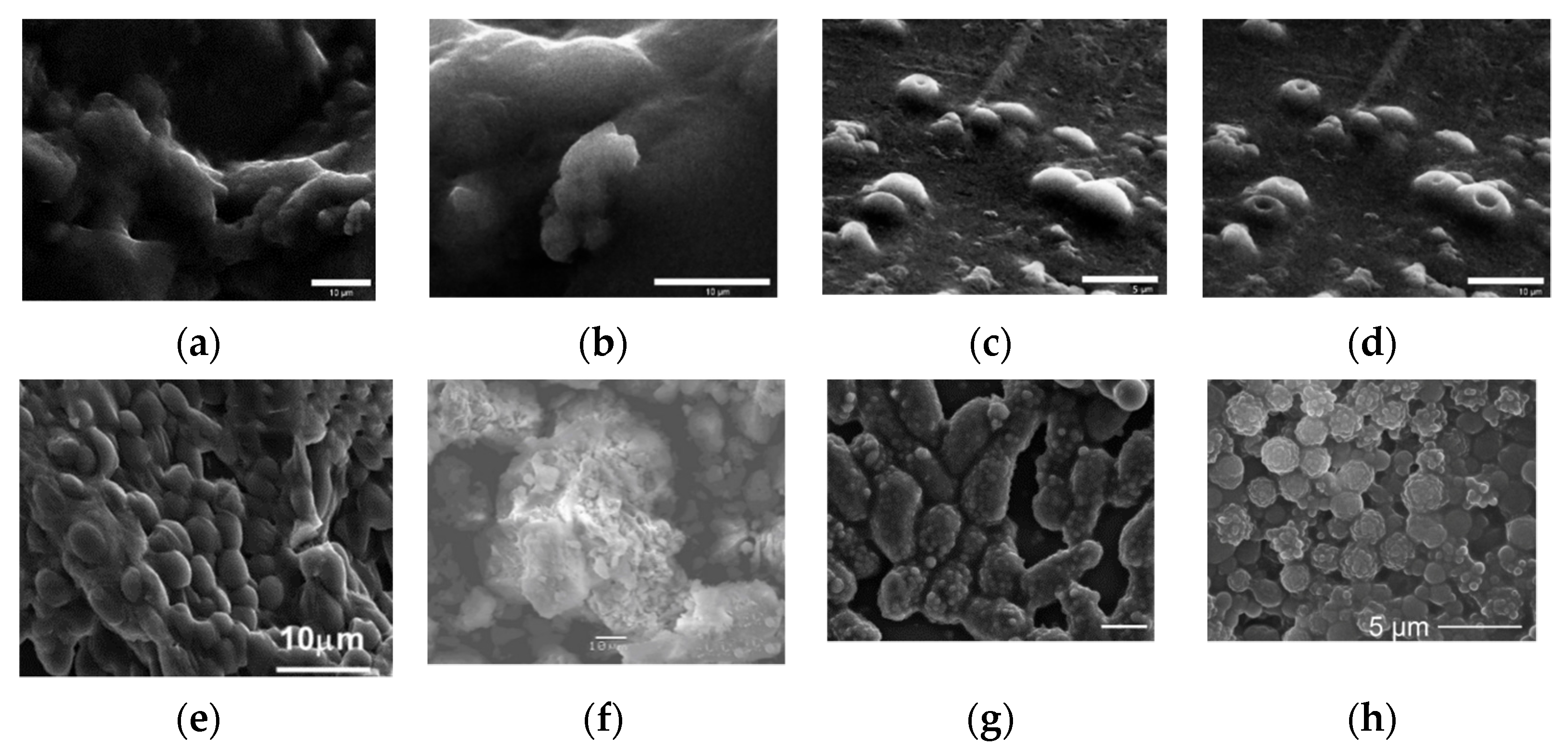
3. Classification of Hybrid Materials According to the Precursors Used in the Formation of the Sol-Gel Matrix
3.1. Silicon-Containing Precursors
3.2. Titanium-Containing Precursors
3.3. Aluminum-Containing Precursors
3.4. Cerium-Containing Precursors
4. Classification of Hybrid Materials According to the Type of Nanomaterial Obtained
4.1. Bioceramics
4.2. Thin Films
5. Classification of Hybrid Materials According to the Application of the Resulting Nanomaterial
5.1. In Ecology
5.2. In Medicine
5.3. For Batteries
5.4. As Templates
5.5. Application of Sol-Gel Hybrid Materials in Catalysis
6. Use of Organic Matter to Improve Microbial Viability
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ananikov, V.P. Organic–Inorganic Hybrid Nanomaterials. Nanomaterials 2019, 9, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máková, V.; Holubová, B.; Krabicová, I.; Kulhánková, J.; Řezanka, M. Hybrid Organosilane Fibrous Materials and Their Contribution to Modern Science. Polymer 2021, 228, 123862. [Google Scholar] [CrossRef]
- Romashov, L.V.; Ananikov, V.P. Self-Assembled Selenium Monolayers: From Nanotechnology to Materials Science and Adaptive Catalysis. Chem. (Weinh. Bergstr. Ger.) 2013, 19, 17640–17660. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.A.d.T.; Ladeira, Y.F.X.; França, A.d.S.; Souza, R.O.M.A.d.; Moraes, A.H.; Wojcieszak, R.; Itabaiana, I., Jr.; Miranda, A.S.d. Multicatalytic Hybrid Materials for Biocatalytic and Chemoenzymatic Cascades—Strategies for Multicatalyst (Enzyme) Co-Immobilization. Catalysts 2021, 11, 936. [Google Scholar] [CrossRef]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Prosa, M.; Bolognesi, M.; Fornasari, L.; Grasso, G.; Lopez-Sanchez, L.; Marabelli, F.; Toffanin, S. Nanostructured Organic/Hybrid Materials and Components in Miniaturized Optical and Chemical Sensors. Nanomaterials 2020, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Hayta, E.N.; Ertelt, M.J.; Kretschmer, M.; Lieleg, O. Bacterial Materials: Applications of Natural and Modified Biofilms. Adv. Mater. Interfaces 2021, 8, 2101024. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lim, J.; Kim, D.; Lee, T.; Choi, J.-W. Nanobiohybrid Material-Based Bioelectronic Devices. Biotechnol. J. 2020, 15, 1900347. [Google Scholar] [CrossRef]
- Palomo, J.M. Nanobiohybrids: A New Concept for Metal Nanoparticles Synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Richardson, J.J.; Kong, B.; Liang, K. Nanobiohybrids: Materials Approaches for Bioaugmentation. Sci. Adv. 2020, 6, eaaz0330. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent Advances and Future Perspectives of Sol–Gel Derived Porous Bioactive Glasses: A Review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Prasad, T.; Halder, S.; Dhar, S.S. Process Parameter Effects on Particle Size Reduction of Sol-Gel Synthesized Silica Nanoparticles. Mater. Today Proc. 2020, 22, 1669–1675. [Google Scholar] [CrossRef]
- Benson, J.J.; Sakkos, J.K.; Radian, A.; Wackett, L.P.; Aksan, A. Enhanced Biodegradation of Atrazine by Bacteria Encapsulated inorganically Modified Silica Gels. J. Colloid Interface Sci. 2018, 510, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.N.W. Sol--Gel Technology for Innovative Fabric Finishing—A Review. J. Sol-Gel Sci. Technol. 2016, 78, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Bhattacharyya, S.; Ducheyne, P. 5.10 Encapsulation of Cells (Cellular Delivery) Using Sol–Gel Silica. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 175–186. ISBN 978-0-08-100692-4. [Google Scholar]
- Grandi, S.; Tomasi, C.; Mustarelli, P.; Clemente, F.; Carbonaro, C.M. Characterisation of a New Sol-Gel Precursor for a SiO2-Rhodamine 6G Hybrid Class II Material. J. Sol-Gel Sci. Technol. 2007, 41, 57–63. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Nedelec, J.-M.; Seisenbaeva, G.A.; Kessler, V.G. Controlling Nucleation and Growth of Nano-CaCO3 via CO2 Sequestration by a Calcium Alkoxide Solution to Produce Nanocomposites for Drug Delivery Applications. Acta Biomater. 2017, 57, 426–434. [Google Scholar] [CrossRef]
- Chouler, J.; Di Lorenzo, M. Water Quality Monitoring in Developing Countries; Can Microbial Fuel Cells Be the Answer? Biosensors 2015, 5, 450–470. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-P.; Lin, Y.-S. Sol–Gel-Immobilized Recombinant E. coli for Biosorption of Cd2+. J. Chin. Inst. Chem. Eng. 2007, 38, 235–243. [Google Scholar] [CrossRef]
- Ciriminna, R.; Ilharco, L.M.; Fidalgo, A.; Campestrini, S.; Pagliaro, M. The Structural Origins of Superior Performance in Sol–Gel Catalysts. Soft Matter 2005, 1, 231–237. [Google Scholar] [CrossRef]
- Pannier, A.; Mkandawire, M.; Soltmann, U.; Pompe, W.; Böttcher, H. Biological Activity and Mechanical Stability of Sol–Gel-Based Biofilters Using the Freeze-Gelation Technique for Immobilization of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2012, 93, 1755–1767. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Mas Ayu, H.; Redzuan, N.; Izman, S. Research Progress of Sol-Gel Ceramic Coating: A Review. Mater. Today Proc. 2021, 48, 1849–1854. [Google Scholar] [CrossRef]
- Blondeau, M.; Coradin, T. Living Materials from Sol-Gel Chemistry: Current Challenges and Perspectives. J. Mater. Chem. 2012, 22, 22335–22343. [Google Scholar] [CrossRef]
- Subasri, R.; Soma Raju, K.R.C.; Samba Sivudu, K. 12—Applications of Sol–Gel Coatings: Past, Present, and Future. In Handbook of Modern Coating Technologies; Aliofkhazraei, M., Ali, N., Chipara, M., Bensaada Laidani, N., De Hosson, J.T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 425–451. ISBN 978-0-444-63237-1. [Google Scholar]
- Lavrova, D.G.; Kamanina, O.A.; Alferov, V.A.; Rybochkin, P.V.; Machulin, A.V.; Sidorov, A.I.; Ponamoreva, O.N. Impact of Hydrophilic Polymers in Organosilica Matrices on Structure, Stability, and Biocatalytic Activity of Immobilized Methylotrophic Yeast Used as Biofilter Bed. Enzym. Microb. Technol. 2021, 150, 109879. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.; Ely, R. Silica Sol-Gel Encapsulation of Cyanobacteria: Lessons for Academic and Applied Research. Appl. Microbiol. Biotechnol. 2013, 97, 1809–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, M.L.; Garcia-Carvajal, Z.Y.; Yuste, L.; Rojo, F.; del Monte, F. Bacteria Viability in Sol−Gel Materials Revisited: Cryo-SEM as a Suitable Tool To Study the Structural Integrity of Encapsulated Bacteria. Chem. Mater. 2006, 18, 1458–1463. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, H.; Tran-Minh, C. Sol–Gel Process for Vegetal Cell Encapsulation. Mater. Sci. Eng. C 2007, 27, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Soltmann, U.; Böttcher, H. Utilization of Sol–Gel Ceramics for the Immobilization of Living Microorganisms. J. Sol-Gel Sci. Technol. 2008, 48, 66–72. [Google Scholar] [CrossRef]
- Amoura, M.; Brayner, R.; Perullini, M.; Sicard, C.; Roux, C.; Livage, J.; Coradin, T. Bacteria Encapsulation in a Magnetic Sol–Gel Matrix. J. Mater. Chem. 2009, 19, 1241–1244. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lee, C.-Y.; Chiu, H.-T. Porous Inorganic Materials from Living Porogens: Channel-like TiO2 from Yeast-Assisted Sol–Gel Process. ACS Appl. Mater. Interfaces 2014, 6, 31–35. [Google Scholar] [CrossRef]
- Eleftheriou, N.M.; Ge, X.; Kolesnik, J.; Falconer, S.B.; Harris, R.J.; Khursigara, C.; Brown, E.D.; Brennan, J.D. Entrapment of Living Bacterial Cells in Low-Concentration Silica Materials Preserves Cell Division and Promoter Regulation. Chem. Mater. 2013, 25, 4798–4805. [Google Scholar] [CrossRef]
- Yulizar, Y.; Juliyanto, S.; Sudirman; Apriandanu, D.O.B.; Surya, R.M. Novel Sol-Gel Synthesis of CeO2 Nanoparticles Using Morinda Citrifolia L. Fruit Extracts: Structural and Optical Analysis. J. Mol. Struct. 2021, 1231, 129904. [Google Scholar] [CrossRef]
- Harper, J.C.; Lopez, D.M.; Larkin, E.C.; Economides, M.K.; McIntyre, S.K.; Alam, T.M.; Tartis, M.S.; Werner-Washburne, M.; Brinker, C.J.; Brozik, S.M.; et al. Encapsulation of S. cerevisiae in Poly(Glycerol) Silicate Derived Matrices: Effect of Matrix Additives and Cell Metabolic Phase on Long-Term Viability and Rate of Gene Expression. Chem. Mater. 2011, 23, 2555–2564. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.S.; Rigit, A.R.H.; Bhawani, S.A. Recent Developments in Immobilizing Titanium Dioxide on Supports for Degradation of Organic Pollutants in Wastewater—A Review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Amoura, M.; Nassif, N.; Roux, C.; Livage, J.; Coradin, T. Sol–Gel Encapsulation of Cells Is Not Limited to Silica: Long-Term Viability of Bacteria in Alumina Matrices. Chem. Commun. 2007, 39, 4015–4017. [Google Scholar] [CrossRef] [PubMed]
- Kessler, V.G.; Seisenbaeva, G.A.; Unell, M.; Håkansson, S. Chemically Triggered Biodelivery Using Metal-Organic Sol-Gel Synthesis. Angew. Chem. (Int. Ed. Engl.) 2008, 47, 8506–8509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Su, J.; Lv, X.-Y.; Long, Y.-F.; Wen, Y.-X. Yeast Bio-Template Synthesis of Porous Anatase TiO2 and Potential Application as an Anode for Sodium-Ion Batteries. Electrochim. Acta 2015, 182, 596–603. [Google Scholar] [CrossRef]
- Sicard, C.; Perullini, M.; Spedalieri, C.; Coradin, T.; Brayner, R.; Livage, J.; Jobbagy, M.; Bilmes, S.A. CeO2 Nanoparticles for the Protection of Photosynthetic Organisms in Silica Gels. Chem. Mater. 2011, 23, 1374–1378. [Google Scholar] [CrossRef]
- Samuneva, B.; Kabaivanova, L.; Chernev, G.; Djambaski, P.; Kashchieva, E.; Emanuilova, E.; Salvado, I.M.M.; Fernandes, M.H.V.; Wu, A.; Miranda Salvado, I.M.; et al. Sol–Gel Synthesis and Structure of Silica Hybrid Materials. J. Sol-Gel Sci. Technol. 2008, 48, 73–79. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Podsevalova, E.; Ponamoreva, O. Application of Organosilicate Matrix Based on Methyltriethoxysilane, PVA and Bacteria Paracoccus Yeei to Create a Highly Sensitive BOD. 3 Biotech 2021, 11, 331. [Google Scholar] [CrossRef]
- Bagheri Lotfabad, T.; Ebadipour, N.; Roostaazad, R.; Partovi, M.; Bahmaei, M. Two Schemes for Production of Biosurfactant from Pseudomonas Aeruginosa MR01: Applying Residues from Soybean Oil Industry and Silica Sol–Gel Immobilized Cells. Colloids Surf. B Biointerfaces 2017, 152, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttolomondo, M.V.; Alvarez, G.S.; Desimone, M.F.; Diaz, L.E. Removal of Azo Dyes from Water by Sol–Gel Immobilized Pseudomonas Sp. J. Environ. Chem. Eng. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Sakkos, J.K.; Mutlu, B.R.; Wackett, L.P.; Aksan, A. Adsorption and Biodegradation of Aromatic Chemicals by Bacteria Encapsulated in a Hydrophobic Silica Gel. ACS Appl. Mater. Interfaces 2017, 9, 26848–26858. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, S.; Melo, J.S.; Sen, D.; Bahadur, J. Enhancement in β-Galactosidase Activity of Streptococcus Lactis Cells by Entrapping in Microcapsules Comprising of Correlated Silica Nanoparticles. Colloids Surf. B Biointerfaces 2020, 195, 111245. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, I.V.N.; Megharaj, M.; Naidu, R. Green Fluorescent Protein Based Whole Cell Bacterial Biosensor for the Detection of Bioavailable Heavy Metals in Soil Environment. Environ. Technol. Innov. 2021, 23, 101785. [Google Scholar] [CrossRef]
- Nassif, N.; Bouvet, O.; Noelle Rager, M.; Roux, C.; Coradin, T.; Livage, J. Living Bacteria in Silica Gels. Nat. Mater. 2002, 1, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.; Roux, C.; Coradin, T.; Rager, M.-N.; Bouvet, O.M.M.; Livage, J. A Sol-Gel Matrix to Preserve the Viability of Encapsulated Bacteria. J. Mater. Chem. 2003, 13, 203–208. [Google Scholar] [CrossRef]
- Kim, K.O.; Lim, S.Y.; Hahn, G.H.; Lee, S.H.; Park, C.B.; Kim, D.M. Cell-Free Synthesis of Functional Proteins Using Transcription/Translation Machinery Entrapped in Silica Sol-Gel Matrix. Biotechnol. Bioeng. 2009, 102, 303–307. [Google Scholar] [CrossRef]
- Molnar, Z.; Farkas, E.; Lako, A.; Erdelyi, B.; Kroutil, W.; Vertessy, B.G.; Paizs, C.; Poppe, L. Immobilized Whole-Cell Transaminase Biocatalysts for Continuous-Flow Resolution of Amines. Catalysts 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Biocompatible Sol−Gel Route for Encapsulation of Living Bacteria in Organically Modified Silica Matrixes. Chem. Mater. 2003, 15, 3614–3618. [Google Scholar] [CrossRef]
- Alvarez, G.S.; Pieckenstain, F.L.; Desimone, M.F.; Estrella, M.J.; Ruiz, O.A.; Díaz, L.E. Evaluation of Sol-Gel Silica Matrices as Inoculant Carriers for Mesorhizobium Spp. Cells. In Current Reseach, Technology and Education Topics in Applied Microbiology and Microbioal Biotechnology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2010; pp. 160–166. [Google Scholar]
- Zhou, H.; Fan, T.; Ding, J.; Zhang, D.; Guo, Q. Bacteria-Directed Construction of Hollow TiO2 Micro/Nanostructures with Enhanced Photocatalytic Hydrogen Evolution Activity. Opt. Express 2012, 20 (Suppl. 2), A340–A350. [Google Scholar] [CrossRef] [PubMed]
- Boettcher; Soltmann, U.; Mertig, M. Biocers: Ceramics with Incorporated Microorganisms for Biocatalytic, Biosorptive and Functional Materials Development. Mater. Chem. 2004, 14, 2176–2188. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S. An Optical Microplate Biosensor for the Detection of Methyl Parathion Pesticide Using a Biohybrid of Sphingomonas Sp. Cells-Silica Nanoparticles. Biosens. Bioelectron. 2017, 87, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Jaroch, D.; McLamore, E.; Zhang, W.; Shi, J.; Garland, J.; Banks, M.K.; Porterfield, D.M.; Rickus, J.L. Cell-Mediated Deposition of Porous Silica on Bacterial Biofilms. Biotechnol. Bioeng. 2011, 108, 2249–2260. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Xin, J.; Li, S.; Xia, C.; Cui, J. Efficient Immobilization of Whole Cells of Methylomonas Sp. Strain GYJ3 by Sol–Gel Entrapment. J. Mol. Catal. B Enzym. 2004, 30, 167–172. [Google Scholar] [CrossRef]
- Carturan, G.; Campostrini, R.; Dire, S.; Scardi, V.; De Alteriis, E. Inorganic Gels for Immobilization of Biocatalysts: Inclusion of Invertase-Active Whole Cells of Yeast (Saccharomyces cerevisiae) into Thin Layers of Silica Gel Deposited on Glass Sheets. J. Mol. Catal. 1989, 57, 6–13. [Google Scholar] [CrossRef]
- Inama, L.; Diré, S.; Carturan, G.; Cavazza, A. Entrapment of Viable Microorganisms by SiO2 Sol-Gel Layers on Glass Surfaces: Trapping, Catalytic Performance and Immobilization Durability of Saccharomyces cerevisiae. J. Biotechnol. 1989, 30, 197–200. [Google Scholar] [CrossRef]
- Podrazky, O.; Ripp, S.; Trogl, J.; Sayler, G.; Demnerova, K.; Vankova, R. Monitoring the Viability of Cells Immobilized by the Sol-Gel Process. J. Sol-Gel. Sci. Technol. 2004, 31, 335–342. [Google Scholar]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent Bio-Applications of Sol-Gel Materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Kato, K.; Nakamura, H.; Nakanishi, K. Asymmetric Bioreduction of Acetophenones by Baker’s Yeast and Its-Free Extract Encapsulated in Sol-Gel Silica Materials. Appl. Surf. Sci. 2014, 293, 312–317. [Google Scholar] [CrossRef]
- Shukla, P.; Mishra, A.; Manivannan, S.; Melo, J.S.; Mandal, D. Parametric Optimization for Adsorption of Mercury (II) Using Self Assembled Bio-Hybrid. J. Environ. Chem. Eng. 2020, 8, 103725. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liu, G.; Cheng, X.; Chi, Z.; Madzak, C.; Liu, C.; Chi, Z. Genetical Surface Display of Silicatein on Yarrowia Lipolytica Confers Living and Renewable Biosilica-Yeast Hybrid Materials. ACS Omega 2020, 5, 7555–7566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shang, L.; Guo, S.; Li, D.; Liu, C.; Qi, L.; Dong, S. Organic-Inorganic Hybrid Material for the Cells Immobilization: Long-Term Viability Mechanism and Application in BOD Sensors. Biosens. Bioelectron. 2009, 25, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Gyor, L.; Farkas, E.; Lacatus, M.; Toth Gergoand Incze, D.; Hornyanszky, G.; Bodai, V.; Paizs, C.; Poppe, L.; Balogh-Weiser, D. Conservation of the Biocatalytic Activity of Whole Yeast Cells by Supported Sol—Gel Entrapment for Efficient Acyloin Condensation. Period. Polytech. Chem. Eng. 2020, 64, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, D.; Hager, U.; Franke, H.; Soltmann, U.; Bottcher, H. Algae Biocers: Astaxanthin Formation in Sol-Gel Immobilised Living Microalgae. J. Mater. Chem. 2007, 17, 261–266. [Google Scholar] [CrossRef]
- Trögl, J.; Jirková, I.; Kuráň, P.; Akhmetshina, E.; Brovdyová, T.; Sirotkin, A.; Kirilina, T. Phospholipid Fatty Acids as Physiological Indicators of Paracoccus Denitrificans Encapsulated in Silica Sol-Gel Hydrogels. Sensors 2015, 15, 3426–3434. [Google Scholar] [CrossRef] [Green Version]
- Varshney, S.; Nigam, A.; Pawar, S.J.; Mishra, N. Structural, Optical, Cytotoxic, and Anti-Microbial Properties of Amorphous Silica Nanoparticles Synthesised via Hybrid Method for Biomedical Applications. Mater. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Pannier, A.; Oehm, C.; Fischer, A.R.; Werner, P.; Soltmann, U.; Böttcher, H. Biodegradation of Fuel Oxygenates by Sol–Gel Immobilized Bacteria Aquincola Tertiaricarbonis L108. Enzym. Microb. Technol. 2010, 47, 291–296. [Google Scholar] [CrossRef]
- Djambaski, P.; Aleksieva, P.; Emanuilova, E.; Chernev, G.; Spasova, D.; Nacheva, L.; Kabaivanova, L.; Salvado, I.M.M.; Samuneva, B. Sol-Gel Nanomaterials with Algal Heteropolysaccharide for Immobilization of Microbial Cells, Producing A-Galactosidase and Nitrilase. Biotechnol. Biotechnol. Equip. 2009, 23, 1270–1274. [Google Scholar] [CrossRef] [Green Version]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD Using Paracoccus Yeei Bacteria Isolated from Activated Sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, A. Molecular Imprinting in Sol-Gel Matrix. Biotechnol. Adv. 2008, 26, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Irani, M.; Keshtkar, A.R.; Moosavian, M.A. Removal of Cadmium from Aqueous Solution Using Mesoporous PVA/TEOS/APTES Composite Nanofiber Prepared by Sol–Gel/Electrospinning. Chem. Eng. J. 2012, 200–202, 192–201. [Google Scholar] [CrossRef]
- Pereira, A.P.V.; Vasconcelos, W.L.; Oréfice, R.L. Novel Multicomponent Silicate–Poly(Vinyl Alcohol) Hybrids with Controlled Reactivity. J. Non-Cryst. Solids 2000, 273, 180–185. [Google Scholar] [CrossRef]
- Pérez-Moreno, A.; Reyes-Peces, M.V.; Vilches-Pérez, J.I.; Fernández-Montesinos, R.; Pinaglia-Tobaruela, G.; Salido, M.; de la Rosa-Fox, N.; Piñero, M. Effect of Washing Treatment on the Textural Properties and Bioactivity of Silica/Chitosan/TCP Xerogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 8321. [Google Scholar] [CrossRef]
- Song, J.-H.; Yoon, B.-H.; Kim, H.-E.; Kim, H.-W. Bioactive and Degradable Hybridized Nanofibers of Gelatin-Siloxane for Bone Regeneration. J. Biomed. Mater. Res. Part A 2008, 84, 875–884. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Kharkova, A.S.; Kurbanaliyeva, S.K.; Kuznetsova, L.S.; Machulin, A.V.; Tarasov, S.E.; Melnikov, P.V.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. Use of Biocompatible Redox-Active Polymers Based on Carbon Nanotubes and Modified Organic Matrices for Development of a Highly Sensitive BOD Biosensor. Enzym. Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Influence of the Polymer Amount on Bioactivity and Biocompatibility of SiO2/PEG Hybrid Materials Synthesized by Sol-Gel Technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 548–555. [Google Scholar] [CrossRef]
- Homburg, S.V.; Kruse, O.; Patel, A.V. Growth and Photosynthetic Activity of Chlamydomonas Reinhardtii in Lens-Shaped Silica Hydrogels. J. Biotechnol. 2019, 302, 58–66. [Google Scholar] [CrossRef]
- Kambala, V.S.R.; Naidu, R. Disinfection Studies on TiO2 Thin Films Prepared by a Sol-Gel Method. J. Biomed. Nanotechnol. 2009, 5, 121–129. [Google Scholar] [CrossRef]
- Leroux, G.; Neumann, M.; Meunier, C.F.; Fattaccioli, A.; Michiels, C.; Arnould, T.; Wang, L.; Su, B.-L. Hybrid Alginate@TiO2 Porous Microcapsules as a Reservoir of Animal Cells for Cell Therapy. ACS Appl. Mater. Interfaces 2018, 10, 37865–37877. [Google Scholar] [CrossRef] [PubMed]
- Leroux, G.; Neumann, M.; Meunier, C.F.; Voisin, V.; Habsch, I.; Caron, N.; Michiels, C.; Wang, L.; Su, B.-L. Alginate@TiO2 Hybrid Microcapsules with High in Vivo Biocompatibility and Stability for Cell Therapy. Colloids Surf. B Biointerfaces 2021, 203, 111770. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, J.; Zhang, P.; Chai, Z. Speciation and Subcellular Location of Se-Containing Proteins in Human Liver Studied by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Hydride Generation-Atomic Fluorescence Spectrometric Detection. Anal. Bioanal. Chem. 2002, 372, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Hsiao, C.-H.; Hsieh, P.-L.; Huang, X.-F.; Huang, M.H. Growth of CeO2 Nanocubes Showing Size-Dependent Optical and Oxygen Evolution Reaction Behaviors. Dalton Trans. 2021, 50, 15170–15175. [Google Scholar] [CrossRef]
- Wan Mohd Zawawi, W.N.I.; Mansor, A.F.; Othman, N.S.; Mohidem, N.A.; Nik Malek, N.A.N.; Mat, H. Synthesis and Characterization of Immobilized White-Rot Fungus Trametes Versicolor in Sol–Gel Ceramics. J. Sol-Gel Sci. Technol. 2016, 77, 28–38. [Google Scholar] [CrossRef]
- Soltmann, U.; Matys, S.; Kieszig, G.; Pompe, W.; Boettcher, H. Algae-Silica Hybrid Materials for Biosorption of Heavy Metals. J. Water Resour. Prot. 2010, 2, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Nandy, P.; Basu, R.; Das, S. Development of nano mullite based mesoporous silica biocer with incorporated bacteria for arsenic remediation. Ceram. Silik. 2016, 60, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Dickson, D.J.; Luterra, M.D.; Ely, R.L. Transcriptomic Responses of Synechocystis Sp. PCC 6803 Encapsulated in Silica Gel. Appl. Microbiol. Biotechnol. 2012, 96, 183–196. [Google Scholar] [CrossRef]
- Ritenberg, M.; Kolusheva, S.; Ganin, H.; Meijler, M.; Jelinek, R. Biofilm Formation on Chromatic Sol–Gel/Polydiacetylene Films. ChemPlusChem 2012, 77, 752–757. [Google Scholar] [CrossRef]
- Ghach, W.; Etienne, M.; Urbanova, V.; Jorand, F.P.A.; Walcarius, A. Sol–Gel Based ‘Artificial’ Biofilm from Pseudomonas Fluorescens Using Bovine Heart Cytochrome c as Electron Mediator. Electrochem. Commun. 2014, 38, 71–74. [Google Scholar] [CrossRef]
- Chernev, G.; Christova, N.; Kabaivanova, L.; Nacheva, L. New Approach for N-Hexadecane Biodegradation by Sol-Gel Entrapped Bacterial Cells. Ecol. Chem. Eng. S 2018, 25, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Fazal, Z.; Pelowitz, J.; Johnson, P.E.; Harper, J.C.; Brinker, C.J.; Jakobsson, E. Three-Dimensional Encapsulation of Saccharomyces Cerevisiae in Silicate Matrices Creates Distinct Metabolic States as Revealed by Gene Chip Analysis. ACS Nano 2017, 11, 3560–3575. [Google Scholar] [CrossRef] [PubMed]
- Pannier, A.; Soltmann, U.; Soltmann, B.; Altenburger, R.; Schmitt-Jansen, M. Alginate/Silica Hybrid Materials for Immobilization of Green Microalgae Chlorella Vulgaris for Cell-Based Sensor Arrays. J. Mater. Chem. B 2014, 2, 7896–7909. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, I.V.N.; Munagamage, T.; Pathirathne, A.; Megharaj, M. Whole Cell Microalgal-Cyanobacterial Array Biosensor for Monitoring Cd, Cr and Zn in Aquatic Systems. Water Sci. Technol. 2021, 84, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Habib, O.; Demirkol, D.O.; Timur, S. Sol–Gel/Chitosan/Gold Nanoparticle-Modified Electrode in Mediated Bacterial Biosensor. Food Anal. Methods 2012, 5, 188–194. [Google Scholar] [CrossRef]
- Ghach, W.; Etienne, M.; Billard, P.; Jorand, F.P.A.; Walcarius, A. Electrochemically Assisted Bacteria Encapsulation in Thin Hybrid Sol-Gel Films. J. Mater. Chem. B 2013, 1, 1052–1059. [Google Scholar] [CrossRef]
- Depagne, C.; Masse, S.; Link, T.; Coradin, T. Bacteria Survival and Growth in Multi-Layered Silica Thin Films. J. Mater. Chem. 2012, 22, 12457–12460. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Kamanina, O.A.; Alferov, V.A.; Machulin, A.V.; Rogova, T.V.; Arlyapov, V.A.; Alferov, S.V.; Suzina, N.E.; Ivanova, E.P. Yeast-Based Self-Organized Hybrid Bio-Silica Sol–Gels for the Design of Biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Afonina, E.L.; Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Boronin, A.M.; Arliapov, V.; Alferov, V.A.; Boronin, A.M. Yeast Debaryomyces Hansenii within ORMOSIL Shells as a Heterogeneous Biocatalyst. Appl. Biochem. Microbiol. 2018, 54, 736–742. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H. Application of Non-Ionic Surfactant in the Microwave-Assisted Extraction of Alkaloids from Rhizoma Coptidis. Anal. Chim. Acta 2008, 612, 160–164. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, L.L.; Huang, S.; Zhao, L.; Cui, J.S.; Wang, X.H.; Chen, X. Novel BOD Optical Fiber Biosensor Based on Co-Immobilized microorganisms in Ormosils Matrix. Biosens. Bioelectron. 2006, 21, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tang, M.; Chen, X.; Qi, L.; Dong, S. Co-Immobilized Microbial Biosensor for BOD Estimation Based on Sol–Gel Derived Composite Material. Biosens. Bioelectron. 2003, 18, 1023–1029. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Identification of NAD-Dependent Xylitol Dehydrogenase from Gluconobacter Oxydans WSH-003. ACS Omega 2019, 4, 15074–15080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy-Győr, L.; Abaházi, E.; Bódai, V.; Sátorhelyi, P.; Erdélyi, B.; Balogh-Weiser, D.; Paizs, C.; Hornyánszky, G.; Poppe, L. Co-Immobilized Whole Cells with ω-Transaminase and Ketoreductase Activities for Continuous-Flow Cascade Reactions. ChemBioChem 2018, 19, 1845–1848. [Google Scholar] [CrossRef]
- Nagy-Gyor, L.; Lacatus, M.; Balogh-Weiser, D.; Csuka, P.; Bodai, V.; Erdelyi, B.; Molnar, Z.; Hornyanszky, G.; Paizs, C.; Poppe, L.Y. How to Turn Yeast Cells into a Sustainable and Switchable Biocatalyst? On-Demand Catalysis of Ketone Bioreduction or Acyloin Condensation. Acs Sustain. Chem. Eng. 2019, 7, 19375–19383. [Google Scholar] [CrossRef] [Green Version]
- Seisenbaeva, G.A.; Kessler, V.G. Chapter 3—Silica and Titania Nanoadsorbents for Application in Molecular Recognition Technology. In Biocompatible Hybrid Oxide Nanoparticles for Human Health; Melnyk, I.V., Vaclavikova, M., Seisenbaeva, G.A., Kessler, V.G., Eds.; Micro and Nano Technologies; Elsevier: New York, NY, USA, 2019; pp. 33–49. ISBN 978-0-12-815875-3. [Google Scholar]
- Pogorilyi, R.P.; Melnyk, I.V.; Zub, Y.L.; Seisenbaeva, G.A.; Kessler, V.G. Enzyme Immobilization on a Nanoadsorbent for Improved Stability against Heavy Metal Poisoning. Colloids Surf. B Biointerfaces 2016, 144, 135–142. [Google Scholar] [CrossRef]
- Haroun, A.A.; Elnahrawy, A.M.; Abd-Alla, H.I. Sol-Gel Preparation and in Vitro Cytotoxic Activity of Nanohybrid Structures Based on Multi-Walled Carbon Nanotubes and Silicate. Inorg. Nano-Met. Chem. 2017, 47, 1023–1027. [Google Scholar] [CrossRef]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-Based Systems for Oral Delivery of Drugs, Macromolecules and Cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef]
- Biswas, A.; Mukhopadhyay, S.; Singh, R.S.; Kumar, A.; Rana, N.K.; Koch, B.; Pandey, D.S. Manipulating Metallogel Properties by Luminogens and Their Applications in Cell Imaging. ACS Omega 2018, 3, 5417–5425. [Google Scholar] [CrossRef]
- dos Santos, C.; Vargas, A.; Fronza, N.; Zimnoch dos Santos, J.H. Structural, Textural and Morphological Characteristics of Tannins fromAcacia Mearnsii Encapsulated Using Sol-Gel Methods: Applications asantimicrobial Agents. Colloids Surf. B-Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y.; Hatton, B.D. Drug Self-Assembly for Synthesis of Highly-Loaded Antimicrobial Drug-Silica Particles. Sci. Rep. 2018, 8, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Q.; Luo, D.; Li, X.; Zhang, Z.; Yang, L.; Hirano, S. Elaborately Prepared Hierarchical Structure Titanium Dioxide for Remarkable Performance in Lithium Storage. J. Power Sources 2016, 313, 189–197. [Google Scholar] [CrossRef]
- Weng, Z.; Guo, H.; Liu, X.; Wu, S.; Yeung, K.W.K.; Chu, P.K. Nanostructured TiO2 for Energy Conversion and Storage. RSC Adv. 2013, 3, 24758–24775. [Google Scholar] [CrossRef]
- Fireman-Shoresh, S.; Turyan, I.; Mandler, D.; Avnir, D.; Marx, S. Chiral Electrochemical Recognition by Very Thin Molecularly Imprinted Sol−Gel Films. Langmuir 2005, 21, 7842–7847. [Google Scholar] [CrossRef]
- Diab, M.; Shreteh, K.; Volokh, M.; Abramovich, S.; Abdu, U.; Mokari, T. Calcareous Foraminiferal Shells as a Template for the Formation of Hierarchal Structures of Inorganic Nanomaterials. ACS Appl. Mater. Interfaces 2019, 11, 6456–6462. [Google Scholar] [CrossRef]
- Cohen, T.; Starosvetsky, J.; Cheruti, U.; Armon, R. Whole Cell Imprinting in Sol-Gel Thin Films for Bacterial Recognition in Liquids: Macromolecular Fingerprinting. Int. J. Mol. Sci. 2010, 11, 1236–1252. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, E.F.; Awad, G. Hollow N-TiO2/MnO2 Nanocomposite Based Yeast Biomass for Gaseous Formaldehyde Degradation under Visible Light. J. Ind. Eng. Chem. 2021, 98, 366–374. [Google Scholar] [CrossRef]
- Du, X.; Qiao, S.Z. Dendritic Silica Particles with Center-Radial Pore Channels: Promising Platforms for Catalysis and Biomedical Applications. Small (Weinh. Der Bergstr. Ger.) 2015, 11, 392–413. [Google Scholar] [CrossRef]
- Ciriminna, R.; Ilharco, L.M.; Pandarus, V.; Fidalgo, A.; Béland, F.; Pagliaro, M. Towards Waste Free Organic Synthesis Using Nanostructured Hybrid Silicas. Nanoscale 2014, 6, 6293–6300. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. The Chemical Effects of Molecular Sol–Gel Entrapment. Chem. Soc. Rev. 2007, 36, 932–940. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.N.; Lavrova, D.G.G.; Kamanina, O.A.A.; Rybochkin, P.V.V.; Machulin, A.V.V.; Alferov, V.A.A. Biohybrid of Methylotrophic Yeast and Organically Modified Silica Gels from Sol-Gel Chemistry of Tetraethoxysilane and Dimethyldiethoxysilane. J. Sol-Gel Sci. Technol. 2019, 92, 359–366. [Google Scholar] [CrossRef]
- Wang, X.; Ahmed, N.B.; Alvarez, G.S.; Tuttolomondo, M.V.; Hélary, C.; Desimone, M.F.; Coradin, T. Sol-Gel Encapsulation of Biomolecules and Cells for Medicinal Applications. Curr. Top. Med. Chem. 2015, 15, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Ulfa, M.; Aristia, K.S.; Prasetyoko, D. Synthesis of Mesoporous Silica Materials via Dual Templating Method from Starch of Waste Rice and Their Application for Drug Delivery System. AIP Conf. Proc. 2018, 2049, 020002. [Google Scholar] [CrossRef]
- Krasňan, V.; Stloukal, R.; Rosenberg, M.; Rebroš, M. Immobilization of Cells and Enzymes to LentiKats®. Appl. Microbiol. Biotechnol. 2016, 100, 2535–2553. [Google Scholar] [CrossRef]
- Homburg, S.V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A. V Entrapment and Growth of Chlamydomonas Reinhardtii in Biocompatible Silica Hydrogels. Colloids Surf. B Biointerfaces 2019, 173, 233–241. [Google Scholar] [CrossRef]
- Desmet, J.; Meunier, C.F.; Danloy, E.P.; Duprez, M.-E.; Hantson, A.-L.; Thomas, D.; Cambier, P.; Rooke, J.C.; Su, B.-L. Green and Sustainable Production of High Value Compounds via a Microalgae Encapsulation Technology That Relies on CO2 as a Principle Reactant. J. Mater. Chem. A 2014, 2, 20560–20569. [Google Scholar] [CrossRef]
- Rehbein, P.; Raguz, N.; Schwalbe, H. Evaluating Mechanical Properties of Silica-Coated Alginate Beads for Immobilized Biocatalysis. Biochem. Eng. J. 2019, 141, 225–231. [Google Scholar] [CrossRef]
- Reid, A.; Buchanan, F.; Julius, M.; Walsh, P.J. A Review on Diatom Biosilicification and Their Adaptive Ability to Uptake Other Metals into Their Frustules for Potential Application in Bone Repair. J. Mater. Chem. B 2021, 9, 6728–6737. [Google Scholar] [CrossRef]
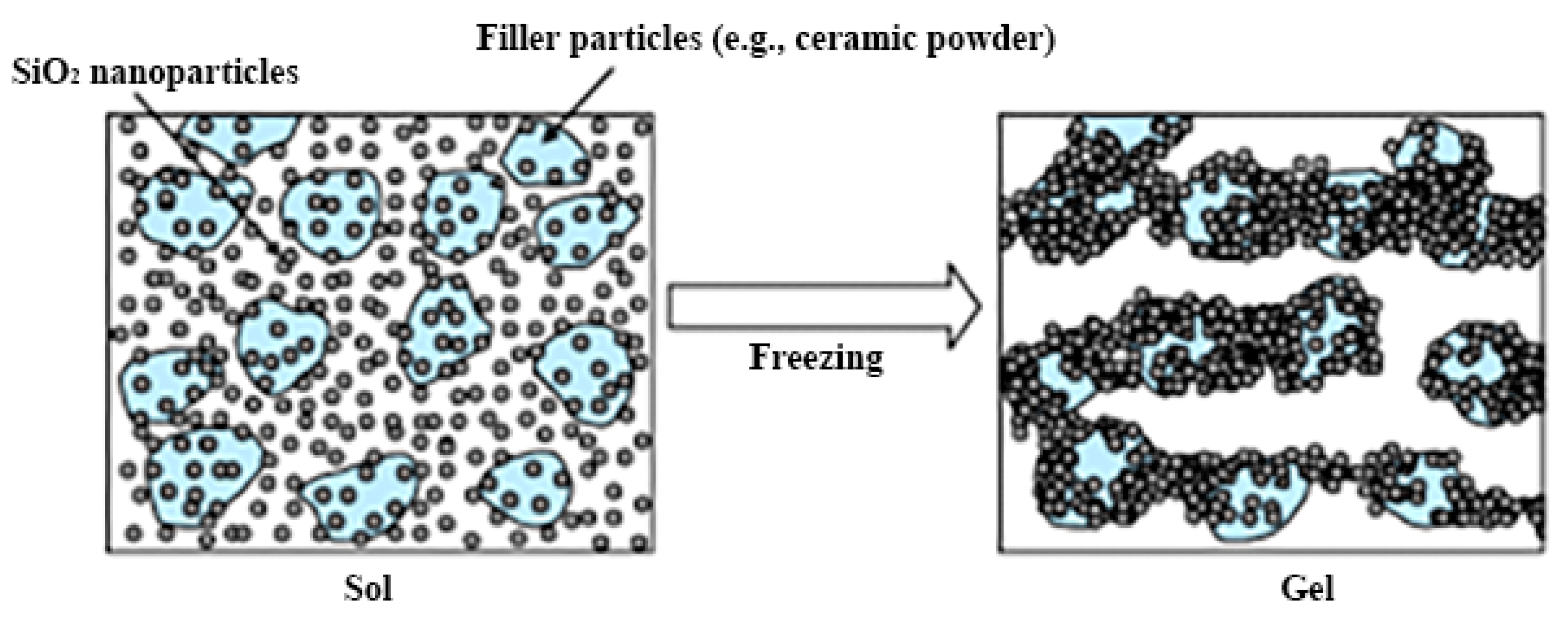
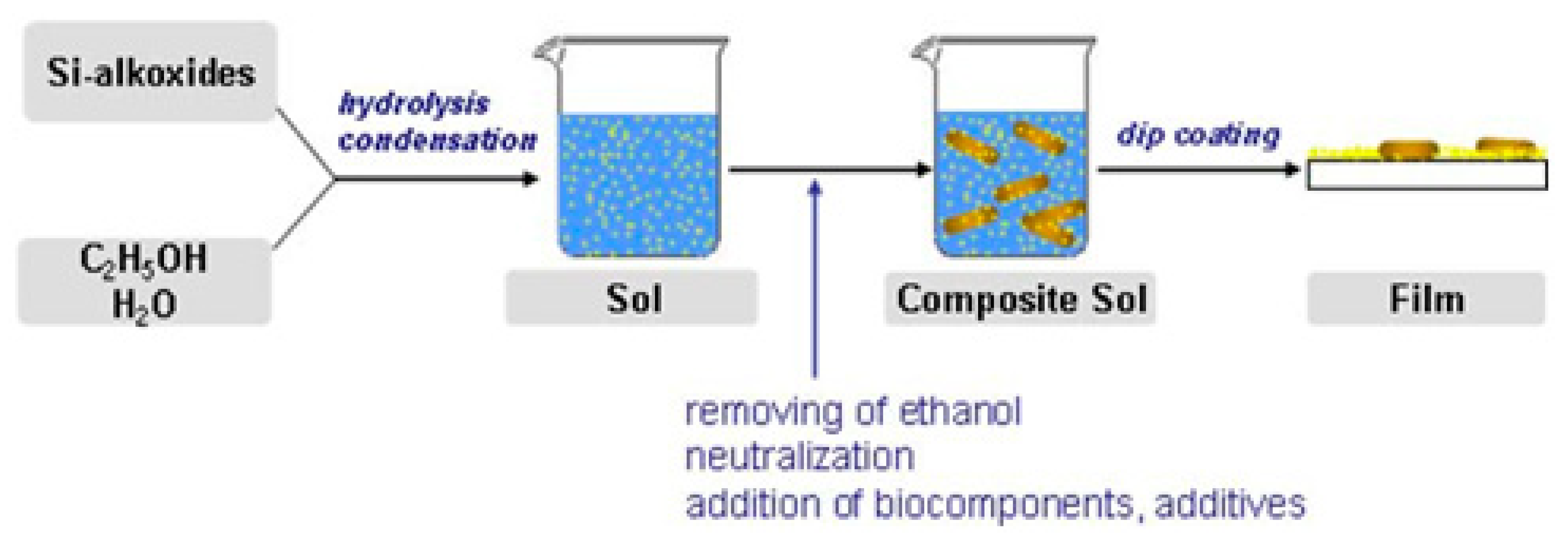
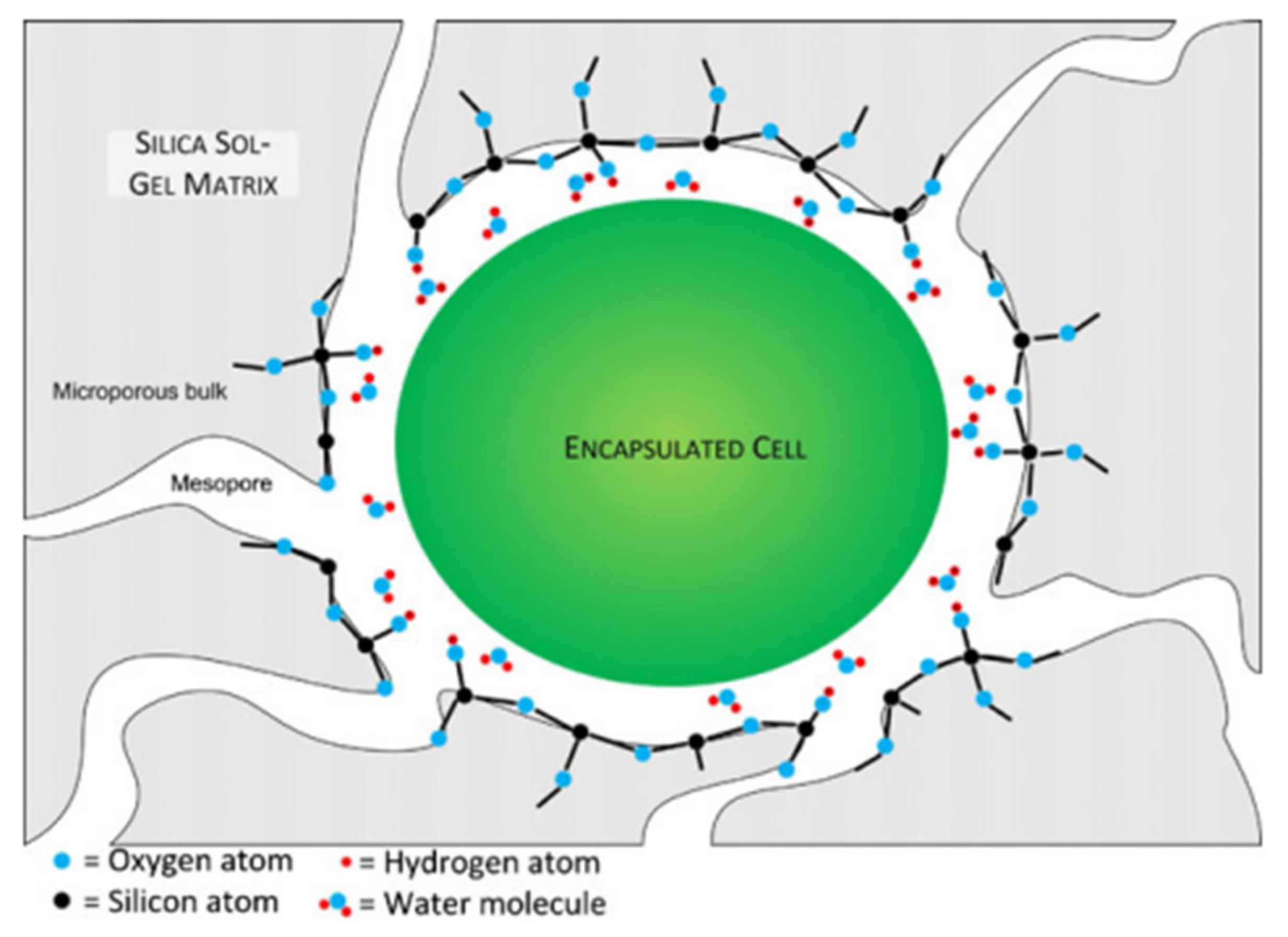
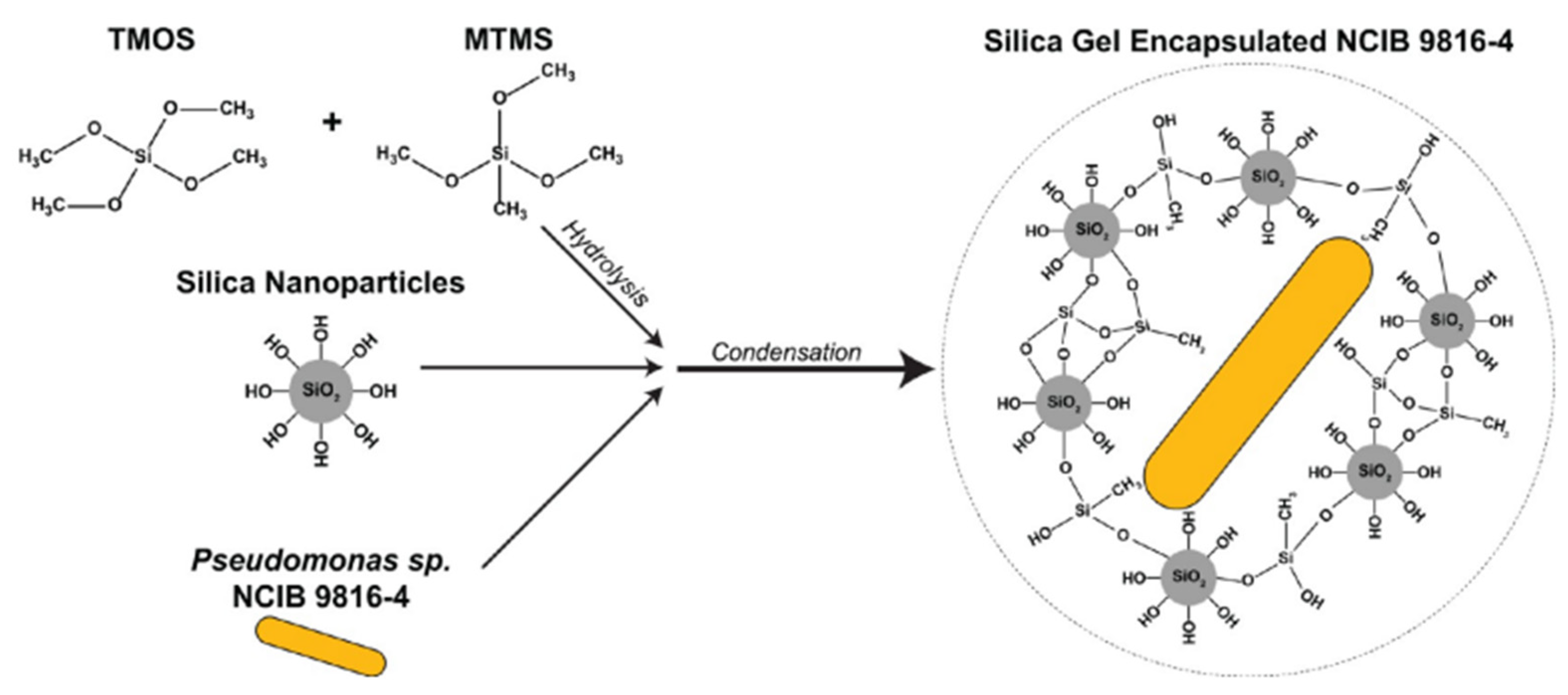


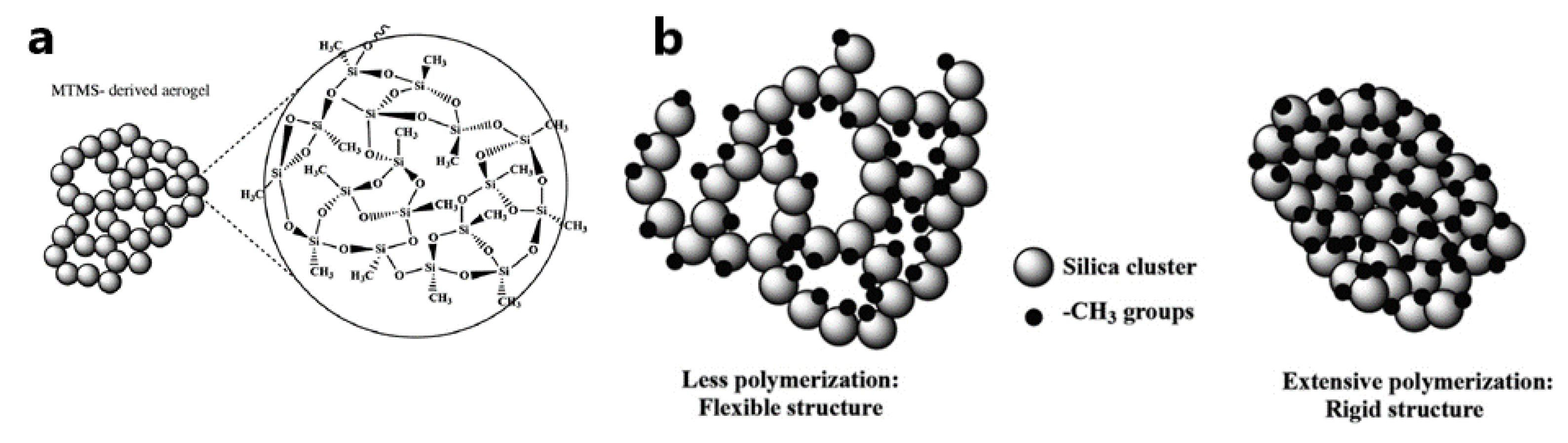
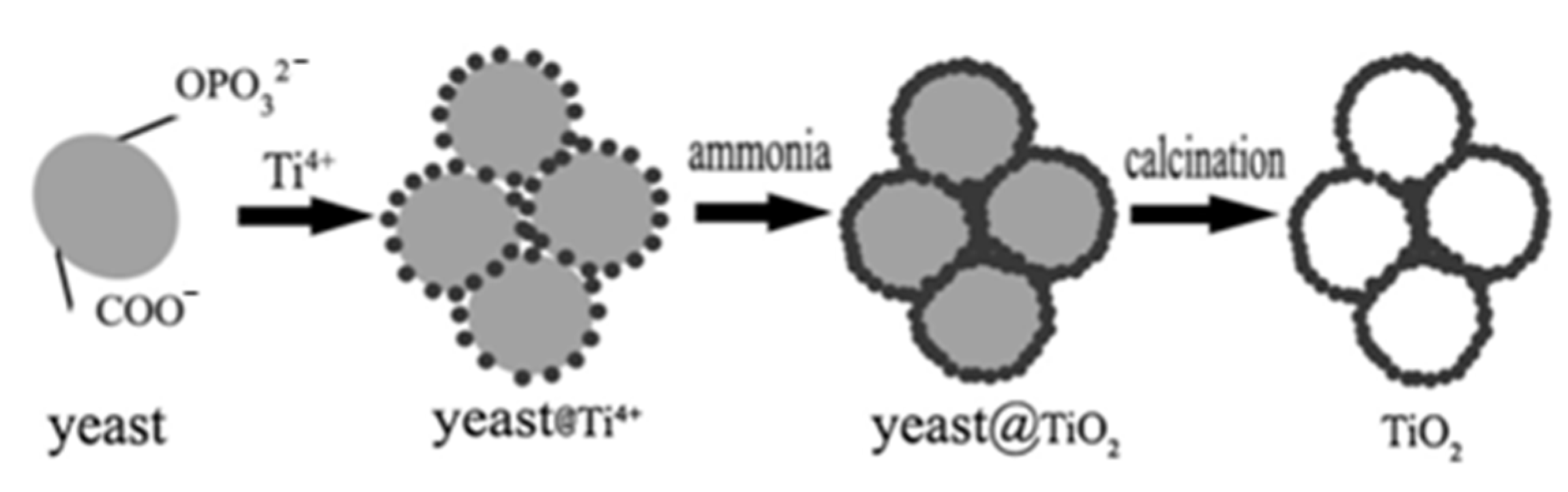
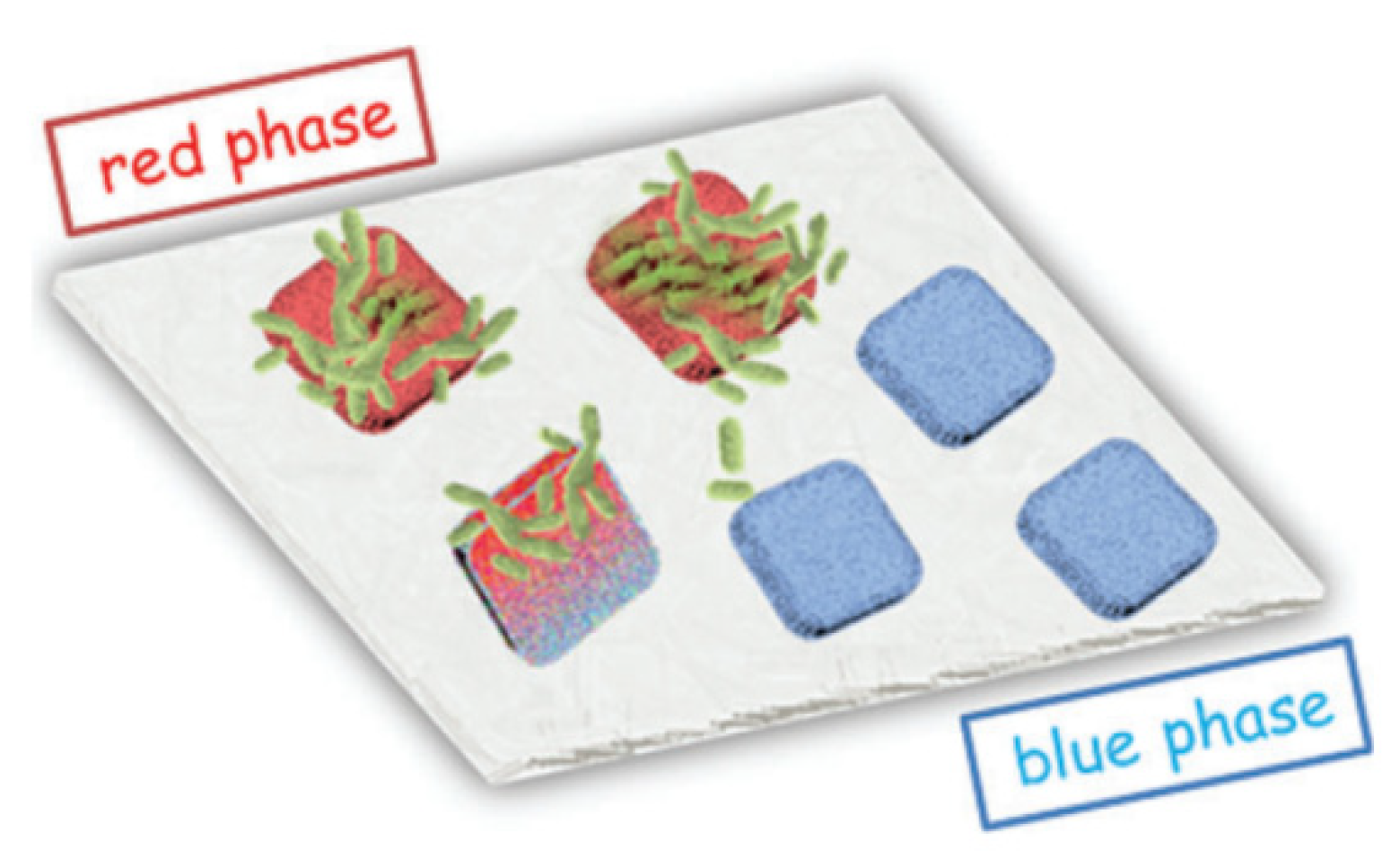
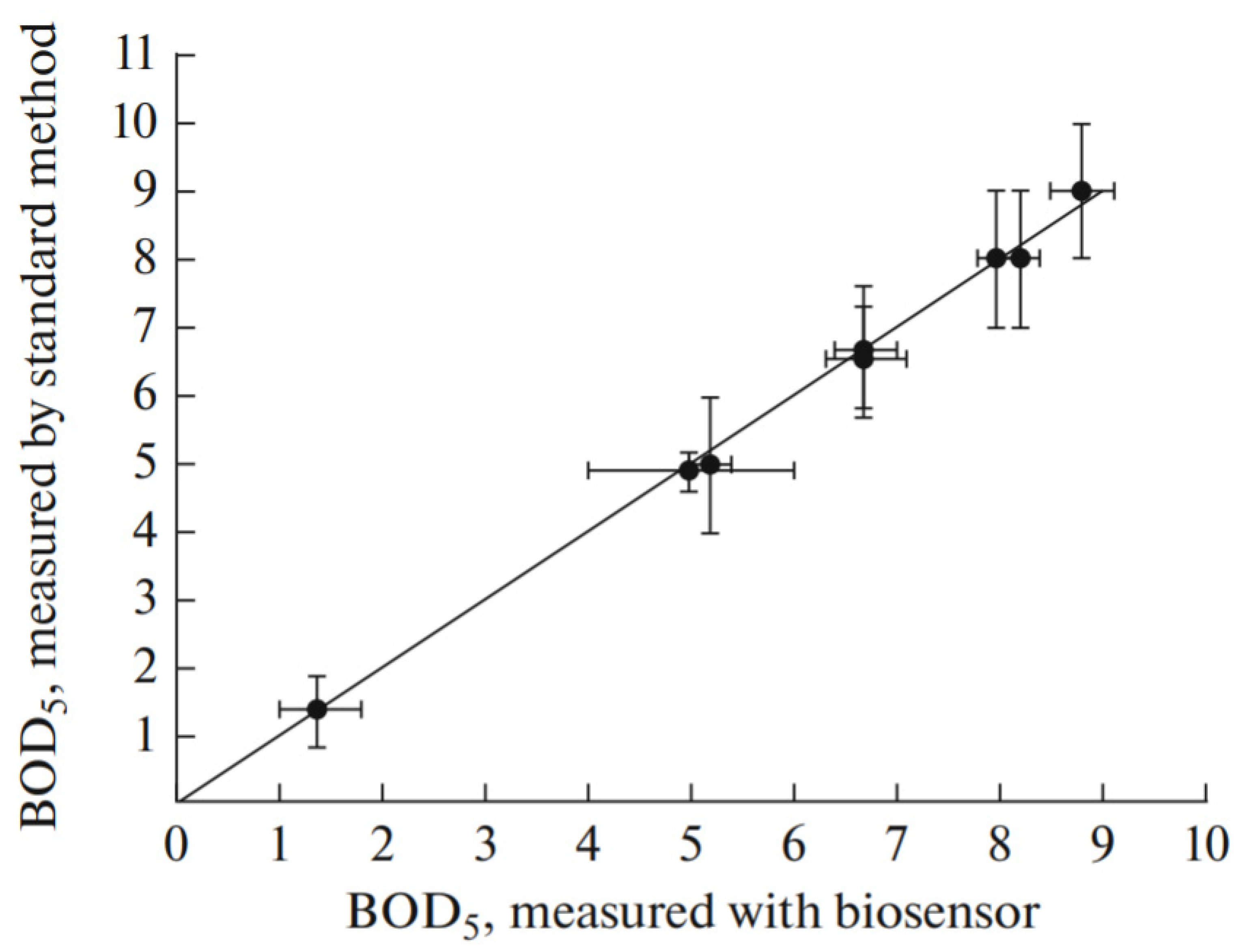
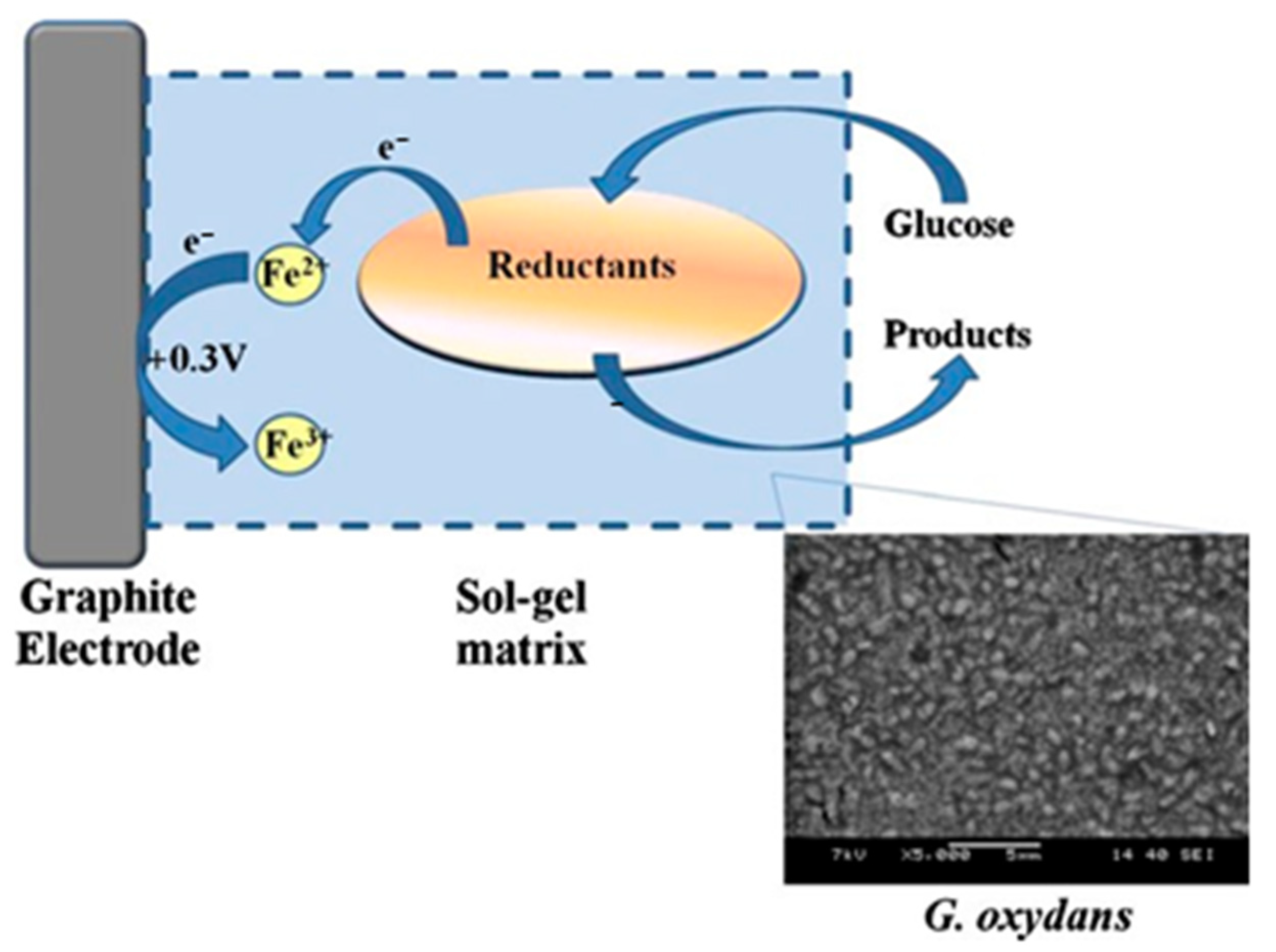
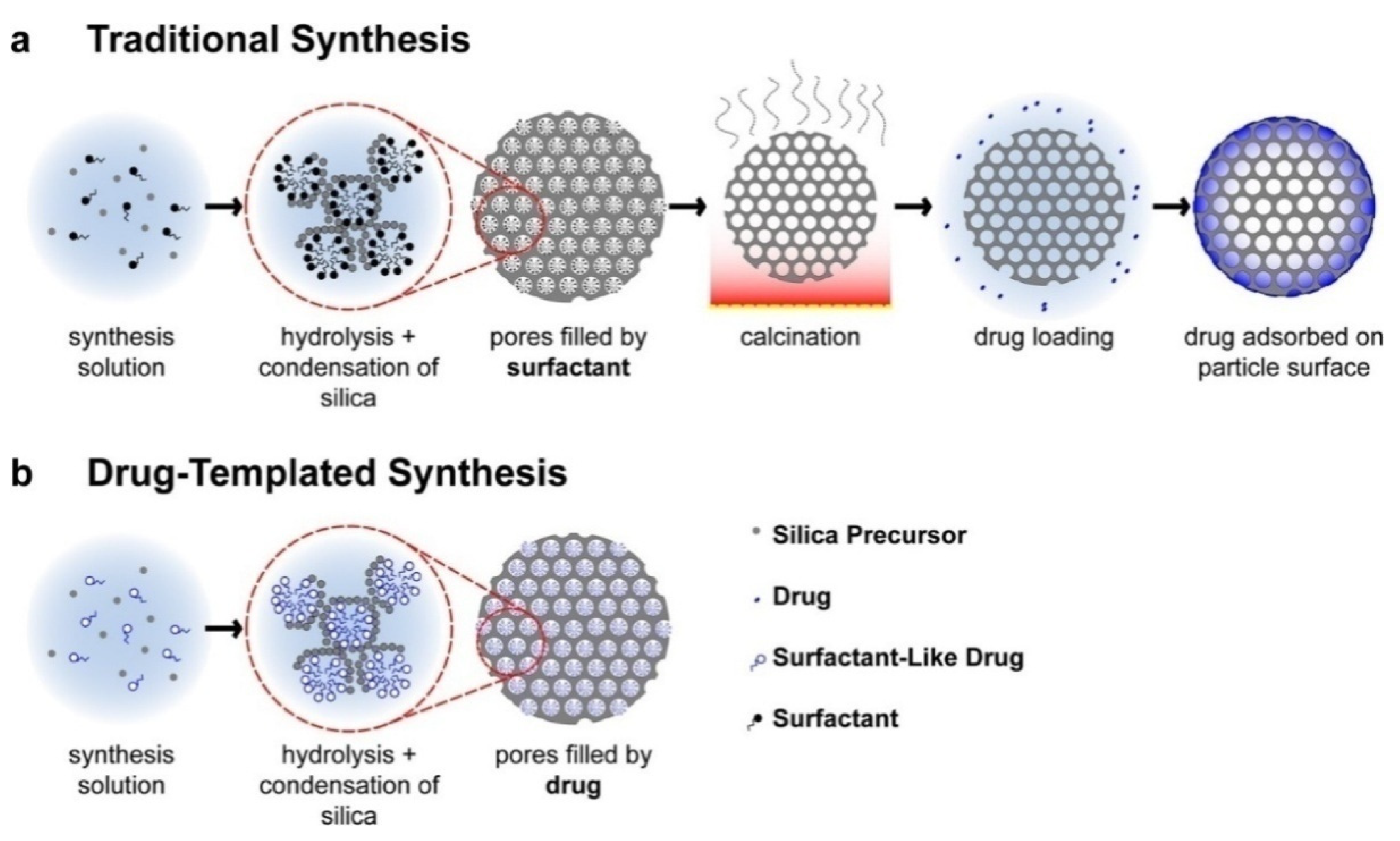
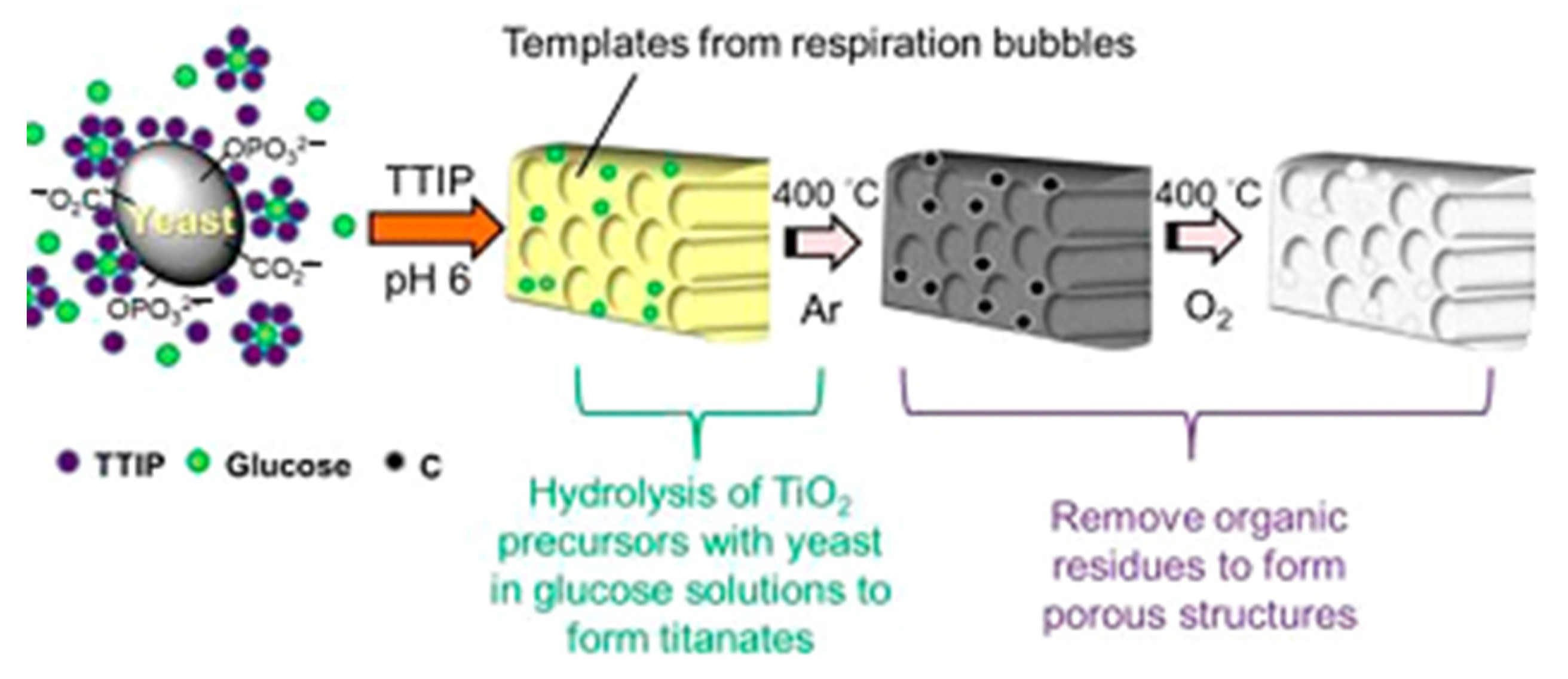
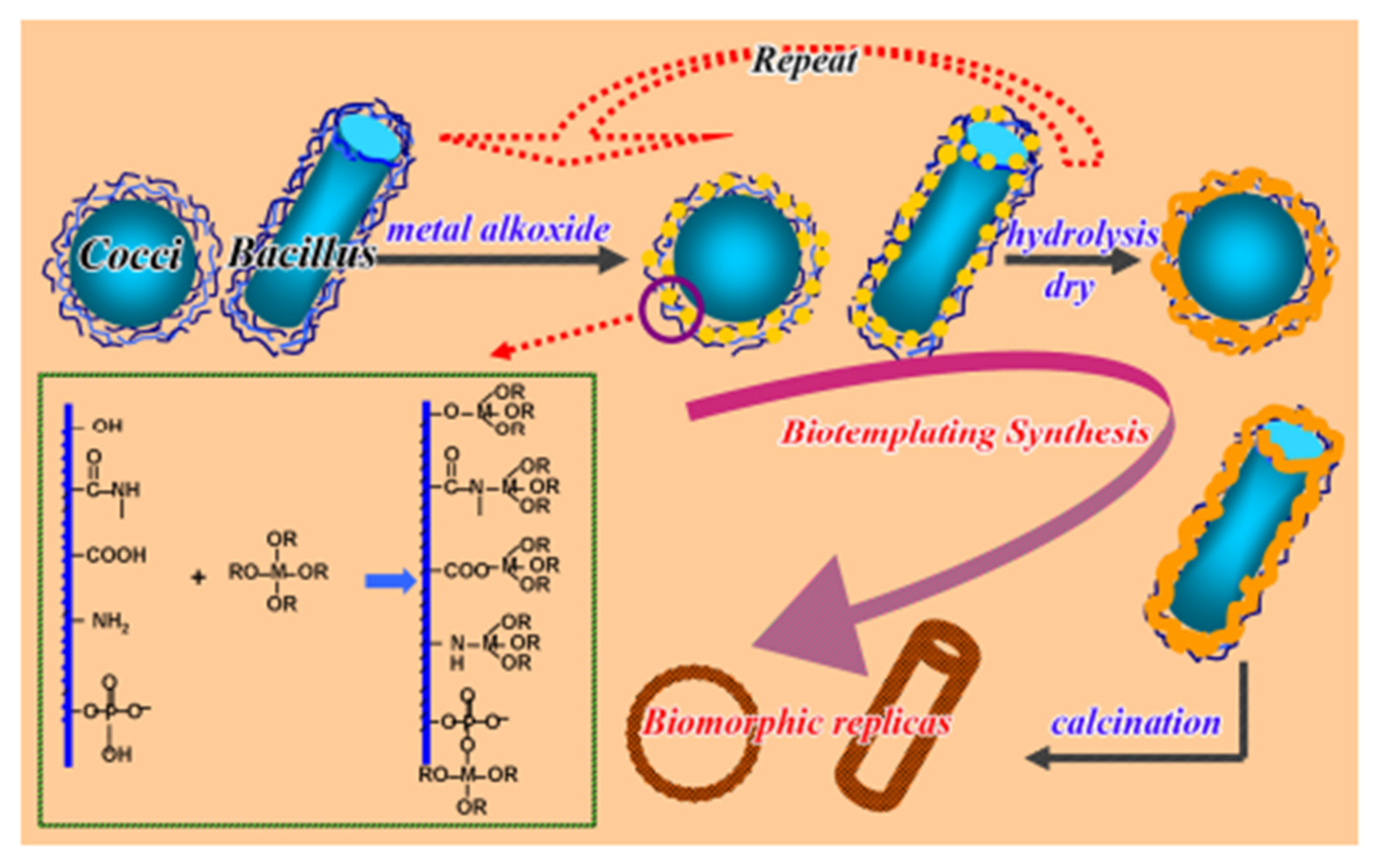
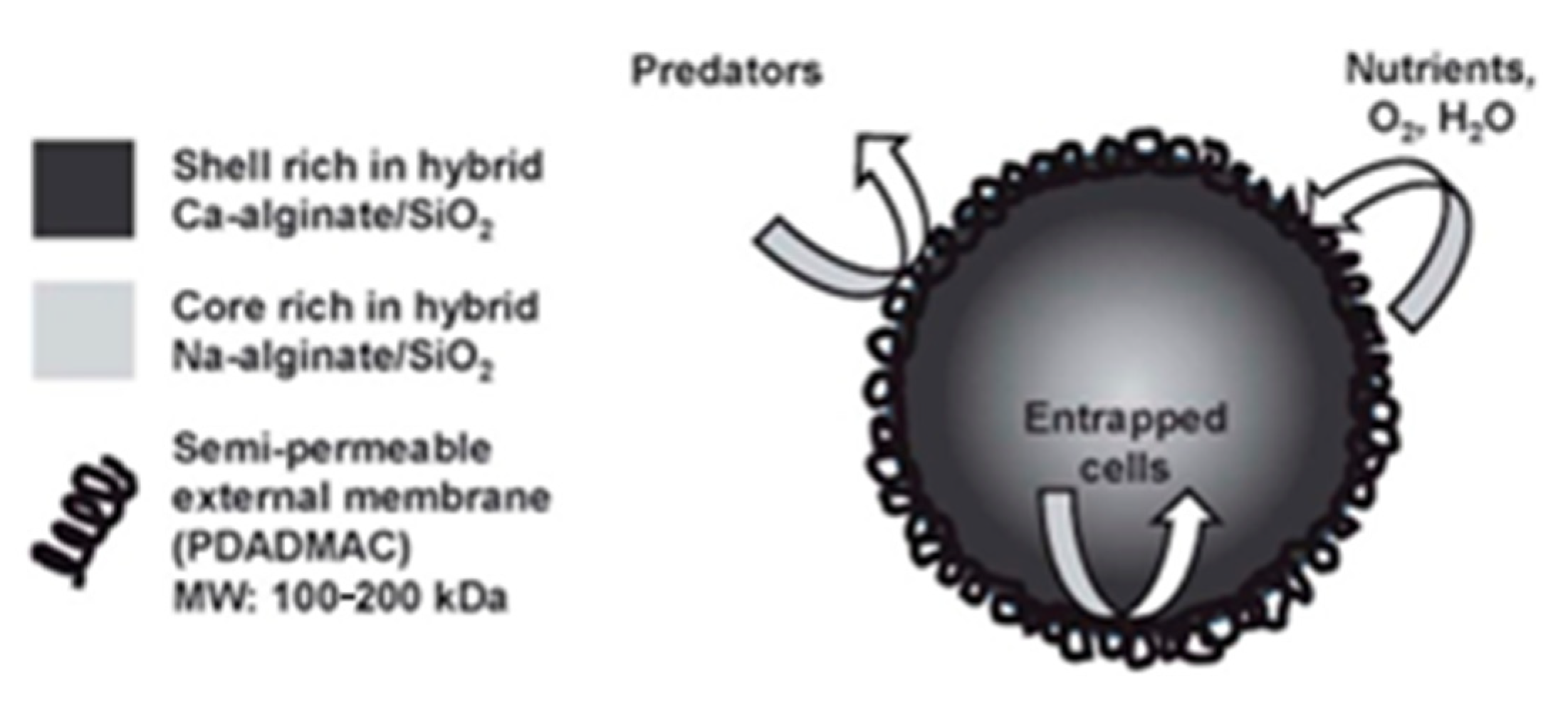
| Precursors | Organic Component | Biomaterial | Properties and Applications | Reference |
|---|---|---|---|---|
| SiO2 | chitosan derivative (CHT) containing completely natural quaternary amine fragments | human mesenchymal stem cells (hASC) | To get new generations of hybrid materials with silica shell functionalization by modifying the cell surface. These materials can be applied in various fields such as tissue engineering, biosensors, drug delivery, and targeted cell therapy. | [132] |
| TEOS, MTES, phenyltriethoxysilane, TMOS, aminopropyltriethoxysilane, colloidal silica, sodium silicate | polyethylene glycol, glycerin, chitosan, trehalose, N-(3-triethoxysilylpropyl)gluconamide (GLTES) | Escherichia coli | To use bacteria for the atrazine utilization and biosorption of Cd2+ ions. Efficiency is due to the presence of a hydrophobic additive in the required ratio. Search for such a ratio for the most efficient utilization of atrazine. To study the processes of encapsulated bacteria division and the preservation of their biological activity. To study the structure of the obtained material in the presence of various organic components to increase the long-term operation of the biomaterial, as well as for research the activity of microorganisms immobilized in the sol-gel matrix during the aging of the immobilizing material. | [13,19,27,32,48,49,50,51,52,99] |
| TEOS, GLYEO (3-glycidyloxypropyl)-triethoxysilane | Pseudomonas fluorescens, Rhodococcus ruber, Haematococcus pluvialis, Nannochloropsis limnetica, Botryococcus braunii, Chlorella vulgaris | To obtain thin films. The catalytic activity of microorganisms was studied by the intensity of glucose oxidation. | [29] | |
| TEOS, titanium isopropoxide, triethoxysilane (TREOS), diethoxydimethylsilane (DEDMS), diethoxymethylsilane (DEMS) | glycerol | Saccharomyces cerevisiae | To study the long-term stability of immobilized material. To assess the rate of gene expression. For the development of biosensors. To study the morphology of the resulting structures and the thickness of the resulting films. | [34,50,51,63,64] |
| Aluminosilicate | Rhodococcus ruber | To study the biological activity, mechanical strength, and structure of biologically active ceramic composites derived from Rhodococcus ruber bacteria and capable of degrading phenol. The immobilized cells showed no decrease in activity when stored for several months at 4 °C. They can be stored for up to 12 months without losing their biological activity. | [21] | |
| Silicon oxide | Mesotaenium sp. Synechococcus sp. | To produce optical biosensor for detection of heavy metal ions Cd2+, Cr6+ and Zn2 + in aqueous media. | [97] | |
| Colloidal SiO2 SiNa/LUDOX 1/1 | Genus Bacillus | To maintain the viability of immobilized microorganisms for several months. To improve mechanical stability. For the determination of heavy metal ions. | [41,47] | |
| TEOS, MTMS, TMOS, sodium silicate | glycerol | Pseudomonas sp. | The cells retain high viability for 365 days after immobilization when stored in phosphate buffer at 4 °C. Immobilized cells are able to efficiently produce biosurfactants and can participate in the biodegradation of azo dyes. | [43,44,45,93] |
| Colloidal silicon dioxide | Streptococcus lactise | To increase the catalytic activity of immobilized cells and the protective function of the matrix. | [46] | |
| Sodium silicate | nodule bacteria of the genus Rhyzobium | Immobilization of microorganisms in a sol-gel matrix can be considered as an alternative for long-term storage of nodule bacteria. | [53] | |
| TEOS | glycerol | cyanobacteria | To obtain a porous organosilicon capsule. This capsule protects each cell of cyanobacteria from mechanical damage but does not prevent the rapid diffusion of low molecular weight substances through the pores of the capsule. | [26] |
| Sodium silicate MTMS | Metylomonas sp. GYJ3 | The activity of encapsulated microorganisms was maintained at 4 °C for 45 days. | [58] | |
| TMOS | Paracoccus denitrificans | To determine the content of phospholipids of fatty acids using an optical sensor. | [27] | |
| TEOS | Yarrowia lipolytica | The resulting biohybrid material has the ability to remove Cr (III) and Cr (VI) pollutants with high efficiency and without special pre-treatment from water. | [65] | |
| Polyvinyl alcohol and 4-vinylpyridine | Trichosporon cutaneum | To maintain the viability of encapsulated cells. Arthroconidia are formed in extracellular material and play an important role in maintaining the long-term viability of microorganisms. This may be due to the fact that arthroconidia have the ability to withstand environmental stresses. A biosensor based on encapsulated yeast has been used to analyze biochemical oxygen demand in contaminated wastewater. | [66] | |
| TEOS | Paracoccus denitrificans | Monitoring the state of cells (live/dead) immobilized in silica gel by determining phospholipid fatty acids. | [69] | |
| TEOS | E. coli, Staphylococcus aureus | SiO2 nanoparticles are biologically inert and have an antimicrobial effect against E. coli and Staphylococcus aureus bacteria. Nanoparticles are non-toxic, which was shown in a study on a human lung epithelial cell line (A549) | [63] | |
| TEOS, aluminum silicate | glycerin, trehalose | Aquincolatertiaricarbonis L108 | Development of biofilters that are able to decompose difficult-to-oxidize methyl tret-butyl ether and ethyl tret-butyl ether. Immobilized biomaterial can be stored up to 8 months. | [71] |
| TEOS | Lodderomyceselongisporus, Candida norvegica, Debaryomyces fabryi, Pichia carsonii | Development biocatalysts of the next generation. They provide longer catalytic activity of immobilized cells. | [67] | |
| TEOS | Humicola lutea, Bacillus sp. | [72] | ||
| TEOS, tetra(n-propylamino)silane | Chlamydomonas reinhardtii ent | Comparison of cell viability immobilized with silane precursors and immobilized with sodium silicate. | [82] | |
| Tetraethyl orthosilicate | Trametes versicolor | The characterization of the free silica and Trametes versicolor cells in ceramic matrices was carried out by using scanning electron microscope, transmission electron microscope, Fourier transform infrared spectrophotometer, nitrogen adsorption–desorption measurement, and catalytic activity assay. | [77] | |
| TEOS | Algae | Biosorption of heavy metals | [78] | |
| TEOS | Ralstonia eutropha MTCC 2487 | Immobilized bacteria utilize arsenic As (V) | [90] | |
| TEOS, GLYEO (3-glycidyloxypropyl), TEOS | Microalgae cells Haematococcus pluvialis | Microalgae immobilized in sol-gel layers can be used for the biotechnological production of astaxanthin. It has been shown that the formation of astaxanthin during cultivation can be increased by the combined use of Fe2+ compounds with NaCl or hydrogen peroxide as stress factors. | [68] | |
| Sodium silicate | Synechocystis sp. PCC 6803 | To study gene expression of encapsulated microorganisms. | [91] | |
| diamino-functional silane N-(2-aminoethyl)-3-aminopropyltrimethoxysilan, TEOS | Sodium alginate | Chlorella vulgaris | To improve the stability of Chlorella vulgaris cells in saline solutions, as well as to achieve stable reproducibility of the obtained materials. | [96] |
| TEOS | chitosan | Gluconobacter oxydans | A mediator whole-cell biosensor with acetic acid bacteria was created and characterized. Bacteria were immobilized on a graphite electrode using a hybrid composite obtained by the sol-gel method. | [98] |
| TEOS, MTES | PEG | Pichia angusta, Cryptococcus curvatus | To create a biosensor for the utilization of lower alcohols and determine their concentration. | [101] |
| 3-aminopropyl trimethoxysilane TMOS, DiMe-DMOS, TEOS, silicon oxide | poly(vinylalcohol), 4-vinylpyridine (PVA-g-P(4-VP)) PVA, 4-vinylpyrrolidone | E. marius, B. horikoshii, H. Marina B. licheniformis, D. marisand, M. marinus, Trichosporon cutaneum, Bacillus subtilis | To create a BOD biosensor. | [66,103,104,105] |
| Silica nanoparticles | polyethyleneimine (PEI) | Sphingomonas sp. | To improve the previously created optical microplate biosensor for methyl parathion based on Sphingomonas sp. | [56] |
| TEOS | Escherichia coli, Chromobacterium violaceum, Lodderomyces elongisporus | To create biocatalysts capable of joint utilization of organic substances. | [107] | |
| TEOS | Lodderomyceselongisporus, Pichia carsonii, Candida norvegica, Debaryomyces fabryi | To create biocatalysts for organic synthesis. | [108] | |
| TEOS | Citrus aurantium Lextract | Citrus flavonoids were immobilized in a sol-gel matrix. Sol-gel synthesis and structure formation were investigated using X-ray diffraction patterns (XRD), Fourier transform infrared spectroscopy (FTIR), scanning and transmission electron microscopes (TEM). The resulting nanohybrid materials had an agglomerated amorphous structure with a particle size of 171–199 nm. | [111] | |
| TEOS | Tannins from Acacia mearnsii | The best results were obtained using the silicate sol-gel method. Only hybrid materials prepared using the silicate route have demonstrated good antimicrobial activity. The bactericidal activity of the materials was close to that of pure tannins. Thus, the sol-gel process prevents the loss of tannin through oxidation and hydrolysis. The tannin can be released in an aquatic environment in a controlled manner. | [114] | |
| TEOS | Chlamydomonas reinhardtii | To increase cell viability. To develop a low ethanol synthesis method. | [129] | |
| Titanium tetraisopropoxide, TiSO4 | Yeast cells | Cells were used to form a material with a given structure (they were then burned out). The material can be applied as the anode of a lithium-ion battery. | [31,38] | |
| Titanium (IV) oxide (immobilized on silicon oxide or activated carbon support) | Material-catalyst for utilization of organic pollutants. | [35] | ||
| Ti(OEt)4 | triethanolamine | A. chlorophenolicus, P. anomala, Lb. plantarum | [37] | |
| Bis(ammonium lactato) titanium dihydroxide (IV) | Poly (diallyldimethylammonium)chloride, alginate | To create a mesoporous and biocompatible material as a repository of animal cells for use in cell therapy. | [85] | |
| Butoxide tetraethyl titanium | Yeast | Catalytic tests have shown that the new N-TiO2/MnO2 hollow nanosphere has a higher photodegradation activity against formaldehyde gas under visible irradiation than commercial TiO2. This is explained by the higher surface area (160 m2g−1) of the hollow structure. The catalytic efficiency of the developed material was more than 90%, which is about 10 times higher than that of the traditional TiO2-P25 catalyst. | [112] | |
| Aluminum chloride (thermohydrolysis in alkaline medium) | glycerol | Escherichia coli | To use alumina, the rate of formation of the material is higher, and the survival of microorganisms is lower compared to the material obtained on the basis of silicon oxide precursors. | [36] |
| Aluminosilicate | Rhodococcus ruber | To study the biological activity, mechanical strength, and structure of biologically active ceramic composites obtained on the basis of Rhodococcus ruber bacteria capable of degrading phenol. The immobilized cells showed no decrease in activity when stored for several months at 4 °C. They can be stored for up to 12 months without loss of their biological activity. | [21] | |
| Incubated wet yeast | To create a BOD-biosensor | [86] | ||
| Ce(NO3)3 | Morinda citrifolia | IR spectroscopy has proven the production of cerium oxide nanoparticles, which are formed due to the extract of Morinda citrifolia. The TEM method demonstrated the formation of spherical nanoparticles. | [33] | |
| CeO2 nanoparticles (embedded in transparent silica hydrogel, TEOS) | Chlorella vulgaris | The resulting materials have protective properties due to the applied precursors. The immobilized cells were protected from UV, H2O2. | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamanina, O.A.; Saverina, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology. Nanomaterials 2022, 12, 1086. https://doi.org/10.3390/nano12071086
Kamanina OA, Saverina EA, Rybochkin PV, Arlyapov VA, Vereshchagin AN, Ananikov VP. Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology. Nanomaterials. 2022; 12(7):1086. https://doi.org/10.3390/nano12071086
Chicago/Turabian StyleKamanina, Olga A., Evgeniya A. Saverina, Pavel V. Rybochkin, Vyacheslav A. Arlyapov, Anatoly N. Vereshchagin, and Valentine P. Ananikov. 2022. "Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology" Nanomaterials 12, no. 7: 1086. https://doi.org/10.3390/nano12071086








